Back to Journals » Cancer Management and Research » Volume 12
Synergistic Effects of Resveratrol and Temozolomide Against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications
Authors Liu Y, Song X, Wu M, Wu J, Liu J
Received 17 April 2020
Accepted for publication 16 July 2020
Published 11 September 2020 Volume 2020:12 Pages 8341—8354
DOI https://doi.org/10.2147/CMAR.S258584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yusi Liu, Xue Song, Moli Wu, Jiao Wu, Jia Liu
Liaoning Laboratory of Cancer Genetics and Epigenetics and Department of Cell Biology, College of Basic Medical Sciences, Dalian Medical University, Dalian, People’s Republic of China
Correspondence: Jia Liu
Liaoning Laboratory of Cancer Genetics and Epigenetics and Department of Cell Biology, College of Basic Medical Sciences, Dalian Medical University, Dalian 116044, People’s Republic of China
Tel +86-411-86110318
Email [email protected]
Purpose: Temozolomide (TMZ) is a commonly used anti-glioblastoma (GBM) drug. However, glioblastoma cells frequently show primary and acquired resistance to TMZ. As a promising anti-GBM candidate, resveratrol (Res) faces the similar problem as TMZ. Although resveratrol combined with TMZ (Res/TMZ) has been reported to be used to treat GBMs, it remains unclear whether this combination is broad-spectrum for all glioma cells until now, especially for GBM cells/cases with dual drug resistance. The study aimed to evaluate the synergistic effects of resveratrol and TMZ against GBMs and identify the underlying mechanisms.
Materials and Methods: Drug sensitivities of rat RG-2, human LN-18 and LN-428 cell lines and effectiveness of Res/TMZ combinations were investigated via multiple experimental methods. O6-methylguanine-DNA methyltransferase (MGMT) was observed by Western blotting and immunocytochemistry (ICC). Transducer and activator of transcription 3 (STAT3) signaling pathway and expression changes of STAT3-related gene were detected to explore the possible synergistic mechanism.
Results: One hundred micromolar resveratrol and 500 μM TMZ inhibited the growth of RG-2 cells and the low-dose combination (25 μM/250 μM) showed similar suppressive effects. LN-18 and, especially, LN-428 cells were neither sensitive to 100 μM resveratrol nor to 500 μM TMZ, while their growth was suppressed by combination of 75 μM Res/750 μM TMZ with the suppressive rates of 62.5% and 28.6% and apoptosis rates of 11.9% and 7.4%, respectively. Resveratrol had regulatory effect on the expression of MGMT and it could significantly down-regulate MGMT overexpression caused by TMZ. In addition, STAT3/Bcl-2/survivin signaling pathway was also remarkably inhibited in Res/TMZ-treated GBM cells.
Conclusion: Our results demonstrated synergistic effects of Res/TMZ on RG-2 cells and their bilaterally sensitizing effects to LN-18 and LN-428 cells. Frequent upregulation of MGMT and activation of STAT3 are the unfavorable factors for the treatment of GBMs and they may be the potential targets of Res/TMZ therapy.
Keywords: resveratrol, temozolomide, synergistic effects, glioblastoma, MGMT, STAT3, Bcl-2, survivin
Introduction
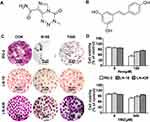
Glioblastoma (GBM) is the most common malignant tumor with extremely poor prognosis.1 The combination of surgery, radiotherapy and/or chemotherapy is the main strategy of GBM therapy.2 However, the difficulty of eradicating high aggressive GBM tissues always leads to the recurrence after surgery. Therefore, post-operative adjuvant chemotherapy has to be employed to prevent tumor relapse and to prolong the survival time of GBM patients.3 Currently, the second-generation oral alkylation agent temozolomide (TMZ) (Figure 1A) is the standard first-line chemotherapeutic drug because of its strong capability to cross the blood-brain barrier,4 relatively weaker adverse effects and higher effectiveness to extend patients’ life span.5
It has been recognized that TMZ methylates guanine at position O6 of DNA, resulting in DNA cross-linking and finally cell death.6 MGMT, a 22 kD DNA repair protein, can bind with methyl groups to repair TMZ-caused DNA damage and therefore prevent the apoptosis of cancer cells. For instance, MGMT can reduce the efficacy of treating GBM of TMZ via removing TMZ-caused methyl groups at the site of DNA O6 guanine within 10 minutes.7 Consequently, more than half of GBM patients with expression of MGMT relapse after TMZ chemotherapy, while the recurrent rate of GBM patients negative in MGMT expression is less than 10%.8 Apparently, the status of the expression of MGMT may influence the therapeutic outcome of TMZ and, alternatively, well control of the expression of MGMT would be helpful in improving the treatment effects of TMZ for GBM. Based on the fact that GBM frequently shows primary and secondary resistance to TMZ, it would be of clinical values to explore the way(s) for improving the TMZ-based therapy strategies for GBM.
