Back to Journals » Drug Design, Development and Therapy » Volume 8
Synthesis and antibacterial activities of acylide derivatives bearing an aryl-tetrazolyl chain
Authors Shan L, Sun P, Guo B, Xu X, Li Z, Sun J, Zhou S, Chen W
Received 7 April 2014
Accepted for publication 21 May 2014
Published 24 September 2014 Volume 2014:8 Pages 1515—1525
DOI https://doi.org/10.2147/DDDT.S65673
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ling-Xing Shan,1 Ping-Hua Sun,1,2 Bao-Qin Guo,1 Xing-Jun Xu,1 Zhi-Qiang Li,1 Jia-Zhi Sun,2 Shu-Feng Zhou,2 Wei-Min Chen1
1Guangdong Province Key Laboratory of Pharmacodynamic Constituents of Traditional Chinese Medicine and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, People's Republic of China; 2College of Pharmacy, University of South Florida, Tampa, FL, USA
Abstract: Seventeen acylides bearing an aryl-tetrazolyl alkyl-substituted side chain were synthesized, starting from clarithromycin, via several reactions including hydrolysis, acetylating, esterification, carbamylation, and Michael addition. The structures of all new compounds were confirmed by 1H nuclear magnetic resonance spectroscopy, 13C nuclear magnetic resonance spectroscopy, and mass spectrometry. All these synthesized acylides were evaluated for in vitro antimicrobial activities against gram-positive pathogens (Staphylococcus aureus, Staphylococcus epidermidis) and gram-negative pathogens (Pseudomonas aeruginosa, Escherichia coli), using the broth microdilution method. Results showed that compounds 10e, 10f, 10g, 10 h, 10o have good antibacterial activities.
Keywords: acylide, clarithromycin, synthesis, antibacterial activity
Introduction
Since the 1950s, macrolide antibiotics have been widely used for the clinical treatment of respiratory tract or soft tissue infections, but because of the extensive use of antibiotics, bacterial resistance has increased year by year.1–4 Allen proved that cladinose is the key group for bacterial resistance, and deglycosylation or modification of its cladinose is a feasible way to change a drug’s resistance.5 Modification at C-3 could enhance activity against efflux resistance. Tanikawa et al synthesized a series of clarithromycin derivatives in which hydrolysis and acylation occur at the C-3 position.6 It has been proven that compounds TEA-0769 and FMA-481 have better antibacterial activity than clarithromycin for macrolide-susceptible strains. They also have antibacterial activity against MLSB-resistant Staphylococcus aureus and efflux-resistant Streptococcus pneumoniae. After ketolide, acylide is another class of erythromycin derivatives that has antibacterial activity against macrolide resistance strains and has the potential to become a new generation of macrolide antibiotics.
Tetrazole, a bioisostere of carboxyl group or a peptide bond,7,8 is an important pharmacophore widely used in drugs such as matrix metalloproteinase inhibitors,9 monoamine oxidase B inhibitors,10 and antibacterial, antifungal, and antiproliferative agents.11 Water solubility can be improved by introducing tetrazole, because of its hydrophility, and its basicity will be also increased. Therefore, the introduction of tetrazole into acylide may not only enhance antibacterial activity but also improve its pharmacokinetic properties. On the basis of the previous study about ketolides, performed in our laboratory,12 the cladinosyl of the C-3 position in macrolide was substituted by aroyl, and the carbamate ring was connected with some different alky side chains bearing aryl-tetrazolyl. As a consequence, 17 new acylide derivatives were synthesized and evaluated for in vitro antimicrobial activities.
Materials and methods
Chemistry
Unless otherwise noted, all materials were commercially available and used without further purification. Dichloromethane and triethylamine were distilled from calcium hydride and sodium, respectively. The reactions were monitored by thin-layer chromatography (silica gel 60 GF254; Qingdao Haiyang Chemical Co, Ltd, Qingdao, Shandong, People’s Republic of China) at 254 nm. 1H nuclear magnetic resonance spectroscopy (NMR) and 13C NMR spectra were recorded on Bruker-AV300MHz nuclear magnetic resonance spectrometers (Bruker Biosciences Corporation, Billerica, MA, USA). Chemical shifts are provided in parts per million downfield from tetramethylsilane (internal standard) with a coupling constant in hertz. Mass spectra and high-resolution mass spectrometry (HRMS) results were recorded on Thermo Finnigan LCQ Advantage MAX (Thermo Fisher Scientific, Waltham, MA, USA).
The compounds 2-(2H-tetrazol-5-yl) pyridine (3a), 3-(2H-tetrazol-5-yl) pyridine (3b), 4-(2H-tetrazol-5-yl) pyridine (3c), 5-phenyl-2H-tetrazole (3d), and 5-(thiophen-2-yl)-2H-tetrazole (3e) were prepared by a known procedure,13 and 1-methyl-1H-tetrazole-5-thiol (3f) was commercially available. The compounds 4a–4q and 5a–5q have been synthesized via a method we previously published.12
General procedure for preparation of compounds 4a–4q
To a solution of compounds 3a–3f (1 mmol) in anhydrous N, N-dimethyl formamide (5 mL) were added, successively, N-bromo-phthalimide (1 mmol) and anhydrous potassium carbonate (1 mmol), and the mixture was stirred for 10 hours at 80°C under nitrogen. The reaction mixture was quenched with ice water (10 mL). A crystalline solid was precipitated, and the filtered product was purified by silica-gel column chromatography (ether/ethyl acetate acetate =1:1 to petroleum ether/ethyl acetate/dichloromethane =1:1:1) to afford 4a–4q as white solid.
General procedure for the synthesis of compounds 5a–5q
To a solution of compounds 4a–4q (1 mmol) in the mixture of anhydrous ethanol (7 mL) and acetonitrile (5 mL) was added hydrazine monohydrate (2 mmol), and the mixture was then refluxed for 6 hours. After cooling, the mixture was filtered and the filtrate was evaporated in vacuo. Aqueous NaOH (20 mmol) was subsequently added to the residue and then extracted with dichloromethane (10 mL three times), washed with a saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The purification of the crude product by silica gel column chromatography (dichloromethane/methanol/triethylamine =9:1:0.1) afforded the desired compounds 5a–5q.
3-O-descladinosyl-10,11-anhydro-6-O-methyl- erythromycin A (compound 6)
To a solution of ethylene carbonate (51.6 mmol) and triethylamine (40 mL), clarithromycin (13.4 mmol) was added, and the reaction mixture was refluxed for 24 hours. Then additional ethylene carbonate (34.4 mmol) was added and the mixture was refluxed for 18 more hours. The solvent was removed under reduced pressure, and 23 mmol HCl aqueous solution, 40 mL ethanol, and 100 mL water were added. The reaction mixture was stirred for 24 hours at room temperature and then basified with NaOH aqueous solution (1 mol/L) to Ph 10–11. The precipitated, crude product was filtered off and recrystallized from the mixture of petroleum ether and acetone to afford compound 6, a white solid (7.2 g, 94.1%). 1H NMR (300 MHz, CDCl3) δ 6.39 (s, 1H), 5.00 (d, J=9.1 Hz, 1H), 4.69 (d, J=5.0 Hz, 2H), 3.85 (s, 1H), 3.74 (t, J=9.3 Hz, 1H), 3.49 (dd, J=10.8, 5.5 Hz, 1H), 3.15 (dd, J=27.9, 12.8 Hz, 1H), 3.08 (s, 3H), 2.74–2.56 (m, 3H), 2.44 (s, 3H), 2.22 (s, 6H), 2.09 (s, 3H), 2.02 (s, 3H), 1.94 (dd, J=9.2, 4.9 Hz, 1H), 1.88–1.77 (m, 1H), 1.72 (dd, J=12.9, 2.6 Hz, 1H), 1.52 (dd, J=12.7, 6.6 Hz, 3H), 1.37 (s, 3H), 1.27 (d, J=3.9 Hz, 6H), 1.16 (d, J=6.3 Hz, 3H), 0.91 (s, 3H), 0.88 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 207.59, 176.68, 141.29, 138.77, 106.77, 91.96, 80.90, 79.17, 77.65,77.22,73.63, 70.50,69.69, 65.58, 48.26, 44.33, 40.29, 38.32, 36.93, 36.40, 30.93, 21.34, 20.86, 20.37, 20.22, 16.13, 15.88, 12.94, 10.50, 7.62.
