Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Synthesis and in vitro studies of PLGA-DTX nanoconjugate as potential drug delivery vehicle for oral cancer
Authors Gupta P, Singh M , Kumar R, Belz J, Shanker R , Dwivedi PD, Sridhar S, Singh SP
Received 18 October 2016
Accepted for publication 9 January 2017
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 67—69
DOI https://doi.org/10.2147/IJN.S124995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Parul Gupta,1 Manjri Singh,2 Rajiv Kumar,3 Jodi Belz,3 Rishi Shanker,4 Premendra D Dwivedi,1 Srinivas Sridhar,3 Surinder P Singh2
1CSIR-Indian Institute of Toxicological Research, Lucknow, 2CSIR-National Physical Laboratory, New Delhi, India; 3Nanomedicine Science and Technology Center, Northeastern University, Boston, MA, USA; 4Institute of Life Sciences, Ahmedabad University, Ahmedabad, India
Abstract: Advances in nanotechnology have led to the design of multifunctional nanoparticles capable of cellular imaging, targeted drug delivery, and diagnostics for early cancer detection. We synthesized poly(lactic-co-glycolic acid) nanoparticles encapsulating a model radiosensitizing drug docetaxel accomplishing localized in situ delivery of the sensitizer to the tumor site. The synthesized nanoparticles have been characterized for their physicochemical properties. The in vitro cytotoxicity of drug-loaded nanoparticles has been studied on human tongue carcinoma cell line SCC-9 (ATCC-CRL-1629).
Keywords: docetaxel, PLGA nanoformulation
Introduction
Head and neck squamous cell carcinoma is a highly destructive cancer with poor diagnosis and low overall survival rate.1 Besides known risk factors (eg, alcohol and smoking), the entirety of the relevant tumor biology is still unclear. Chemotherapeutic agents are cytotoxic drugs used to treat cancer that function by targeting fast growing malignant cells and by inhibiting the cell division process impairing mitosis as well as promoting apoptosis. However, these pharmacologically active cancer drugs reach the tumor tissue with poor specificity and dose limiting toxicity.2 In order to enhance the potency of these promising drugs, new formulations have been proposed which overcome the voids of conventional drug therapy. Docetaxel (DTX) is known as one of the most efficient anticancer drugs. However, its poor water solubility and systemic toxicity have greatly restricted its clinical application. Recently, with the development of nanotechnology, new drug delivery systems for various chemotherapeutic agents have been emerged, which can increase their water solubility, reduce the side effects, and increase the tumor-targeting distribution by passive or active targeting.
In this study, we synthesized poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) loaded with a model radiosensitizing drug DTX and administered intratumorally to provide localized in situ delivery of the sensitizer to the tumor site. The synthesized NPs have been characterized for their physicochemical properties. The in vitro cytotoxicity of drug-loaded NPs has been studied on human tongue carcinoma cell line SCC-9 (ATCC-CRL-1629).
Materials and methods
The reagents used were purchased from Sigma Chemical Co. Ltd. (St Louis, MO, USA). The SCC-9 (ATCC® CRL-1629™) cell line was purchased from American Type Culture Collection (ATCC, University Boulevard, Manassas, VA, USA).
Formulation of NPs
We have synthesized PLGA NPs encapsulating DTX using simple nanoprecipitation method in a single step. Briefly, 1:1 (wt. ratio) of PLGA and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine, conjugated polyethylene glycol, (DSPE-PEG) were mixed in 250 μL of dichloromethane. A total of 75 μL of DTX was added to the clear transparent PLGA/DSPE-PEG solution and sonicated for 15 s. Then, 0.4 mL of 2.5% aqueous polyvinyl alcohol (PVA) solution was added to the reaction mixture while vortexing. The obtained viscous emulsion was further sonicated for 30 s and added to 10 mL of 0.3% aqueous PVA solution with vigorous stirring. The optically transparent appearance of the reaction mixture results in the formation of PEGylated PLGA NPs.