Resveratrol (Res) (Figure 1B), a polyphenolic compound, inhibits the growth of various human tumors in vitro.9 Unlike most anticancer agents, resveratrol shows little adverse effects on normal neuronal cells in vitro and the brain tissues in vivo at the anticancer doses (100 μM to 200 μM).10 A large quantity of evidence has demonstrated that resveratrol can exhibit its antitumor effects on cancer cells by changing multiple molecular targets.11 For example, it can inhibit the growth and induce apoptosis of human glioma cells by inhibiting the activation and transcription of STAT3.12 However, all current GBM cell lines respond to resveratrol and TMZ differently,13 indicating the limitation of their wide use in GBM treatment. Because the combination of anticancer drugs can improve the primary and secondary drug resistance, it may be possible to overcome the dual drug resistance of GBM cells to TMZ as well as resveratrol. Previous studies on this issue mainly focused on the study of resveratrol to reverse resistance to temozolomide. However, this study aims to address the synergistic sensitization effect between resveratrol and temozolomide against glioma cells. Evaluation of MGMT and STAT3 signaling pathway were detected to investigate the underlying mechanism of reversed resistance of both resveratrol and temozolomide through effective combination treatment of Res/TMZ.
Materials and Methods
Agents
Resveratrol and TMZ (3,4-dihydro-3-methyl-4-oxoimidazo [5,1-d]-as-tetrazine-8-carboxamide, TMZ) were purchased from Sigma (SigmaChem Co., St. Louis, USA). Resveratrol was dissolved in dimethyl sulfoxide (DMSO; Sigma Chem Co.) to prepare a stock solution at concentration of 100 mM, TMZ was dissolved in DMSO to prepare a stock solution at concentration of 1 mM, both were wrapped in aluminum foil for protection against light and stored at −20°C. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide, MTT) was purchased from Sigma (SigmaChem Co., St. Louis, USA) and prepared at a concentration of 5 mg/mL in phosphate buffered saline (PBS; Beyotime, Shanghai, China) and stored at 4°C.
Glioblastoma Cell Lines and Cell Culture
Rat RG-2 glioblastoma cell line and human LN-18 and LN-428 cell lines14,15 were selected in this study, which were kindly provided by Professor Nicolas de Tribolet, Department of Neurosurgery, Central Hospital of Lausanne University (CHUV), Switzerland. The use of the three kinds of cell lines was approved by the Institutional Review Board of Dalian Medical University (DMU). All cell lines used in the study were authenticated for accuracy by short tandem repeat (STR) test in February 2019 by GENEWIZ, Inc., Beijing, China, and tested periodically for mycoplasma by PCR. The three kinds of cell lines were cultured in Dulbecco’s modified Eagle’s essential medium (DMEM; Invitrogen Co., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand island, NY, USA) and 100 mg·mL–1 of penicillin-streptomycin (Invitrogen Co.) under a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cell Growth Evaluation
To determine the cell response to drug treatments, novel coverslip preparation dishes (China Patent for Invention: ZL201520113833.9; Guangzhou JET Bio-filtration Co., Guangzhou, China) were employed to prepare dozens of cell-bearing coverslips under the same experimental condition. Briefly, 5×104 cells/mL of the three GBM cells were seeded to the culture-dishes. When the cells grew to 75% confluent, the coverslips bearing RG-2, LN-18 or LN-428 cells were collected from the main dish and re-incubated in the medium containing different concentrations of resveratrol, TMZ or their combinations, respectively (Table 1). The treatments lasted for 48 h, and the coverslips were collected, properly fixed and then methyl thiazolyl tetrazolium (MTT) cell proliferation assay, hematoxylin-eosin (HE) morphological evaluation, terminal deoxynucleotidyl transferase dUTP-nick end labeling assay (TUNEL; all from SigmaChem Co., St. Louis, USA) and ICC staining were performed. The effects of resveratrol and TMZ on cell proliferation were determined by MTT assay and shown in cell viability (% of control). HE staining was performed on cell-bearing coverslips to evaluate the morphological features of the three kinds of GBM cell lines with different treatments. TUNEL assay was employed to detect apoptotic cells according to the producer’s instructions (Promega Corporation, USA), the coverslips were photographed using a fluorescence microscope (Leica DMI4000B, Germany).