2′-Acetyl-3-O-descladinosyl -10,11-anhydro-6-O- methyl-erythromycin A (compound 7)
To a solution of acetic anhydride(16.8 mmol) and anhydrous triethylamine (16.8 mmol) in dichloromethane (30 mL), compound 6 (8.4 mmol) was added and stirred for 24 hours at room temperature. The reaction mixture was poured into saturated aqueous NaHCO3 (30 mL) and extracted with CH2Cl2 (20 mL ×3). The organic layer was washed with brine, dried over anhydrous Na2SO4, and filtered, and the solvent was removed under reduced pressure to yield white solid 7 (4.68 g, 90%). 1H NMR (300 MHz, CDCl3) δ 6.40 (s, 1H), 5.28 (s, 1H), 5.07–4.93 (m, 1H), 4.68 (dd, J=9.5, 5.3 Hz, 2H), 3.86 (d, J=2.7 Hz, 1H), 3.76 (d, J=10.4 Hz, 1H), 3.49 (dd, J=9.4, 5.9 Hz, 1H), 3.12 (d, J=7.0 Hz, 1H), 3.08 (s, 2H), 2.63 (dd, J=10.4, 6.8 Hz, 2H), 2.18 (d, J=17.9 Hz, 6H), 2.08 (s, 3H), 2.02 (s, 2H), 1.92 (dd, J=14.7, 7.1 Hz, 1H), 1.84–1.65 (m, 2H), 1.64–1.41 (m, 3H), 1.37 (d, J=7.7 Hz, 3H), 1.33–1.18 (m, 12H), 1.16 (d, J=6.5 Hz, 3H), 0.93–0.82 (m, 6H).13C NMR (75 MHz, CDCl3) δ 207.80, 175.83, 170.25, 140.93, 139.43, 102.53, 79.97, 79.38, 77.15, 73.29, 71.80, 69.18, 64.09, 48.84, 44.26, 40.69, 37.69, 37.43, 37.18, 30.12, 29.69, 21.45, 21.29, 21.19, 20.66, 20.27, 17.00, 15.55, 13.51, 10.58, 7.94.
2′-Acetyl-3-O-descladinosyl-3-O-(3-pyridyl)acetyl-10, 11-anhydro-6-O-methyl-erythromycin A (compound 8)
Triethylamine (10.8 mmol) was dissolved in a solution of 3-pyridine acetic acid hydrochloride (10.8 mmol) in anhydrous dichloromethane (20 mL). The mixture was cooled to −15°C, and pivaloyl chloride (21.6 mmol) was added drop wise, with intensive stirring under nitrogen. After completion of dripping, the resulting reaction mixture was stirred for 1 hour at −15°C, followed by the drop-wise addition of a solution of compound 7 (3.2 mmol) in anhydrous dichloromethane (10 mL) over a period of 15 minutes. 4-Dimethylaminopyridine (0.32 mmol) was then added and stirred for 5 hours at room temperature. The reaction mixture was finally poured into saturated aqueous NaHCO3 (30 mL) and extracted with dichloromethane. The organic layer was washed with brine, dried over anhydrous sodium sulfate, filtered, and evaporated in vacuo. The crude product was purified by column chromatography (petroleum ether/acetone/triethylamine =6:1:1 to 4:1:1) to afford the desired product 8 as white solid (1.6 g, 66.8%). NMR (300 MHz, CDCl3) δ 8.55–8.44 (m, 2H), 7.66 (dt, J=7.9, 1.9 Hz, 1H), 7.32–7.21 (m, 2H), 6.55 (d, J=0.9 Hz, 1H), 5.58 (dd, J=5.3, 1.8 Hz, 1H), 5.05 (dd, J=10.8, 2.5 Hz, 1H), 4.66 (dd, J=10.5, 7.5 Hz, 1H), 4.17 (d, J=7.4 Hz, 1H), 3.65 (s, 2H), 3.43 (d, J=7.6 Hz, 1H), 3.31 (dd, J=10.1, 6.2 Hz, 1H), 3.03 (s, 2H), 2.86 (s, 3H), 2.66–2.55 (m, 3H), 2.23 (s, 6H), 2.10 (d, J=3.9 Hz, 1H), 2.04 (s, 3H), 1.92 (d, J=0.6 Hz, 3H), 1.83 (ddd, J=14.1, 7.5, 2.6 Hz, 1H), 1.69 (dd, J=13.0, 2.5 Hz, 1H), 1.47 (ddd, J=14.1, 10.7, 7.3 Hz, 2H), 1.34 (s, 3H), 1.23 (s, 3H), 1.20–1.17 (m, 6H), 0.99 (d, J=7.1 Hz, 3H), 0.94 (d, J=7.0 Hz, 3H), 0.88 (t, J=7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 206.94, 173.49, 172.39, 169.82, 150.25, 148.64, 139.73, 137.12, 129.28, 123.50, 101.78, 93.47, 82.62, 79.39, 77.23, 75.96, 73.99, 71.38, 70.43, 69.12, 63.12, 49.97, 42.41, 40.81, 40.42, 40.16, 38.37, 31.78, 30.50, 27.31, 23.09, 22.17, 21.39, 21.17, 20.99, 20.41, 19.72, 13.52, 12.76, 10.70, 10.14.
2′-Acetyl-3-O-descladinosyl-3-O-(3-pyridyl) acetyl-10,11-anhydro-11-deoxy-12-O-(1H-1- imidazoylcarbonyl)-6-O-methyl-erythromycin A (compound 9)
Compound 8 (1.4 mmol), carbonyl diimidazole (5.6 mmol), and 4-dimethylamino pyridine (0.14 mmol) were dissolved in anhydrous dichloromethane (20 mL). The solution was stirred for 48 hours at room temperature. The reaction mixture was quenched with saturated aqueous NaHCO3 (20 mL) and extracted with dichloromethane. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was recrystallized from the mixture of petroleum ether and acetone (4:1) to afford white solid 9 (0.82 g, 80%). 1H NMR (300 MHz, CDCl3) δ 8.60–8.53 (m, 2H), 8.06 (s, 1H), 7.77–7.70 (m, 1H), 7.38–7.27 (m, 2H), 7.05 (dd, J=1.6, 0.8 Hz, 1H), 6.64 (s, 1H), 5.84 (dd, J=10.2, 3.0 Hz, 1H), 5.10 (d, J=10.3 Hz, 1H), 4.65 (dd, J=10.5, 7.4 Hz, 1H), 3.90 (s, 1H), 3.70 (s, 2H), 3.60 (d, J=4.8 Hz, 1H), 3.11 (s, 3H), 3.08–3.02 (m, 1H), 2.86 (d, J=5.9 Hz, 1H), 2.65–2.52 (m, 1H), 2.24 (s, 6H), 2.15 (s, 1H), 2.04 (s, 3H), 1.85 (s, 3H), 1.77 (s, 3H), 1.70–1.61 (m, 2H), 1.35 (dd, J=9.8, 4.8 Hz, 2H), 1.23 (s, 3H), 1.20 (d, J=4.1 Hz, 3H), 1.13 (dd, J=6.1, 3.9 Hz, 6H), 1.01 (d, J=6.7 Hz, 3H), 0.89 (d, J=1.5 Hz, 3H), 0.86 (d, J=5.3 Hz, 3H).13C NMR (75 MHz, CDCl3) δ 170.19, 169.80, 150.24, 148.85, 145.84, 137.63, 137.08, 130.82, 129.34, 123.60, 117.13, 100.99, 84.37, 78.35, 75.82, 71.16, 69.13, 63.21, 50.60, 42.90, 40.52, 38.50, 30.28, 29.69, 27.42, 21.44, 20.89, 19.92, 15.11, 13.57, 10.26.
