Back to Journals » Open Access Journal of Sports Medicine » Volume 6
Synovial chondromatosis caused mechanical snapping elbow: a case report
Authors Karaman I, Guney A , Dogar F, Oner M, Bılal O
Received 28 February 2015
Accepted for publication 12 May 2015
Published 7 July 2015 Volume 2015:6 Pages 225—228
DOI https://doi.org/10.2147/OAJSM.S83717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Freddie H Fu
Ibrahim Karaman,1 Ahmet Guney,1 Fatih Dogar,2 Mithat Oner,1 Okkes Bilal3
1Department of Orthopaedics and Traumatology, Erciyes University Medical Faculty, 2Department of Orthopaedics and Traumatology, Training and Research Hospital, Kayseri, 3Department of Orthopaedics and Traumatology, Kahramanmaras Sütçü Imam University Medical Faculty, Kahramanmaras, Turkey
Abstract: Synovial chondromatosis is a rare and benign proliferative disorder of the synovial membrane in joints and bursae. Herein, we present the case of a 34-year-old male with synovial chondromatosis that caused limitation in the elbow joint in terms of mechanical function.
Keywords: chondromatosis, elbow, loose bodies, mechanical block
Introduction
Synovial chondromatosis (SC) resulting from the metaplasia of synovial tissue is a rare proliferative disorder with monoarticular involvement that is found in the joints and bursae. Although the etiology is unclear, it is considered as a metaplastic formation. SC can develop secondary to osteoarthritis, although it is generally a primary condition. It is most commonly seen between 30 and 50 years of age, and the prevalence in men is double of that in women. The knee joint is the most frequently involved site, but it may also involve other joints in a lesser degree.1 Pain and progressive limitations of movements occur with effusion and occasional snapping of the joint.2 SC expands the joint and can occur in extra-articular locations, but if it invades beyond the joint capsule, one would suspect a different entity.3
Case report
A 34-year-old male presented to our clinic with pain, mechanical limitation, and swelling in the right elbow, that had become increasingly severe over the previous 3 years. In physical examination, there was limitation in extension by 30° and in flexion by 20° when compared to the contralateral elbow. It was detected that there was a palpable, painful, rigid, immobile mass at the anterior and posterior aspects of the elbow joint.
In radiological evaluation, it was seen that there was narrowing in the joint space and cortical irregularity in the anterior region (Figure 1). On computerized tomography (CT) scan, it was seen that there was soft tissue swelling at the right elbow joint, and cortical erosive changes in the distal humerus (Figure 2). On magnetic resonance (MR) imaging, hyperintense, well-defined, nodular mass lesions were seen in fat suppression sequences (Figure 3).
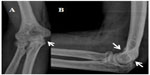  | Figure 1 Anterioposterior (A) and lateral (B) radiographs of elbow. |
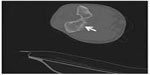  | Figure 2 On CT scan, chondral erosions can be seen posterior to the elbow joint in axial sections with arrow. |
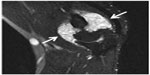  | Figure 3 On MR imaging, hyperintense, well-defined, nodular mass lesions can be seen in fat suppression sequences (arrows). |
Surgery was scheduled for the patient with an initial diagnosis of SC. First, olecranon fossa was exposed through the lateral and medial tricep muscles following a midline incision at the posterior aspect of the elbow. Numerous chondral loose bodies, which resulted in limitation of extension of the elbow and filled the olecranon fossa leading to enlargement of the joint capsule, were removed after capsulotomy. A second incision was made at the anterior aspect of the elbow for lesion in the anterior region of the elbow. The anterior capsule was exposed by preserving the vascular and neural components. Again, numerous rigid, free chondral loose bodies, which extended to the radius head and a process inside the joint, were removed following capsulotomy (Figure 4). Both anterior and posterior synovial excisions were made. After excision, the range of motion was assessed intraoperatively. It was noted that preoperative flexion and extension limitations were relieved.
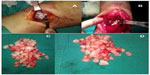  | Figure 4 Chondral components removed during (A and B) and after (C and D) surgery can be seen. |
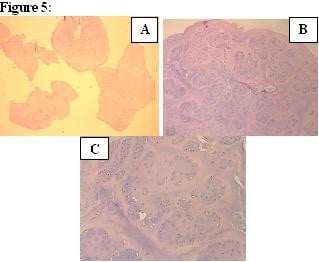
In the histopathological evaluation of the chondral loose bodies that were removed, SC was confirmed by observation of well-defined chondrocytes and mature cartilage tissues with lobulation (Figure 5). No recurrence was detected during 24-month follow-up.