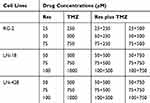 |
Table 1 Different Concentrations of Res, TMZ or Res Plus TMZ |
Annexin V/PI Staining-Based Apoptosis Assay
The Annexin V-FITC Apoptosis Detection Kit (Nanjing Key Gen Biotech Co., Nanjing, China) was used for quantification of apoptosis. Cells (2 × 105/well) were seeded into 6-well plates and were collected after 48 h drug treatments. Externalized phosphatidylserine was labeled with annexin-V-FITC-conjugated for 15 min on ice. Propidium iodide (PI, 1 μg/mL) was added 10 min prior to FACS analysis. Viable (annexin-/PI-), early (annexin+/PI-) and late apoptotic (annexin+/PI+) and necrotic cells (annexin-/PI+) were assigned. The labeled cells were identified by flow cytometry BD Accuri C6 (BD Biosciences, San Jose, CA, USA).
Cell Cycle Analysis
Cells (2 × 105/well) were seeded into 6-well plates and were collected after drug treatment for 48 h. The harvested cells were fixed in 70% ethanol for staining with DNA dye, and then suspended in 0.5 mL to 1 mL of PI solution containing RNase and incubated at 37°C for 30 min. Cell cycle profiles and cell apoptotic fractionations were obtained with a FACSvantage flow cytometer and the data were analyzed with ModFit software (Verity Software House, Inc, Topsham, ME). The analyses were repeated for three times to establish confidential conclusion.
Western Blot Analysis
Cells were lysed using RIPA lysis buffer (Beyotime, Shanghai, China). The sample proteins (50 μg/well) were separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to polyvinylidene difluoride membrane (Amersham, Buckinghamshire, UK). The membrane was blocked with 5% skimmed milk in TBS-T (10 mM Tris–HCl, pH 8.0, 150 mM NaCl and 0.5% Tween 20) at 4°C, and then rinsed 10 min for three times with TBS-T, followed by incubation for 3 h at room temperature with the first antibody in appropriate concentrations (MGMT: 1:1000; STAT3: 1:800; p-STAT3: 1:800; Bcl-2: 1:800; survivin: 1:800; all from Santa Cruz Biotech, Inc., Santa Cruz, CA, USA), and then incubation with HRP conjugated anti-mouse or anti-rabbit IgG (Proteintech, Chicago, IL, USA) 1 h. The bound antibody was detected using the enhanced chemiluminescence system (Roche GmbH, Mannheim, Germany). After removing the labeling signal by incubation with stripping buffer,16,17 the membrane was reprobed with other antibodies one by one until all of the parameters were examined. Image J was used to measure the density of bands (National Institutes of Health, Bethesda, MD).
Immunocytochemical Staining
ICC staining was performed on the cell-bearing coverslips of the different experimental groups. The antibodies used were rabbit anti-rat and human MGMT (1:500), rabbit anti-rat and human STAT3 (1:500), rabbit anti-rat and human p-STAT3 (1:800), rabbit anti-rat and human survivin (1:500), rabbit anti-rat and human Bcl-2 (1:500; all from Bioss Biotech, Inc., Beijing, China). Color reaction was performed using 3.3′-diaminobenzidine tetrahydrochloride (DAB; Dako, Glostrup, Denmark). According to the labeling intensity, the staining results were evaluated by two independent researchers and scored as negative (-) if no immunolabeling was observed in target cells, weakly positive (+) if the labeling was faint, moderately positive (++), and strongly positive (>++) when the labeling was stronger or distinctly stronger than (++).
Statistical Analysis
Each experiment was conducted for three times, and the data obtained were analyzed together. The statistical significance between different experimental groups was evaluated with independent samples t-test. p < 0.05 was considered to be statistically significant. The bar graphs present the mean ± standard deviation (SD) of experiments data.
Results
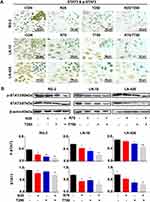
Different Drug Sensitivities of the Three Kinds of GBM Cell Lines
In order to determine the sensitivities of RG-2, LN-18 and LN-428 cells to resveratrol and temozolomide, the conventional in vitro treatment doses of Res18 (100 μM; R100) and TMZ19 (500 μM; T500) were used to treat the three cells, respectively. Compared with the control group without drug treatment, R100 and T500 showed growth inhibitory effect on RG-2 cells with the inhibitory rate of 50% and 26%, which decreased to 24% and 16% in LN-18 and further to 13% and 5% in LN-428 cells (Figure 1C and D). The rank of the sensitive degree of the three kinds of GBM cell lines to R100 and T500 were RG-2 > LN-18 > LN-428, and all of them were more susceptible to resveratrol than TMZ at two conventional doses of treatment drugs (Figure 1C and D).