General procedure for the synthesis of compounds 10a–10q
To a solution of compound 9 (0.30 mmol) in acetonitrile (1 mL), compounds 5a–5q (0.60 mmol) were added, and the resulting mixture was stirred for 16–25 hours at 55°C. After cooling, 5% KH2PO4 (2 mL) was added. The mixture was extracted with ethyl acetate ester. The organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting residue was dissolved in methanol (1 mL), and the solution was refluxed for 25 hours. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography (petroleum ether/acetone/triethylamine =5:1:1) to afford compounds 10a–10q as white foam.
Characterization of compounds 10a–10q, of which some solid or powder compounds like 10c, 10d, 10g, 10k, 10n, 10o, and 10q give melting point data
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(3- [5-(pyridin-2-yl)-1H-tetrazol-1-yl])propyl]imino) erythromycin A (compound 10a)
Yield 53.7%. 1H NMR (300 MHz, CDCl3) δ 8.69 (dd, J=4.8, 0.7 Hz, 1H), 8.55–8.42 (m, 2H), 8.27 (d, J=7.9 Hz, 1H), 7.82 (td, J=7.8, 1.7 Hz, 1H), 7.70 (d, J=7.9 Hz, 1H), 7.37 (ddd, J=7.6, 4.9, 1.0 Hz, 1H), 7.25 (dd, J=7.9, 4.7 Hz, 1H), 5.26 (s, 3H), 5.10–4.97 (m, 2H), 4.96–4.88 (m, 2H), 3.81 (d, J=7.2 Hz, 1H), 3.67–3.61 (m, 3H), 3.13 (dd, J=10.1, 7.2 Hz, 1H), 3.05–2.94 (m, 2H), 2.85 (s, 3H), 2.82–2.74 (m, 1H), 2.41 (ddd, J=20.6, 12.6, 5.9 Hz, 3H), 2.28 (s, 6H), 2.10 (s, 3H), 1.90–1.76 (m, 1H), 1.58–1.47 (m, 3H), 1.33 (s, 3H), 1.19 (d, J=5.2 Hz, 3H), 1.12 (s, 6H), 1.08 (s, 3H), 1.07 (d, J=6.4 Hz, 4H), 1.03 (d, J=7.1 Hz, 3H), 0.93 (d, J=6.8 Hz, 3H), 0.83 (d, J=6.7 Hz, 3H), 0.71 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.55, 174.06, 170.49, 157.22, 151.75, 150.06, 149.55, 148.51, 144.89, 137.37, 129.52, 125.22, 124.46, 123.59, 103.68, 82.84, 81.07, 78.34, 77.58, 77.16, 76.74, 76.38, 70.12, 69.22, 65.80, 60.73, 49.97, 47.62, 45.47, 42.87, 41.54, 39.97, 38.71, 38.32, 36.32, 30.93, 29.64, 28.51, 27.57, 21.94, 20.98, 19.52, 18.74, 15.01, 14.15, 10.11, 8.87. HRMS (ESI) m/z calcd for C47H68N8O11 [M + H]+: 921.5080, found: 921.5081.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(3- [5-(pyridin-2-yl)-2H-tetrazol-2-yl])propyl]imino) erythromycin A (compound 10b)
Yield 54.6%. 1H NMR (300 MHz, CDCl3) δ 8.70 (d, J=4.2 Hz, 1H), 8.53–8.41 (m, 2H), 8.17 (d, J=7.9 Hz, 1H), 7.77 (td, J=7.8, 1.7 Hz, 1H), 7.70 (d, J=7.9 Hz, 1H), 7.36–7.21 (m, 2H), 5.48 (s, 3H), 5.04–4.87 (m, 2H), 4.84–4.67 (m, 2H), 3.81 (d, J=7.1 Hz, 1H), 3.66 (dd, J=8.0, 3.9 Hz, 3H), 3.14 (dd, J=9.7, 6.9 Hz, 1H), 3.06–2.92 (m, 2H), 2.91 (s, 3H), 2.48–2.38 (m, 3H), 2.29 (s, 6H), 2.09 (s, 3H), 1.86 (dd, J=11.1, 6.2 Hz, 1H), 1.56–1.46 (m, 3H), 1.35 (s, 3H), 1.17 (d, J=2.9 Hz, 3H), 1.12 (s, 6H), 1.08 (d, J=5.3 Hz, 3H), 1.02 (s, 3H), 0.94 (d, J=6.7 Hz, 3H), 0.85 (d, J=6.7 Hz, 3H), 0.77 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.68, 174.15, 170.52, 164.66, 157.28, 150.08, 148.44, 146.85, 137.47, 137.05, 129.55, 124.69, 123.60, 122.46, 103.65, 82.90, 81.07, 78.34, 76.53, 70.08, 69.12, 65.79, 60.56, 51.20, 50.18, 45.47, 42.88, 41.36, 39.91, 38.73, 38.29, 36.37, 30.92, 29.64, 28.58, 27.52, 27.04, 21.96, 20.95, 19.44, 18.75, 15.02, 14.19, 10.15, 8.89. HRMS (ESI) m/z calcd for C47H68N8O11 [M + H]+: 921.5080, found: 921.5083.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(3- [5-(pyridin-3-yl)-2H-tetrazol-2-yl])propyl]imino) erythromycin A (compound 10c)
Yield 55.8%. melting point (Mp) =173°C–175°C; 1H NMR (300 MHz, CDCl3) δ 9.33 (s, 1H), 8.57 (d, J=40.9 Hz, 3H), 8.39 (d, J=7.9 Hz, 1H), 7.69 (d, J=7.7 Hz, 1H), 7.43–7.32 (m, 1H), 7.26 (s, 1H), 4.98 (t, J=11.6 Hz, 2H), 4.76 (dd, J=10.1, 6.8 Hz, 2H), 3.82 (d, J=7.0 Hz, 1H), 3.67 (d, J=4.0 Hz, 4H), 3.13–2.98 (m, 3H), 2.91 (s, 3H), 2.85 (dd, J=11.1, 6.8 Hz, 1H), 2.49–2.36 (m, 3H), 2.23 (s, 6H), 2.13–2.05 (m, 2H), 1.90 (dd, J=13.6, 6.9 Hz, 1H), 1.67–1.60 (m, 1H), 1.58–1.46 (m, 4H), 1.38 (s, 3H), 1.20 (d, J=4.7 Hz, 5H), 1.09 (dd, J=12.4, 6.6 Hz, 10H), 0.98 (d, J=6.7 Hz, 3H), 0.88 (d, J=6.6 Hz, 3H), 0.79 (t, J=7.2 Hz, 3H). HRMS (ESI) m/z calcd for C47H68N8O11 [M + H]+: 921.5080, found: 921.5082.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(3- [5-(pyridin-4-yl)-2H-tetrazol-2-yl])propyl]imino) erythromycin A (compound 10d)