Discussion
SC is an extremely rare proliferative disorder resulting from the metaplasia of cartilage tissue, which has a mesenchymal subintimal layer of synovium. The disease is characterized by the formation of multiple intrasynovial and osteochondral cartilaginous nodules. SC is generally associated with monoarticular involvement, while it is more frequently seen in large joints. The etiology is unclear.4,5 It is extremely rare for it to involve the elbow joint.6 In a study by Mueller et al and Kamineni et al, only 20 cases and 12 cases of elbow involvement were reported in recent years, respectively.7,8
The HLA-DR and CD68 gene expression seen in previous studies supports the view that a reactive condition may play a role in the etiopathogenesis of SC; also many other abnormalities have been reported as non-diploid karyotypes, rearrangements, losses, or gains of chromosomes.9,10 However, no cytogenetic analysis was performed in our patient.
Malign transformation was reported in a few cases during long-term follow-up although spontaneous regression and transformation to chondrosarcoma are extremely rare.11 Anract et al suggested that malign transformation should be suspected in the presence of osseous involvement and progressive clinical findings.12 In our case, no apparent osseous involvement was observed despite occasional cortical erosions on CT scan and MR imaging. No changes indicating malign transformation were observed during 24-month follow-up.
In SC, the most common symptoms include pain, swelling, limitation, and snapping.6 Clinical evaluation, plain radiographs, sonography, CT scan, and MR imaging are helpful in diagnosis. In addition, histopathological evaluation is warranted. In plain radiographs, chondral loose bodies inside the joint can be visualized depending on the degree of calcification or ossification. However, lack of radiopacity makes diagnosis challenging. In our case, CT scan and MR imaging were used for further evaluation due to the lack of radiopacity on plain radiographs. MR imaging is the most effective modality in early diagnosis.
In SC, the differential diagnosis includes pigmented villonodular synovitis, rheumatoid arthritis, synovial hemangioma, and chondrosarcoma.13 It can also develop secondary to other articular disorders, including osteochondritis dissecans, tuberculosis arthritis, degenerative joint disease, and osteochondral fractures.4 No mitotic changes, pleomorphism, or atypia suggesting malignant transformation were observed; in addition, no underlying condition was identified in our case.
The treatment involves the removal of loose bodies via pen or arthroscopic synovectomy. Arthroscopic treatment has been generally preferred in recent years due to early rehabilitation and less morbidity.5,14 However, in our case, open synovectomy was preferred as arthroscopy did not seem to be possible due to numerous loose bodies spreading to the anterior and posterior of the elbow region.
Conclusion
In conclusion, SC with monoarticular involvement is an extremely rare, proliferative disorder. As it is difficult to make a diagnosis based solely on clinical evaluation, the diagnosis should be supported by consistent radiological and histopathological findings. After establishing diagnosis, it is treated with complete synovial excision and the removal of loose bodies. Long-term follow-up should be performed due to the likelihood of recurrence.
Disclosure
The authors report no conflicts of interest in this work.
References
Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151–158. | |
Kural C, Akyildiz MF, Ertürk H, Bayraktar K. Synovial chondromatosis of the ankle joint and elbow in two cases. Acta Orthop Traumatol Turc. 2005;39(5):441–444. | |
Hermann G, Abdelwahab IF, Klein M, Kenan S, Lewis M. Synovial chondromatosis. Skeletal Radiol. 1995;24(4):298–300. | |
Chillemi C, Marinelli M, de Cupis V. Primary synovial chondromatosis of the shoulder: clinical, arthroscopic and histopathological aspects. Knee Surg Sports Traumatol Arthrosc. 2005;13(6):483–488. | |
Bruggeman NB, Sperling JW, Shives TC. Arthroscopic technique for treatment of synovial chondromatosis of the glenohumeral joint. Arthroscopy. 2005;21(5):633. | |
Jazrawi LM, Ong B, Jazrawi AJ, Rose D. Synovial chondromatosis of the elbow. Am J Orthop. 2001;30(3):223–224. | |
Mueller T, Barthel T, Cramer A, Werner A, Gohlke F. Primary synovial chondromatosis of the elbow. J Shoulder Elbow Surg. 2000;9(4):319–322. | |
Kamineni SS, O’Driscoll W, Morrey BF. Synovial osteochondromatosis of the elbow. J Bone Joint Surg Br. 2002;84-B:961–966. | |
Apte SS, Athanasou NA. An immunohistological study of cartilage and synovium in primary synovial chondromatosis. J Pathol. 1992;166(3):277–281. | |
Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18–22. | |
Hallam P, Ashwood N, Cobb J, Fazal A, Heatley W. Malignant transformation in synovial chondromatosis of the knee? Knee. 2001; 8(3):239–242. | |
Anract P, Katabi M, Forest M, Benoit J, Witvoët J, Tomeno B. Synovial chondromatosis and chondrosarcoma. A study of the relationship between these two diseases. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(3):216–224. | |
Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243–245. | |
Griesser MJ, Harris JD, Likes RL, Jones GL. Synovial chondromatosis of the elbow causing a mechanical block to range of motion: a case report and review of the literature. Am J Orthop. 2011;40(5):253–256. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