Low-Dose Res/TMZ Combination Suppressed RG-2 Proliferation
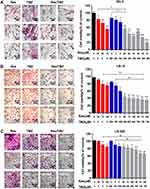
Because RG-2 cells were more sensitive to 100 μM resveratrol and 500 μM TMZ (Figure 1) among the three kinds of cell lines, they were further treated by lower concentrations of resveratrol (25, 50 and 75 μM), TMZ (250, 500 and 750 μM) and their different combinations, respectively. MTT results showed that both relatively low concentrations of resveratrol (25 μM, p < 0.05; 50 μM, p < 0.01; 75 μM, p < 0.01) and TMZ (250 μM, p < 0.05; 500 μM, p < 0.05; 750 μM, p < 0.05) suppressed growth of RG-2 cells in dose-dependent manner (Figure 2A). HE staining revealed reduction of cell number and distinct morphological alteration of RG-2 cells (Figure 2A). The significant growth suppression could be achieved by the combination of 25 μM resveratrol and 250 μM TMZ (p < 0.01), accompanied with cellular shrinkage, chromatin condensation and formation of apoptotic body (Figure 2A).
Suppression of LN-18 and LN-428 Cells by High-Dose Res/TMZ Combination
MTT cell proliferation assay was further performed on LN-18 and LN-428 cells. The results revealed that neither the single doses of R50 μM, R75 μM and R100 μM nor T500 μM and T750 μM showed apparent inhibitory effect on the two kinds of cell lines (Figure 2B, C and Table 2). When those doses of resveratrol (R50 μM, R75 μM and R100 μM) and TMZ (T500 μM, T750 μM) were used in combination on LN-18 and LN-428 cells, the inhibitory effects were both apparently improved in dose-related manner. As shown in Figure 2B, C and Figure 3A, the growth of LN-18 cells was remarkably suppressed by R50+T500 (p < 0.01) and R75+T750 (p < 0.01), LN-428 cells were suppressed by R50+T500 (p < 0.05), R100+T500 (p < 0.01), and R75+T750 (p < 0.01), respectively. Extensive death was observed in those Res/TMZ-treated cells (Figure 3B).
 |
Table 2 Different Inhibition Effect of Res Plus TMZ on Glioblastoma Cell Lines |
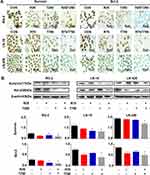
Res/TMZ-Caused Apoptosis and G1 or S Arrest
TUNEL assay demonstrated that a high percentage of damage in nuclei DNA appeared in RG-2 cells after treated by R25/T250 for 48 h, which also appeared in LN-18 and LN-428 cells treated by R75/T750 combination after 48 h (Figure 3B). The results of flow cytometry based on Annexin V-FITC/PI double staining are shown in Figure 3C. The proportion of apoptotic cells in control RG-2 cells were 2.73 ± 0.47%, which increased to 16.54 ± 0.56% after treatment by R25/T250 for 48 h (p < 0.001). In contrast, the proportion of apoptotic cells in LN-18 and LN-428 cells after treatment by R75/T750 were 11.90 ± 0.32% and 7.40 ± 0.49%. Flow cytometry analysis (Figure 3D) revealed that 59.75% of the control RG-2 cells were in G0/G1 phase and 32.25% were in S phase. The proportion of cells in G0/G1 phase was decreased while that of the S phase was elevated when RG-2 cells were treated by the combination of R25/T250 (p < 0.05). The percentages of G0/G1 phase and S phase were 33.06% and 62.55% in control LN-18 cells, the proportion of G1 increased (R75: p < 0.05; T750: p < 0.01; R75/T750: p < 0.01) and the proportion of S phase decreased after treatment by R75, T750 and especially their combination (R75+T750: p < 0.01). The percentages of G0/G1 phase and S phase were 62.81% and 30.06% in control LN-428 cells, no distinct change of the fractions of G1 and S phase was detected when LM-428 cells were treated by R75. However, more cells were arrested in the G0/G1 phase (p < 0.01) and less cells in the S phase (p < 0.05) when treated with R75/T750.
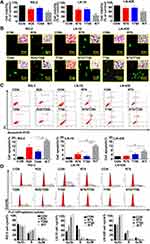
Differential Expression of MGMT in the Experimental Groups
Upregulation of the expression of MGMT is known as the main factor leading to TMZ resistance in GBMs.6 Western blotting and ICC staining were therefore conducted to check the expression of MGMT in GBM cells before and after the drug treatment. ICC staining revealed remarkable increase of production of MGMT in the three kinds of GBM cell lines treated by TMZ, in comparison with that of control cells and the cells treated by Res or combination of Res/TMZ (Figure 4A). The results of Western blotting showed that the expression of MGMT in RG-2 cells was reduced by 25.4% after treatment by 25 μM Res for 48 h, increased by 37.6% after treatment by 250 μM TMZ compared with that of the control group, and decreased by 44.9% in 25 μM Res/250 μM TMZ-treated cells compared with that of 250 μM TMZ group. The level of MGMT was decreased by 15.3% in 75 μM Res treated LN-18 cells and increased by 10.8% in LN-428 cells, which was increased by 12.6% and 35.2% in 750 μM TMZ-treated LN-18 and LN-428 cells, compared with that of the corresponding control group, respectively. When treated by the combination of 75 μM Res/750 μM TMZ, levels of MGMT were distinctly reduced in LN-18 (38.7%) and LN-428 (33.5%) cells in comparison with that of 750 μM TMZ-treated groups (Figure 4B and Supplementary Figure S1).