Yield 47.9%. Mp =170°C–172°C; 1H NMR (300 MHz, CDCl3) δ 9.34 (s, 1H), 8.66 (s, 1H), 8.52 (s, 2H), 8.40 (d, J=7.9 Hz, 1H), 7.70 (d, J=7.9 Hz, 1H), 7.38 (dd, J=7.8, 4.8 Hz, 1H), 7.29 (s, 1H), 4.99 (t, J=11.3 Hz, 2H), 4.84–4.68 (m, 2H), 3.83 (d, J=7.0 Hz, 2H), 3.68 (d, J=3.9 Hz, 3H), 3.12–3.03 (m, 2H), 2.92 (s, 3H), 2.88–2.82 (m, 1H), 2.47–2.38 (m, 2H), 2.25 (s, 6H), 2.13 (s, 2H), 1.95–1.86 (m, 1H), 1.56 (d, J=7.0 Hz, 2H), 1.39 (s, 3H), 1.20 (s, 3H), 1.14–1.09 (m, 6H), 1.06 (s, 1H), 0.99 (d, J=6.7 Hz, 3H), 0.89 (d, J=6.6 Hz, 3H), 0.80 (t, J=7.2 Hz, 3H).13C NMR (75 MHz, CDCl3) δ 215.68, 174.25, 170.43, 162.77,157.32, 151.00, 150.55,150.29, 148.84, 148.13, 137.07, 134.22, 123.64,103.78, 82.92, 80.91, 78.42, 78.35, 70.28, 69.63, 65.96, 60.56, 51.14, 50.06, 45.54, 42.93, 41.32, 40.29, 38.76, 38.45, 38.38, 36.37, 30.92, 28.17, 27.13, 22.01, 19.44, 18.78, 21.05, 14.22, 14.16, 10.20, 8.85. HRMS (ESI) m/z calcd for C47H68N8O11 [M + H]+: 921.5080, found: 921.5083.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(4- [5-(pyridin-2-yl)-1H-tetrazol-1-yl])butyl]imino) erythromycin A (compound 10e)
Yield of 52.3%. 13C NMR (75 MHz, CDCl3) δ 215.74, 182.80, 176.89, 174.13, 170.75, 157.31, 151.58, 149.73, 148.14, 144.82, 137.83, 137.32, 129.84, 125.24, 124.39, 123.69, 103.32, 82.75, 81.21, 78.34, 76.47, 69.65, 68.41, 65.82, 60.29, 49.89, 49.18, 45.50, 44.93, 42.88, 39.47, 38.66, 38.14, 36.37, 29.37, 27.52, 22.65, 20.80, 19.48, 18.73, 15.05, 14.12, 10.15, 8.86, 8.41. HRMS (ESI) m/z calcd for C48H70N8O11 [M + H]+: 935.5237, found: 935.5232.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(4- [5-(pyridin-2-yl)-2H-tetrazol-2-yl])butyl]imino) erythromycin A (compound 10f)
Yield 55.4%. 1H NMR (300 MHz, CDCl3) δ 8.72 (d, J=4.0 Hz, 1H), 8.57 (s, 1H), 8.51 (d, J=3.6 Hz, 1H), 8.18 (d, J=7.9 Hz, 1H), 7.79 (ddd, J=10.1, 5.9, 2.0 Hz, 2H), 7.39–7.28 (m, 2H), 6.27 (s, 3H), 5.06–4.86 (m, 2H), 4.73 (t, J=7.1 Hz, 2H), 3.86 (d, J=7.1 Hz, 1H), 3.75 (d, J=2.3 Hz, 1H), 3.70–3.62 (m, 3H), 3.28–3.15 (m, 1H), 3.05 (dd, J=14.9, 7.3 Hz, 2H), 2.95 (s, 2H), 2.89–2.73 (m, 2H), 2.45 (s, 3H), 2.18–2.07 (m, 3H), 1.95–1.82 (m, 1H), 1.77–1.45 (m, 6H), 1.37 (s, 3H), 1.22 (d, J=5.7 Hz, 3H), 1.16 (s, 9H), 1.08 (t, J=6.8 Hz, 6H), 0.98 (d, J=6.7 Hz, 3H), 0.86 (d, J=6.8 Hz, 3H), 0.77 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.79, 183.82, 174.10, 170.63, 164.60, 157.42, 150.17, 149.74, 148.07, 146.84, 137.96, 137.09, 124.71, 123.74, 122.42, 103.47, 82.80, 81.38, 78.48, 78.42, 69.87, 68.62, 65.81, 60.36, 53.01, 50.08, 45.53, 42.89, 39.61, 38.72, 38.07, 36.37, 29.66, 27.35, 26.93, 24.13, 21.98, 20.87, 19.53, 18.81, 15.02, 14.21, 10.22, 8.93. HRMS (ESI) m/z calcd for C48H70N8O11 [M + H]+: 935.5237, found: 935.5231.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(4- [5-(pyridin-3-yl)-2H-tetrazol-2-yl]butyl)imino]) erythromycin A (compound 10g)
Yield 51.2%. Mp =133°C–135°C; 1H NMR (300 MHz, CDCl3) δ 9.34 (d, J=1.5 Hz, 1H), 8.67 (dd, J=4.9, 1.6 Hz, 1H), 8.62–8.49 (m, 2H), 8.41 (dt, J=8.0, 1.9 Hz, 1H), 7.76 (d, J=7.9 Hz, 1H), 7.40 (dd, J=8.0, 4.8 Hz, 1H), 7.31 (dd, J=7.7, 4.8 Hz, 1H), 4.97 (dd, J=10.0, 3.7 Hz, 2H), 4.75 (dd, J=14.0, 6.3 Hz, 3H), 3.85 (d, J=7.2 Hz, 1H), 3.80–3.65 (m, 6H), 3.48 (s, 3H), 3.19 (dd, J=9.9, 7.3 Hz, 1H), 3.06 (d, J=6.8 Hz, 2H), 2.99 (s, 3H), 2.87 (dd, J=11.1, 6.9 Hz, 1H), 2.65–2.41 (m, 2H), 2.37 (s, 6H), 2.12 (dd, J=14.7, 7.3 Hz, 3H), 1.88 (s, 4H), 1.73 (d, J=7.6 Hz, 2H), 1.65–1.55 (m, 3H), 1.40 (s, 3H), 1.26 (d, J=6.3 Hz, 3H), 1.14 (d, J=2.3 Hz, 3H), 1.09 (s, 3H), 1.01 (d, J=6.8 Hz, 3H), 0.87 (d, J=6.8 Hz, 3H), 0.81 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.82, 174.13, 170.46, 162.60, 157.40, 150.92, 150.25, 148.72, 147.96,137.22, 134.15, 129.40, 123.83, 123.68, 123.54, 103.57, 82.77, 80.86, 78.38, 78.33, 76.57, 70.16, 69.36, 65.90, 60.29, 52.91, 50.04, 45.56, 42.77, 40.21, 38.75, 38.31, 36.30, 28.47, 26.98, 24.14, 21.95, 20.99, 19.53, 18.79, 14.93, 14.20, 10.23, 8.86. HRMS (ESI) m/z calcd for C48H70N8O11 [M + H]+: 935.5237, found: 935.5234.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(4- [5-(pyridin-4-yl)-2H-tetrazol-2-yl])butyl]imino) erythromycin A (compound 10h)