Characterization
The NP formulation DTX-loaded PLGA NPs were extensively characterized using various techniques. The photophysical characterization was done using UV-visible and photoluminescence spectroscopy and the physicochemical characterization was done using transmission electron microscopy (TEM), dynamic light scattering (DLS), and zeta potential studies.
Release kinetics studies
The encapsulation efficiency (EE) and the rate of release of DTX from DTX/Cy7.5/PEG-PLGA NPs were quantified using high performance liquid chromatography at 30°C with a mobile phase of methanol/H2O (70/30, v/v) and a flow rate of 1.0 mL/min using a C18 reverse phase column. The DTX EE was calculated as the ratio between the encapsulated DTX over the initial drug amount used for synthesis.
Cell culture and in vitro cytotoxicity assay
Human tongue carcinoma cell line SCC-9 (ATCC-CRL-1629) was cultured in mixture of Dulbecco’s Modified Eagle’s Medium and Ham’s F12 medium supplemented with 400 ng/mL hydrocortisone, 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2.
Metabolic activity was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in 96-well culture plates at the density of 5,000 viable cells/well and allowed to adhere for 24 h. After adherence, the cells were treated with different concentrations of free DTX and DTX-loaded PLGA NP for 24 h. At designated time intervals, the medium was removed, and the wells were washed twice with phosphate-buffered saline. Ten microliters of MTT assay solution was added to each well of the plate and incubated for another 2 h. Then, 100 μL of the solubilization solution was added to the wells and the plates were kept in the dark for 1 h at room temperature. After gentle mixing of contents in each well, the absorbance (OD) of each well was recorded at 570 nm using a 96-well plate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Results and discussion
This novel nanoprecipitation method yields PLGA NPs of uniform size with PEG decorated surface. A negatively charged surface due to the presence of PVA and PEG of the NPs will prevent opsonization and thus will evade the reticuloendothelial system. Figure 1 shows the size and morphology of the NPs as studied by TEM and DLS, respectively. The TEM micrograph revealed ~120 nm average particle size of spherical shape NPs. The size of NPs was further correlated with DLS measurements that showed the hydrodynamic diameter of ~150 nm which is in agreement with TEM results.
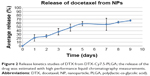
The release of the drug from NPs was monitored by the dialysis method against the infinite pool of buffered Tween 80 solution. It can be analyzed from the Figure 2 that NPs encapsulating DTX showed a slow and sustained release of the encapsulated drug with over 60% of the drug released in 9 days.
The cytotoxicity of NP formulations was assessed by MTT assay using SCC-9 cell line. The percentage of cell growth inhibition was assayed by the number of surviving cells after 24-h incubation. Figure 3 clearly indicates that the PLGA DTX NPs formulation showed higher cytotoxic effect than free drug after 24-h incubation period.
  | Figure 3 In vitro cytotoxicity study of DTX and PLGA-DTX on SCC-9 human carcinoma cell line. |
TEM image shows a spherical morphology of the NPs with a narrow size distribution and concentration (μg/mL).
Conclusion
In this study, PLGA polymers were successfully employed for the nano encapsulation of DTX, by using nanoprecipitation method. The release kinetics of drug reveals the slow release of the drug. The in vitro studies showed an increased antiproliferative efficiency of PLGA DTX NPs compared with free DTX, in a dose dependent manner against SCC-9 human tongue carcinoma cell line.
Acknowledgment
IUSTF funded JC-Nanomedicine for Head & Neck Cancer/34-2012/2013-14, NSF-IGERT grants are gratefully acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
Zigon G, Berrino F, Gatta G, et al; Francisci and the EUROCARE Working Group. Prognoses for head and neck cancers in Europe diagnosed in 1995–1999: a population-based study. Ann Oncol. 2011;22(1):165–174. | ||
Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909–1917. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.