STAT3 Inactivation Caused by Res/TMZ in GBM Cells
STAT3 signaling pathway plays a critical role in the development and regeneration of the central nervous system (CNS).20 The high activation level of the STAT3 signaling pathway is related to the drug resistance of glioma cells to both resveratrol and temozolomide.12 Although the STAT3 inactivation caused by resveratrol has been evidenced in glioma cells, it is still unclear whether it is closely linked with Res/TMZ-caused cell crisis. To address this issue, the levels of STAT3 signaling in sensitive RG-2 cells treated with low effective doses of R25, T250 alone or their combination, in insensitive LN-18 and LN-428 cells treated with relative high doses of R75, T750 alone or their combination were checked. It was found in the results of ICC that STAT3 was expressed in the three kinds of normally cultured GBM cell lines with distinct nuclear translocation (Figure 5A). Western blotting showed that the expression of STAT3 in RG-2 cells was reduced by 25.4% after treatment by R25/T250 for 48 h compared with that of the control group. p-STAT3 was predominantly localized in the nuclei of RG-2 cells and became weakened (71.2% reduction) after treatment by R25/T250 for 48 h. The phosphorylation of STAT3 was also inhibited in LN-18 (45.7%) and LN-428 (32.6%) cells upon treatment by R75/T750 for 48 h (Figure 5B) compared with that of the control group, respectively. Western blotting and ICC results revealed that the levels of activation of STAT3 in the three kinds of GBM cell lines were relatively correlated with their sensitivities to resveratrol and TMZ in terms of their distinct reduction in LN-18 and especially in RG-2 while lesser significant in LN-428 cells.
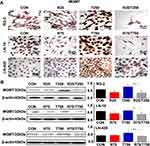
Reduction of Survival Factors in Res/TMZ-Treated Cells
Survivin and Bcl-2 play active roles in cell proliferation and maintenance of gliomas and are known as the common target genes of STAT3 signaling pathway.11 Therefore, their statuses of expression in the three kinds of GBM cell lines with or without Res/TMZ treatment were analyzed. Western blotting and ICC results revealed that the level of survivin was decreased by 56.9% in R25/T250 treated RG-2 cells after 48 h, decreased by 43.5% in R75/T750 treated LN-18 cells and decreased by 24.7% in LN-428 cells compared with that of the corresponding control group, respectively (Figure 6A, B). Moreover, the results showed that the level of Bcl-2 of LN-18 cells, LN-428 cells and, especially, RG-2 cells were 36.4%, 34.2% and 45.9% lower than that in normal glioma cells.
Discussion
GBM is a kind of intracranial malignancy with very poor prognosis. Adjuvant chemotherapy has been widely employed to prevent tumor relapse because of the difficulty to radically remove the highly aggressive GBM tissues by surgery.21 Nevertheless, the recurrent rates still remain very high, and the primary and secondary drug resistance of GBMs is the main cause of postoperative recurrence and death.22 Currently, TMZ is the first choice among anti-GBM drugs,23 while the data from clinics reveals that the initial effective rate of TMZ is about 50% for GBMs, and the frequency of secondary drug resistance is about 35%,24 resulting in the failure of GBM chemotherapy. Given the lack of newly designed anti-GBM drugs, it is in urgent need to explore a safer and more effective approach to improve the sensitivity and even to reverse TMZ resistance of GBM cells.25
Resveratrol, a natural polyphenol compound, possesses multiple anti-cancer effects.26 More importantly, it shows little toxicity and side effects on normal cells within the effective anticancer doses,27 suggesting its values in cancer therapy by itself or especially in combination with other anticancer drugs. It has been known that resveratrol is able to improve the sensitivity of tumor cells to gemcitabine, vincristine, adriamycin and paclitaxel.28 Although resveratrol combined with TMZ has also been reported to be used to treat GBMs,29 it now remains unclear whether this combination is broad-spectrum for all glioma cells or not, especially for GBM cells/cases with dual resistance to both TMZ and resveratrol. Our study aimed to evaluate the synergistic effects of resveratrol and temozolomide against GBMs and identify the underlying mechanisms. Results showed that rat RG-2 cells were either sensitive to Res or TMZ, while human LN-18 and LN-428 GBM cell lines were relatively insensitive to both of them. Further analyses of responses of these three cell lines to different concentrations of Res, TMZ and their combinations revealed that a combination of 1/4 normal in vitro dosages of resveratrol (100 μM) and TMZ (1000 μM) could effectively inhibit the growth and induce the apoptosis of RG-2 cells, suggesting a reciprocal sensitization effect between them. This result was interesting especially for RG-2 cells, which are sensitive to resveratrol and TMZ, because the bioavailability of resveratrol for RG-2 cells by in vivo administration is very low. If it can achieve the anti-cancer effect at a lower concentration through the combination with TMZ, it will provide experimental basis for the practical application of resveratrol in vivo. Furthermore, TMZ has objective side-effects such as severe gastrointestinal reactions30 and bone marrow inhibition.31 If suppression of the growth of GBM cell can be achieved using 1/4 conventional dose of TMZ when combined with nontoxic resveratrol, the patients’ quality of life would be greatly improved. Besides, a large quantity of recent new intracranial drug administration methods such as lumbar puncture and intranasal delivery also provide new strategies for drugs to reach intracranial tumor lesions efficiently and inhibit tumors effectively.