Yield 47.9%. 1H NMR (300 MHz, CDCl3) δ 8.68 (d, J=5.5 Hz, 2H), 8.60–8.37 (m, 2H), 7.97 (d, J=6.0 Hz, 2H), 7.73 (d, J=7.9 Hz, 1H), 7.36–7.21 (m, 1H), 4.91 (d, J=11.0 Hz, 2H), 4.70 (t, J=7.1 Hz, 2H), 3.83 (d, J=7.1 Hz, 1H), 3.77–3.57 (m, 6H), 3.15 (dd, J=10.0, 7.3 Hz, 1H), 3.06–3.00 (m, 1H), 2.93 (d, J=4.3 Hz, 3H), 2.82 (dd, J=11.1, 6.8 Hz, 1H), 2.55–2.39 (m, 2H), 2.29 (s, 3H), 2.10 (s, 3H), 2.05 (dd, J=9.0, 5.7 Hz, 3H), 1.94–1.78 (m, 1H), 1.70 (dd, J=15.6, 7.7 Hz, 2H), 1.58 (d, J=14.9 Hz, 3H), 1.36 (s, 3H), 1.22 (s, 3H), 1.19 (s, 1H), 1.14 (s, 6H), 1.07 (dd, J=8.3, 5.4 Hz, 6H), 0.96 (d, J=6.7 Hz, 3H), 0.81–0.78 (m, 3H), 0.73 (d, J=7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.84, 174.12, 170.51, 162.78, 157.37, 150.11, 148.44, 137.37, 135.13, 129.53, 123.59, 120.87, 103.66, 82.72, 80.96, 78.3, 76.48, 70.08, 69.14, 65.74, 60.23, 53.01, 49.99, 45.54, 44.81, 42.72, 39.86, 38.52, 38.18, 36.31, 30.90, 30.10, 29.62, 28.51, 27.52, 26.93, 24.10, 22.62, 21.91, 20.96, 19.50, 18.76, 14.87, 14.16, 10.21, 8.77. HRMS (ESI) m/z calcd for C48H70N8O11 [M+H]+: 935.5237, found: 935.5236.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(5- [5-(pyridin-2-yl)-1H-tetrazol-1-yl])pentyl]imino) erythromycin A (compound 10i)
Yield 47.8%. 1H NMR (300 MHz, CDCl3) δ 8.70 (d, J=4.8 Hz, 1H), 8.55–8.45 (m, 2H), 8.28 (d, J=7.9 Hz, 1H), 7.84 (td, J=7.8, 1.7 Hz, 1H), 7.69 (d, J=7.9 Hz, 1H), 7.38 (ddd, J=7.6, 4.9, 1.0 Hz, 1H), 7.25 (dd, J=8.0, 4.7 Hz, 1H), 4.99–4.88 (m, 4H), 3.83 (d, J=7.2 Hz, 1H), 3.69 (d, J=2.4 Hz, 3H), 3.63 (s, 1H), 3.59–3.50 (m, 2H), 3.07 (ddd, J=14.4, 8.7, 4.6 Hz, 3H), 2.93 (s, 3H), 2.84 (dd, J=11.1, 6.7 Hz, 1H), 2.24 (s, 6H), 2.12 (s, 6H), 2.03–1.91 (m, 2H), 1.64 (d, J=9.2 Hz, 2H), 1.55 (dd, J=12.9, 4.4 Hz, 3H), 1.35 (d, J=4.0 Hz, 3H), 1.23 (s, 3H), 1.20–1.13 (m, 3H), 1.13–1.08 (m, 6H), 1.06 (d, J=7.1 Hz, 3H), 0.96 (d, J=6.8 Hz, 3H), 0.86 (d, J=6.7 Hz, 3H), 0.74 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.73, 207.12, 174.08, 170.47, 157.29, 151.62, 150.26, 149.60, 148.78, 144.89, 137.36, 137.13, 129.33, 125.24, 124.45, 123.52, 103.71, 82.62, 80.94, 78.39, 76.55, 70.25, 69.57, 65.91, 60.37, 50.00, 49.61, 21.99, 21.05, 19.55, 18.82, 15.00, 14.25, 14.19, 10.24, 8.87, 8.83. HRMS (ESI) m/z calcd for C49H72N8O11 [M + H]+:949.5393, found: 949.5395.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(5- [5-(pyridin-2-yl)-2H-tetrazol-2-yl]pentyl)imino]) erythromycin A (compound 10j)
Yield 62.7%. 1H NMR (300 MHz, CDCl3) δ 8.76 (d, J=4.1 Hz, 1H), 8.58 (s, 1H), 8.56–8.47 (m, 1H), 8.22 (d, J=7.9 Hz, 1H), 7.90–7.74 (m, 2H), 7.41–7.28 (m, 2H), 4.99 (d, J=11.1 Hz, 2H), 4.68 (t, J=7.2 Hz, 2H), 3.88 (d, J=7.1 Hz, 1H), 3.80–3.69 (m, 3H), 3.66 (s, 1H), 3.65–3.52 (m, 2H), 3.25 (dd, J=10.0, 7.2 Hz, 1H), 3.02 (dd, J=14.1, 6.8 Hz, 6H), 2.91–2.81 (m, 1H), 2.77–2.64 (m, 1H), 2.58–2.48 (m, 1H), 2.42 (s, 3H), 2.21 (t, J=5.9 Hz, 3H), 2.17–2.11 (m, 3H), 1.70 (dd, J=18.7, 11.9 Hz, 3H), 1.39 (s, 3H), 1.26 (d, J=1.7 Hz, 3H), 1.24 (s, 3H), 1.21 (s, 3H), 1.18–1.17 (m, 3H), 1.12–1.07 (m, 6H), 0.99 (d, J=6.8 Hz, 3H), 0.92 (d, J=6.7 Hz, 3H), 0.79 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.90, 184.13, 174.19, 170.71, 164.70, 157.45, 150.30, 149.83, 148.17, 146.96, 138.02, 137.19, 129.95, 128.38, 127.94, 125.88, 124.81, 123.81, 122.51, 103.66, 82.79, 81.49, 78.64, 78.55, 77.37, 70.08, 68.85, 65.85, 60.52, 53.49, 50.18, 45.65, 45.06, 43.43, 43.02, 40.73–40.56 (m), 39.71, 38.72, 38.51, 38.19, 36.45, 31.99, 31.50, 30.25, 29.77, 29.09, 27.55, 26.42–26.15 (m), 23.96, 22.76, 22.11, 20.99, 19.64, 18.93, 15.14, 14.49–14.07 (m), 10.35, 9.03, 8.63. HRMS (ESI) m/z calcd for C49H72N8O11 [M + H]+:949.5393, found: 949.5389.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(5- [5-(pyridin-3-yl)-2H-tetrazol-2-yl]pentyl)imino]) erythromycin A (compound 10k)