Multidrug resistance is a very difficult clinical problem for the treatment of GBMs.32 LN-18 and especially LN-428 cell lines selected in this study are the typical examples, because the in vitro inhibition effect of resveratrol and TMZ on them is not promising. In order to seek the possibility of improving the curative effect and reversing the drug resistance, we selected correspondingly two high concentrations of TMZ, three high concentrations of resveratrol compared to the conventional doses and their combinations to treat these two less sensitive cell lines. Our results showed that the effective combination of the two drugs inhibited cell proliferation and induced apoptosis in a dose-dependent manner. Nevertheless, the sensitivity of these two cell lines to the combination therapy of Res/TMZ was different. For example, the combination of 50 μM resveratrol and 500 μM TMZ is sufficient to cause 56.7% inhibition of growth of LN-18 cells while LN-428 cells are more resistant to this combined therapy (Figure 2B), and 100 μM resveratrol combined with as high as 1250 μM TMZ are required to achieve this similar therapeutic outcome. The above results suggested that the combined dosage of resveratrol and TMZ may vary from patients and the individualized medication is necessary. In this context, patient-derived organoids (PDO) would be helpful to achieve that goal. The drug sensitivity test system of PDO based on GBM will be helpful to achieve the above goals.
MGMT is a kind of DNA repair protein that widely exists in the organisms from bacteria to mammals, it can repair DNA alkylation damage caused by TMZ, leading to resistance of TMZ in GBM cells.33 Therefore, suppressing the TMZ-induced upregulation of MGMT may be a potential approach to overcome resistance of TMZ. Similar to the previous reports, TMZ was able to increase the levels of MGMT in the three kinds of GBM cells, including TMZ-sensitive RG-2 cells in our experiments. Resveratrol not only enhanced sensitivity of TMZ in RG-2 cells and improved the efficacy of TMZ to LN-18 and LN-428, but also suppressed the upregulation of MGMT caused by TMZ. These findings explain why RG-2 cell growth can be efficiently inhibited with a combination of 1/4 normal in vitro dosages of resveratrol and TMZ to great degree. It was also found that the level of MGMT in Res/TMZ-treated LN-18 cells was 38.7% lower than that of TMZ-treated cells and 24.3% lower than that of the control group, and the level of MGMT in the corresponding LN-428 cells was 33.5% lower in TMZ group. These phenomena indicated the potential correlation of effects of TMZ-sensitizing and MGMT-suppressing of resveratrol in GBM cells.
As a common molecular target of resveratrol, STAT3 is also significantly associated with chemical resistance to TMZ.34 Compared with normal brain tissues, most of the glioma tissues (about 90%) and cell lines show high degree of phosphorylation of STAT3, which aggravates the potential of multi-drug resistance in malignant glioma cells. The downstream effectors of STAT3 such as survivin and Bcl-2 can regulate multiple processes that are critical for cancer progression, involving tumor initiation and growth, cell death, cell senescence and differentiation.35 The results of ICC staining and Western blotting analysis showed that Res/TMZ treatment restrained the activation of the STAT3 signaling pathway and lowered the expression of Bcl-2 and survivin in sensitive RG-2 cells treated with low effective combination of R25 and T250 as well as in insensitive LN-18 and LN-428 cells treated with relative high dose combination of R75 and T750, thereby enhancing the sensitivities of the three glioma cell lines to both resveratrol and TMZ in varying degrees.
Recent studies have shown that glioma initiating cells (GICs), a small population of stem-like cells aggravate the therapeutic resistance and increase the recurrence of glioblastoma, thus leading to the failure of rational therapy and molecular target therapy.36 Consequently, it is essential to identify and characterize which types of glioma cells can evade therapies and become initiating cells so that they can be targeted. The synergistic sensitization effect between resveratrol and temozolomide in treating gliomas needs to be further verified using GICs. In addition, in vitro studies to further explore more potential pathways related to relapse and resistance and investigate the role of these cellular pathway targets in different cell populations will further help optimize their targeting. In order to simulate the in vivo microenvironment, the establishment of more effective in vivo models is also very necessary and beneficial to the study of drug resistance mechanism of tumor recurrence.
Conclusion
Combination treatment of resveratrol and temozolomide (Res/TMZ) had synergistic effects through inhibiting the expression of MGMT as well as downregulating STAT3/Bcl-2/survivin signaling pathway, further inducing the apoptosis and cell cycle arrest of glioma cells.
Abbreviations
CHUV, central hospital of lausanne university; CNS, central nervous system; DAB, 3.3′-diaminobenzidine tetrahydrochloride; DMEM, Dulbecco’s modified Eagle’s essential medium; DMSO, dimethyl sulfoxide; DMU, Dalian Medical University; FBS, fetal bovine serum; GBM, glioblastoma; HE, hematoxylin-eosin staining; ICC, immunocytochemical staining; MGMT, O6-methylguanine-DNA methyltransferase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PBS, phosphate buffered saline; PDO, patient-derived organoids; PI, propidium iodide; Res, resveratrol; SDS/PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; STAT3, signal transducer and activator of transcription 3; TMZ, 3.4-dihydro-3-methyl-4-oxoimidazo [5,1-d]-as-tetrazine-8-carboxamide; TUNEL, terminal deoxynucleotide transferase (TdT)-mediated dUTP-biotin nick-end labeling.
Ethics Statement
All procedures involving cell lines performed in this study were approved by the Institutional Review Board of Dalian Medical University.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant numbers 81272786 and 81450016), the Program Fund for Liaoning Provincial Department of Education Key Laboratory (grant number LF2017002) and the fund from Natural Science Foundation of Liaoning Province (grant number 2019-ZD-0650).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Waker CA, Lober RM. Brain tumors of glial origin. Adv Exp Med Biol. 2019;1190:281–297.
2. Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(suppl 5):1–49. doi:10.1093/neuonc/nos218
3. Wen PY. Therapy for recurrent high-grade gliomas: does continuous dose-intense temozolomide have a role? J Clin Oncol. 2010;28:1977–1979. doi:10.1200/JCO.2009.27.6014
4. Zhu Y, Fu L, Jing W, et al. Effectiveness of temozolomide combined with whole brain radiotherapy for non-small cell lung cancer brain metastases. Thorac Cancer. 2018;9(9):1121–1128. doi:10.1111/1759-7714.12795
5. Yoshino A, Ogino A, Yachi K, et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. Int J Oncol. 2010;36(6):1367–1377. doi:10.3892/ijo_00000621
6. Spiegl-Kreinecker S, Pirker C, Filipits M, et al. O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro Oncol. 2010;12(1):28–36. doi:10.1093/neuonc/nop003
7. Goellner EM, Grimme B, Brown AR, et al. Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair. Cancer Res. 2011;71(6):2308–2317. doi:10.1158/0008-5472.CAN-10-3213
8. Watanabe R, Nakasu Y, Tashiro H, et al. O6-methylguanine DNA methyl transferase expression in tumor cells predicts outcome of radiotherapy plus concomitant and adjuvant temozolomide therapy in patients with primary glioblastoma. Brain Tumor Pathol. 2011;28:127–135. doi:10.1007/s10014-011-0022-8
9. Matias D, Balça-Silva J, da Graça GC, et al. Microglia/astrocytes–glioblastoma crosstalk: crucial molecular mechanisms and microenvironmental factors. Front Cell Neurosci. 2018;12:235.
10. Dybkowska E, Sadowska A, Świderski F, et al. The occurrence of resveratrol in foodstuffs and its potential for supporting cancer prevention and treatment. Rocz Panstw Zakl Hig. 2018;69(1):5–14.
11. Shu XH, Wang LL, Li H, et al. Diffusion efficiency and bioavailability of resveratrol administered to rat brain by different routes: therapeutic implications. Neurotherapeutics. 2015;12(2):491–501. doi:10.1007/s13311-014-0334-6
12. Song X, Shu XH, Lin S, et al. Lumbar puncture-administered resveratrol inhibits STAT3 activation, enhancing autophagy and apoptosis in orthotopic rat glioblastomas. Oncotarget. 2016;7(46):75790–75799. doi:10.18632/oncotarget.12414
13. Lin GS, Chen YP, Lin ZX, et al. STAT3 serine 727 phosphorylation influences clinical outcome in glioblastoma. Int J Clin Exp Pathol. 2014;7(6):3141–3149.