Yield 56.3%. Mp =139°C–141°C; 1H NMR (300 MHz, CDCl3) δ 9.34 (s, 1H), 8.63 (d, J=33.2 Hz, 2H), 8.55–8.34 (m, 2H), 7.77 (d, J=7.8 Hz, 2H), 7.45–7.40 (m, 1H), 7.37–7.27 (m, 1H), 5.05 (d, J=37.1 Hz, 3H), 4.97 (d, J=11.0 Hz, 2H), 4.65 (t, J=7.0 Hz, 2H), 3.88 (d, J=7.0 Hz, 1H), 3.84–3.69 (m, 3H), 3.65–3.54 (m, 3H), 3.39–3.20 (m, 1H), 3.07 (dd, J=14.5, 7.2 Hz, 6H), 2.97 (s, 3H), 2.85 (d, J=11.0 Hz, 2H), 2.49 (s, 6H), 2.26–2.03 (m, 3H), 1.88 (dd, J=13.4, 7.2 Hz, 1H), 1.68 (s, 3H), 1.57 (d, J=8.3 Hz, 2H), 1.38–1.32 (m, 6H), 1.23 (s, 3H), 1.17 (s, 6H), 1.10 (s, 3H), 0.98 (d, J=6.4 Hz, 3H), 0.91 (d, J=6.4 Hz, 3H), 0.77 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 214.29, 183.33, 173.95, 170.73, 162.55, 157.35, 150.69, 149.77, 148.13, 147.71, 137.95, 134.47, 123.84, 103.36, 82.70, 78.41, 69.87, 68.60, 65.91, 60.39, 53.33, 50.08, 45.61, 42.91, 39.78, 38.72, 38.21, 29.32, 28.97, 27.33, 26.43, 23.82, 20.87, 19.56, 18.84, 15.08, 14.25, 10.27, 8.95, 8.61. HRMS (ESI) m/z calcd for C49H72N8O11 [M + H]+:949.5393, found: 949.5392.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(5- [5-(pyridin-4-yl)-2H-tetrazol-2-yl])pentyl]imino) erythromycin A (compound 10l)
Yield 54.2%. 1H NMR (300 MHz, CDCl3) δ 8.71 (dd, J=4.6, 1.5 Hz, 2H), 8.54 (d, J=1.7 Hz, 1H), 8.49 (dd, J=4.8, 1.4 Hz, 1H), 7.99 (dd, J=4.6, 1.5 Hz, 2H), 7.74 (d, J=7.9 Hz, 1H), 7.28 (d, J=2.9 Hz, 1H), 4.95 (d, J=11.1 Hz, 2H), 4.64 (t, J=7.1 Hz, 2H), 3.85 (d, J=7.1 Hz, 1H), 3.73 (s, 2H), 3.66 (dd, J=11.6, 7.4 Hz, 2H), 3.56 (dd, J=9.2, 6.4 Hz, 2H), 3.22 (dd, J=10.1, 7.2 Hz, 1H), 3.01 (q, J=7.3 Hz, 5H), 2.95 (s, 3H), 2.39 (s, 3H), 2.15–2.03 (m, 3H), 1.92–1.80 (m, 1H), 1.60 (s, 3H), 1.51 (d, J=12.7 Hz, 3H), 1.35 (s, 3H), 1.26 (d, J=7.3 Hz, 3H), 1.21 (d, J=4.0 Hz, 3H), 1.14 (s, 6H), 1.09–1.04 (m, 6H), 0.95 (d, J=6.8 Hz, 3H), 0.87 (d, J=6.7 Hz, 3H), 0.74 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.91, 183.67, 174.21, 170.72, 162.77, 157.34, 150.07, 149.79, 148.17, 137.82, 135.27, 129.76, 123.70, 120.98, 103.53, 82.70, 81.21, 78.37, 69.92, 68.75, 65.76, 60.35, 53.40, 50.04, 45.56, 45.17, 43.26, 42.87, 39.65, 38.72, 38.22, 36.35, 28.95, 27.48, 26.38, 23.75, 21.98, 20.90, 19.53, 18.82, 15.03, 14.21, 10.25, 8.92, 8.56. HRMS (ESI) m/z calcd for C49H72N8O11 [M + H]+:949.5393, found: 949.5392.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O-methyl- 11,12-dideoxy-12,11-(oxycarbonyl[(4-[5-phenyl-2H- tetrazol-2-yl])pentyl]imino)erythromycin A (compound 10m)
Yield 56.4%. 1H NMR (300 MHz, CDCl3) δ 8.60–8.47 (m, 2H), 8.18–8.08 (m, 2H), 7.75 (d, J=8.0 Hz, 1H), 7.46 (d, J=6.6 Hz, 3H), 7.30 (dd, J=7.8, 4.9 Hz, 1H), 5.01 (d, J=11.0 Hz, 2H), 4.65 (t, J=7.2 Hz, 2H), 3.98–3.81 (m, 2H), 3.74 (s, 3H), 3.66–3.54 (m, 2H), 3.26 (ddd, J=24.7, 16.2, 7.2 Hz, 3H), 3.01 (s, 3H), 2.91–2.84 (m, 3H), 2.60–2.45 (m, 2H), 2.37 (s, 3H), 2.26 (d, J=5.0 Hz, 1H), 2.18–2.10 (m, 3H), 1.96–1.78 (m, 2H), 1.72 (d, J=7.1 Hz, 2H), 1.67 (s, 1H), 1.60 (d, J=10.3 Hz, 2H), 1.46 (s, 1H), 1.40 (s, 3H), 1.27 (d, J=2.7 Hz, 3H), 1.20 (s, 3H), 1.18 (s, 3H), 1.15 (d, J=2.8 Hz, 3H), 1.09 (s, 3H), 1.01 (d, J=6.7 Hz, 3H), 0.93 (d, J=6.8 Hz, 3H), 0.80 (t, J=7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.77, 174.13, 170.56, 166.98, 157.33, 150.23, 148.72, 137.35, 130.14, 129.49, 126.85, 123.61, 102.62, 82.67, 81.20, 78.36, 70.12, 69.04, 66.06, 60.42, 53.05, 50.11, 45.60, 43.37, 40.21, 38.82, 36.32, 31.44, 30.17, 29.04, 27.52, 26.50, 23.88, 22.03, 21.01, 19.58, 18.86, 15.08, 14.30, 10.28, 8.95. HRMS (ESI) m/z calcd for C50H73N7O11 [M + H]+:948.5441, found: 948.5444.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(3-[5- (thiophen-2-yl)-2H-tetrazol-2-yl])pentyl]imino) erythromycin A (compound 10n)
Yield 60.6%. Mp =99°C–101°C; 1H NMR (300 MHz, CDCl3) δ 8.51 (dd, J=4.9, 1.4 Hz, 2H), 7.76 (dd, J=3.6, 1.2 Hz, 1H), 7.70 (dt, J=7.8, 1.8 Hz, 1H), 7.40 (dd, J=5.0, 1.2 Hz, 1H), 7.27 (dd, J=7.6, 4.7 Hz, 1H), 7.10 (dd, J=5.0, 3.7 Hz, 1H), 5.06–4.91 (m, 2H), 4.59 (t, J=7.2 Hz, 2H), 3.85 (d, J=7.2 Hz, 1H), 3.74–3.68 (m, 3H), 3.66 (s, 1H), 3.58 (dd, J=8.4, 5.9 Hz, 2H), 3.19–3.01 (m, 3H), 2.98 (d, J=6.2 Hz, 3H), 2.93–2.80 (m, 2H), 2.68–2.59 (m, 1H), 2.23 (d, J=4.4 Hz, 6H), 2.16–2.05 (m, 3H), 1.78–1.66 (m, 2H), 1.57 (t, J=10.9 Hz, 3H), 1.38 (s, 3H), 1.26 (s, 3H), 1.16–1.04 (m, 12H), 0.98 (d, J=6.8 Hz, 3H), 0.90 (d, J=6.8 Hz, 3H), 0.80 (ddd, J=15.0, 5.9, 2.6 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 215.75, 174.10, 170.43, 161.04, 157.32, 150.27, 148.80, 137.11, 129.27, 127.76, 123.52, 103.79, 82.67, 81.01, 78.43, 70.32, 69.66, 65.92, 60.42, 53.12, 50.09, 45.84, 45.59, 43.34, 42.94, 40.29, 38.6, 36.28, 28.97, 28.11, 26.45, 23.82, 22.02, 21.07, 19.56, 18.84, 15.01, 14.24, 10.26, 8.89. HRMS (ESI) m/z calcd for C48H71N7O11S [M + H]+:954.5005, found: 954.5003.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(3- [4-(1-methyl-1H-tetrazol-5-thio)]propyl]imino) erythromycin A (compound 10o)