14. Cho HY, Wang WJ, Niyati J, et al. Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol Cancer Ther. 2012;11(11):2462–2472. doi:10.1158/1535-7163.MCT-12-0321
15. Xia SL, Wu ML, Li H, et al. CRABP-II and FABP5-independent responsiveness of human glioblastoma cells to all-trans retinoic acid. Oncotarget. 2015;6(8):5889–5902. doi:10.18632/oncotarget.3334
16. Li YT, Tian XT, Wu ML, et al. Resveratrol suppresses the growth and enhances retinoic acid sensitivity of anaplastic thyroid cancer cells. Int J Mol Sci. 2018;19(4):1030. doi:10.3390/ijms19041030
17. Wu ML, Li H, Yu LJ, et al. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS One. 2014;9(2):e89806. doi:10.1371/journal.pone.0089806
18. Zhang P, Li H, Yang B, et al. Biological significance and therapeutic implication of resveratrol inhibited Wnt, Notch and STAT3 signaling in cervical cancer cells. Genes Cancer. 2014;5(5–6):154–164. doi:10.18632/genesandcancer.15
19. Li H, Liu Y, Jiao Y, et al. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol Rep. 2015;35(1):343–351.
20. Song H, Man L, Wang Y, et al. The regenerating spinal cord of gecko maintains unaltered expression of beta-catenin following tail amputation. J Mol Neurosci. 2015;55(3):653–662.
21. Song H, Man L, Wang Y. Expression of beta-catenin following tail amputation. J Mol Neurosci. 2015;55(3):653–662. doi:10.1007/s12031-014-0405-5
22. Li G, Zhai Y, Wang Z, et al. Postoperative standard chemoradiotherapy benefits primary glioblastoma patients of all ages. Cancer Med. 2020;9(6):1955–1965. doi:10.1002/cam4.2754
23. Basso J, Miranda A, Sousa J, et al. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 2018;428:173–183. doi:10.1016/j.canlet.2018.04.039
24. Bahadur S, Sahu AK, Baghel P, et al. Current promising treatment strategy for glioblastoma multiform: a review. Oncol Rev. 2019;13(2):417. doi:10.4081/oncol.2019.417
25. Lashford LS, Thiesse P, Jouvet A. Temozolomide in malignant gliomas childhood: a United Kingdom Children ’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J Clin Oncol. 2002;20:(24):
26. Liu T, Li A, Xu Y, et al. Momelotinib sensitizes glioblastoma cells to temozolomide by enhancement of autophagy via JAK2/STAT3 inhibition. Oncol Rep. 2019;41:1883–1892. doi:10.3892/or.2019.6970
27. Pan X, Zhao Y, Cheng T, et al. Monitoring NAD(P)H by an ultrasensitive fluorescent probe to reveal reductive stress induced by natural antioxidants in HepG2 cells under hypoxia. Chem Sci. 2019;10(35):8179–8186. doi:10.1039/C9SC02020A
28. Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998.
29. Sonnemann J, Kahl M, Siranjeevi PM, et al. Reverse chemomodulatory effects of the SIRT1 activators resveratrol and SRT1720 in Ewing’s sarcoma cells: resveratrol suppresses and SRT1720 enhances etoposide- and vincristine-induced anticancer activity. J Cancer Res Clin Oncol. 2016;142(1):17–26. doi:10.1007/s00432-015-1994-2
30. Filippi-Chiela EC, Thomé MP, Bueno e Silva MM, et al. Resveratrol abrogates the Temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the Temozolomide-induced senescence in glioma cells. BMC Cancer. 2013;13(1):147. doi:10.1186/1471-2407-13-147
31. Xin Y, Guo W, Yang CS, et al. Meta-analysis of whole-brain radiotherapy plus temozolomide compared with whole-brain radiotherapy for the treatment of brain metastases from non-small-cell lung cancer. Cancer Med. 2018;7(4):981–990. doi:10.1002/cam4.1306
32. Rizzieri D, LoRusso S, Tse W, et al. Phase I study of temozolomide and laromustine (VNP40101M) in patients with relapsed or refractory leukemia. Clin Lymphoma Myeloma Leuk. 2010;10(3):211–216. doi:10.3816/CLML.2010.n.033
33. Cao H, Li X, Wang F, et al. Phytochemical-mediated glioma targeted treatment: drug resistance and novel delivery systems. Curr Med Chem. 2020;27:599–629.
34. Lipp ES, Healy P, Austin A, et al. MGMT: immunohistochemical detection in high-grade astrocytomas. J Neuropathol Exp Neurol. 2019;78(1):57–64. doi:10.1093/jnen/nly110
35. Piperi C, Papavassiliou KA, Papavassiliou AG. Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells. 2019;8(11):1398. doi:10.3390/cells8111398
36. Lan F, Pan Q, Yu H, et al. Sulforaphane enhances temozolomide-induced apoptosis because of down-regulation of miR-21 via Wnt/β-catenin signaling in glioblastoma. J Neurochem. 2015;134(5):811–818. doi:10.1111/jnc.13174
37. Hao L, Liu YD, Jiao YM, et al. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol Rep. 2016;35(1):343–351. doi:10.3892/or.2015.4346
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.