Yield 53.2%. Mp =202°C–204°C; 1H NMR (300 MHz, CDCl3) δ 8.52 (dd, J=4.9, 1.4 Hz, 2H), 7.78–7.65 (m, 1H), 7.32–7.26 (m, 1H), 4.96 (dd, J=13.5, 6.5 Hz, 2H), 3.90 (s, 3H), 3.85 (d, J=7.2 Hz, 1H), 3.75 (t, J=7.2 Hz, 2H), 3.71 (d, J=1.9 Hz, 3H), 3.65 (s, 1H), 3.51–3.29 (m, 3H), 3.18–3.10 (m, 1H), 3.05 (d, J=7.0 Hz, 2H), 2.99 (s, 3H), 2.86 (dd, J=11.1, 6.7 Hz, 1H), 2.51 (dd, J=9.1, 5.4 Hz, 1H), 2.28 (s, 6H), 2.17–2.04 (m, 3H), 1.96–1.82 (m, 1H), 1.64–1.52 (m, 3H), 1.38 (s, 3H), 1.26 (dd, J=13.7, 10.6 Hz, 6H), 1.10 (dd, J=10.2, 6.6 Hz, 9H), 0.98 (d, J=6.8 Hz, 3H), 0.90 (d, J=6.7 Hz, 3H), 0.79 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.71, 174.17, 170.51, 157.37, 154.17, 150.31, 148.86, 137.13, 129.31, 123.55, 103.73, 82.83, 80.94, 78.40, 76.58, 70.24, 69.58, 65.97, 60.41, 50.23, 45.58, 42.92, 42.29, 40.31, 38.79, 38.40, 36.32, 33.43, 31.07, 28.24, 26.80, 22.00, 21.06, 19.58, 18.85, 15.05, 14.25, 10.22, 8.88. HRMS (ESI) m/z calcd for C43H67N7O11S [M + H]+:890.4692, found: 890.4691.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(4- [4-(1-methyl-1H-tetrazol-5-thio)]butyl]imino) erythromycin A (compound 10p)
Yield 55.9%. 1H NMR (300 MHz, CDCl3) δ 8.53 (d, J=6.0 Hz, 2H), 7.74 (d, J=7.9 Hz, 1H), 7.29 (dd, J=7.8, 4.9 Hz, 1H), 4.97 (t, J=10.2 Hz, 2H), 3.88 (s, 3H), 3.79–3.69 (m, 3H), 3.67–3.58 (m, 3H), 3.47 (s, 2H), 3.39 (dd, J=8.2, 5.2 Hz, 2H), 3.15 (dd, J=10.1, 7.3 Hz, 1H), 3.04 (d, J=6.6 Hz, 2H), 2.96 (d, J=3.8 Hz, 3H), 2.91–2.77 (m, 1H), 2.59–2.48 (m, 1H), 2.46–2.33 (m, 1H), 2.29 (s, 5H), 2.23 (d, J=4.8 Hz, 2H), 2.19–2.14 (m, 2H), 2.09 (dd, J=12.2, 5.1 Hz, 2H), 1.81 (s, 3H), 1.59 (dd, J=18.7, 11.5 Hz, 3H), 1.38 (s, 3H), 1.27 (s, 3H), 1.17 (d, J=3.9 Hz, 3H), 1.14 (s, 3H), 1.09 (d, J=7.2 Hz, 3H), 0.99 (d, J=6.7 Hz, 3H), 0.91 (d, J=6.7 Hz, 3H), 0.78 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.76, 174.12,170.56, 157.32, 154.21, 150.26, 148.75, 137.26, 103.54, 82.70, 81.04, 78.46, 78.39, 77.49, 77.07, 76.64, 70.15, 69.29, 66.04, 60.45, 50.00, 45.58, 42.96, 40.25, 38.80, 38.43, 38.34, 36.31, 33.33, 32.87, 28.66, 26.72, 26.10, 22.03, 21.00, 19.57, 18.81, 15.06, 14.27, 14.21, 10.26, 8.91. HRMS (ESI) m/z calcd for C44H69N7O11S [M + H]+:904.4849, found: 904.4844.
3-O-descladinosyl-3-O-(3-pyridyl)acetyl-6-O- methyl-11,12-dideoxy-12,11-(oxycarbonyl[(5- [4-(1-methyl-1H-tetrazol-5-thio)]pentyl]imino) erythromycin A (compound 10q)
Yield 52.9%. Mp =101°C–104°C; 1H NMR (300 MHz, CDCl3) δ 8.60–8.46 (m, 2H), 7.74 (d, J=7.9 Hz, 1H), 7.35–7.27 (m, 1H), 4.98 (d, J=11.0 Hz, 2H), 3.88 (s, 3H), 3.74–3.67 (m, 3H), 3.65 (s, 1H), 3.61–3.51 (m, 2H), 3.33 (t, J=7.1 Hz, 2H), 3.17 (dd, J=10.0, 7.3 Hz, 1H), 3.08–3.02 (m, 1H), 2.98 (s, 2H), 2.92–2.81 (m, 1H), 2.59–2.40 (m, 2H), 2.33 (s, 5H), 2.19–2.06 (m, 2H), 1.84 (dd, J=15.1, 7.6 Hz, 3H), 1.62 (d, J=11.9 Hz, 3H), 1.53–1.43 (m, 3H), 1.38 (s, 3H), 1.26 (dd, J=13.3, 10.7 Hz, 6H), 1.17 (s, 2H), 1.14–1.06 (m, 9H), 0.99 (d, J=6.8 Hz, 3H), 0.91 (d, J=6.7 Hz, 3H), 0.78 (t, J=7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 215.77, 174.11, 170.57, 157.33, 154.38, 150.17, 148.65, 137.34, 129.48, 123.60, 103.63, 82.65, 81.09, 78.50, 78.39, 70.19, 69.31, 65.94, 60.42, 50.07, 45.58, 43.40, 42.94, 40.14, 38.81, 38.42, 38.32, 36.30, 33.33, 33.28, 28.76, 27.48, 26.52, 25.81, 22.04, 21.00, 19.57, 18.85, 15.04, 14.27, 10.27, 8.91. HRMS (ESI) m/z calcd for C45H71N7O11S [M + H]+:918.5005, found: 918.4993.
Minimum inhibitory concentration assay
The standard antibiotics erythromycin A and clarithromycin were obtained from commercial sources. The antimicrobial susceptibility test in vitro against acylide derivatives and standard antibiotics was performed using broth microdilution method, according to National Committee for Clinical Laboratory Standards guidelines. Acylides and the reference drugs were dissolved in glacial acetic acid as mother liquor; the amount of acetic acid must be less than 2.5 μL/mL to exclude glacial acetic acid effect on antibacterial activity in vitro. Minimum inhibitory concentration (μg/mL) is used to report the antibacterial activities of the compounds in vitro.
All of the synthesized acylides and standard antibiotics were tested for in vitro antibacterial activity against two erythromycin-susceptible strains of S. aureus (American Type Culture Collection [ATCC] 25923, ATCC 6538), one strain of Staphylococcus epidermidis (ATCC 12228), two strains of Pseudomonas aeruginosa (ATCC 9027 and a clinical isolate of resistant PA 1317), and two strains of Escherichia coli (ATCC 8739, ATCC 25922).
Results and discussion
Chemical syntheses
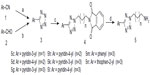
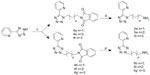
The side chain of alkyl amines 5a–5g (Figures 1 and 2) was synthesized using the method we published previously.12 Starting from aromatic nitriles or aromatic aldehydes as raw material, aromatic tetrazoles 3 were synthesized by (2+3) cycloaddition reaction with sodium azide, followed by reaction with phthalimide N-alkyl bromides N-(3-bromopropyl) phthalimide, N-(4-bromo-butyl) phthalimide, or N-(5-bromopently) phthalimide to get 4a–4g, which were then hydrazinolyzed to the desired tetrazole alkyl amine side chain.
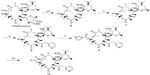
Tanikawa et al14 synthesized similar acylide derivatives from clarithromycin by seven-step reactions in which selective removal of the C-3 cladinose, protection of 2′-OH with acetyl, modification of the C-11,12 hydroxyl group by trichloromethyl chloroformate, acylation of the C-3 hydroxyl group, dehydrogenation of C-10,11, addition of carbonyl imidazole to C-12, and then reaction with side chain amines were performed successively to get the C-11,12 cyclic carbamates clarithromycin derivatives. Trichloromethyl chloroformate used in this routine has high toxicity and can cause serious health damage. Elliott et al15 improved the routine via six steps to synthesize a series of C-11,12 cyclic carbamate acylide derivatives from clarithromycin. On the basis of the two classic methods, we have designed a new approach (Figure 3), less the reaction steps, in which the use of toxic trichloromethyl chloroformate could be avoided.
Treatment of clarithromycin with ethylene carbonate in refluxing triethylamine and vigorously stirring, triethylamine was distilled off, and then the cladinose was selectively removed under dilute aqueous acid to prepare compound 6; the 2′-hydroxyl group was protected with acetic anhydride, using dichloromethane as solvent to obtain compound 7, followed by the esterification of 3-hydroxyl group with 3-pyridylacetic acid hydrochloride to yield compound 8, in which the reaction must be carried out in real-time monitoring to prevent excessive reaction of esterification of the C-12 hydroxyl group. Compound 8, dissolved in anhydrous dichloromethane reacted with carbonyl diimidazole at room temperature, yielded the C-12 imidazolyl carbamate 9, followed by the reaction with excess side chain amines and then deprotection of the acetyl group by refluxing in methanol to yield the desired acylides (10a–10q) shown in Figure 4.
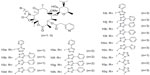  | Figure 4 Structure synthesized acylides. |
Antibacterial activity
The in vitro antimicrobial activities of acylides 10a–10q and standard antibiotics are shown in Table 1. The tabulated results show that all of the acylide derivatives 10a–10q exhibited potent antibacterial activity against the erythromycin-susceptible strains S. aureus ATCC 25923 and ATCC 6538, and most of them displayed excellent minimum inhibitory concentration values in the range of 0.06–0.5 μg/mL, which is better than or comparable to erythromycin and clarithromycin, among which compounds 10g and 10o were found to have the most potent activity against the erythromycin-susceptible strains tested. Compounds 10f and 10g also exhibited excellent activity against S. epidermidis ATCC 12228.
  | Table 1 The antibacterial activities of novel acylides in vitro |
Some of the acylide derivatives showed slightly more potent activity against P. aeruginosa and E. coli. Compounds 10e and 10o seemed to be more potent than other tetrazole-containing acylide derivatives against P. aeruginosa ATCC 9027 and ATCC 1317. Compared with erythromycin, compound 10e and compound 10o also exhibited improved potencies against E. coli strains.
Conclusion
Seventeen acylide derivatives have been synthesized and evaluated for in vitro antibacterial activities against Gram-positive and Gram-negative pathogens. All of them were found to be potent against the strains. In particular, the compounds 10e–10h, with a long side-chain alkyl having four carbon atoms, exhibited better antibacterial activities against erythromycin-susceptible strains. Compound 10o with a side chain of 3 carbon atoms and a sulfur atom also showed better antibacterial activity against the five strains.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81072554, 81373285) and by the 111 Project (No B13038).
Disclosure
The authors report no conflicts of interest in this work.
References
Song JH, Baek JY, Cheong HS, Peck KR, Ko KS. Incorrect identification of Streptococcus pneumoniae and its effect on antimicrobial resistance rates. Int J Antimicrob Agents. 2009;33(1):93–95. | |
Zhanel GG, Dueck M, Hoban DJ, et al. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs. 2001;61(4):443–498. | |
Ma Z, Nemoto PA. Discovery and development of ketolides as a new generation of macrolide antimicrobial agents. Curr Med Chem Anti-Infect Agents. 2002;1(1):15–34. | |
Brvar M, Perdih A, Hodnik V, et al. In silico discovery and biophysical evaluation of novel 5-(2-hydroxybenzylidene) rhodanine inhibitors of DNA gyrase B. Bioorg Med Chem. 2012;20(8):2572–2580. | |
Allen NE. Macrolide resistance in Straphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother. 1977;11(4):669–674. | |
Tanikawa T, Asaka T, Kashimura M, et al. Synthesis and antibacterial activity of acylides (3-O-acyl-erythromycin derivatives): a novel class of macrolide antibiotics. J Med Chem. 2001;44(24):4027–4030. | |
Abell AD. Heterocyclic-based peptidomimetics. Lett Pept Sci. 2002;8(3–5):267–272. | |
Myznikov LV, Hrabalek A, Koldobskii GI. Drugs in the tetrazole series. Chem Heterocycl Compd. 2007;43(1):1–9. | |
Hayashi R, Jin X, Cook GR. Synthesis and evaluation of novel heterocyclic MMP inhibitors. Bioorg Med Chem Lett. 2007;17(24):6864–6870. | |
Lebreton L, Curet O, Gueddari S, et al. Selective and potent monoamine oxidase type B inhibitors: 2-substituted 5-aryltetrazole derivatives. J Med Chem. 1995;38(24):4786–4792. | |
Adamec J, Beckert R, Weiss D, et al. Hybrid molecules of estrone: new compounds with potential antibacterial, antifungal, and antiproliferative activities. Bioorg Med Chem. 2007;15(8):2898–2906. | |
Song QL, Guo BQ, Zhang W, Lan P, Sun PH, Chen WM. Design, synthesis and antibacterial activity of novel ketolides bearing an aryltetrazolyl-substituted alkyl side chain. J Antibiot (Tokyo). 2011;64(8):571–581. | |
Herr RJ. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg Med Chem. 2002;10(11):3379–3393. | |
Tanikawa T, Asaka T, Kashimura M, et al. Synthesis and antibacterial activity of a novel series of acylides: 3-O-(3-pyridyl)acetylerythromycin A derivatives. J Med Chem. 2003;46(13):2706–2715. | |
Elliott RL, Pireh D, Griesgraber G, et al. Anhydrolide macrolides. 1. Synthesis and antibacterial activity of 2,3-anhydro-6-O-methyl 11,12- carbamate erythromycin A analogues. J Med Chem. 1998;41(10):1651–1659. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.