Back to Journals » Infection and Drug Resistance » Volume 13
Synergistic Effect and Mechanism of Plumbagin with Gentamicin Against Carbapenem-Resistant Klebsiella pneumoniae
Authors Chen X, Yin L, Peng L, Liang Y, Lv H, Ma T
Received 10 June 2020
Accepted for publication 24 July 2020
Published 7 August 2020 Volume 2020:13 Pages 2751—2759
DOI https://doi.org/10.2147/IDR.S265753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Xiuli Chen,* Liyuan Yin,* Linxiu Peng, Yanshan Liang, Hang Lv, Tonghui Ma
School of Medicine, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hang Lv; Tonghui Ma
School of Medicine, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, Jiangsu Province, People’s Republic of China
Tel/ Fax +86 25 85811362
Email [email protected]; [email protected]
Background: Aminoglycosides are one of a few susceptible antimicrobials available for carbapenem-resistant Enterobacteriaceae (CRE). However, the altered pharmacokinetics and increasing drug resistance of aminoglycosides will make them hardly effective if used in monotherapy. The purpose of this study was to identify herbal compounds that potentiate the antibacterial effect of gentamicin against carbapenem-resistant Klebsiella pneumoniae (CRKp) with gentamicin resistance and explore the action mechanisms.
Methods: A collection of 280 Chinese herbal compounds was screened for synergistic effect with gentamicin against CRKp by broth microdilution method according to the standard of the Clinical and Laboratory Standards Institute (CLSI). Intracellular gentamicin was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The membrane potential was evaluated by BacLightTM Bacterial Membrane Potential Kit. Plumbagin-induced metabolite changes of vital metabolic pathways were measured by an optimized untargeted metabolomics method based on gas chromatography-mass spectrometer (GC/MS). Intracellular nicotinamide adenine dinucleotide (NADH) was detected via EnzyChrom NAD/NADH assay kit.
Results: We identified plumbagin to remarkably potentiate the antimicrobial activity of gentamicin against the CRKp with gentamicin resistance. Plumbagin at 100 μM could bring the MIC of gentamicin from > 16 μg/mL to ∼ 4 μg/mL despite its minimal inhibitory effect on the CRKp. A similar synergistic effect with gentamicin was also observed in an antibiotics-susceptible strain of Klebsiella pneumoniae. Compared with gentamicin monotreatment, the combination group showed a higher intracellular concentration of gentamicin and increased membrane potential in CRKp. Metabolomics analysis indicated remarkable increases of malate and α-ketoglutarate in the tricarboxylic acid (TCA) cycle in the CRKp upon plumbagin treatment. Further analysis revealed higher intracellular NADH concentration in plumbagin-treated CRKp, supporting increased proton-motive force (PMF) that facilitates aminoglycosides uptake.
Conclusion: Herbal compound plumbagin was identified to stimulate gentamicin uptake by CRKp via enhancing TCA efflux and PMF to achieve a synergistic antibacterial effect. Plumbagin may be used in combination with aminoglycosides for severe CRKp infection by potentiating their therapeutic efficacy and lowering dosage.
Keywords: drug-resistant Enterobacteriaceae, aminoglycosides, Chinese herbal compounds, potentiation mechanism
Introduction
Hospital infections caused by Carbapenem-resistant Klebsiella pneumoniae (CRKp) are increasingly prevalent all over the world, which is associated with high morbidity and mortality.1 Carbapenem antibiotics are the last line of defense in the treatment of CRKp infection with few other choices of effective drugs.2 The World Health Organization (WHO) listed CRKp in the critical priority tier, which needs research and development of novel antibiotics urgently.3
Aminoglycosides have been brought to the frontline of therapy for drug-resistant CRE because currently there are very few susceptible antimicrobials available.4 However, the altered pharmacokinetics and increasing drug resistance of aminoglycosides will make it hardly effective if used in monotherapy. One resolution is development of new generation aminoglycosides, such as plazomicin recently approved by US FDA for infections caused by carbapenem-resistant CRE.5 Another effective approach will be the discovery of reagents that can counteract the drug-resistant mechanisms and lower the dosage of aminoglycosides. Recent studies revealed that regulation of proton-motive force (PMF) to facilitate aminoglycoside uptake is an effective way to potentiate their antibacterial efficacy. It was first reported that specific metabolic stimuli including glucose, fructose, mannitol, or pyruvate can greatly potentiate aminoglycoside killing of both Gram-negative and Gram-positive persisters with multidrug resistance.6 Such potentiation is aminoglycoside-specific and relies on generation of PMF to facilitate aminoglycoside uptake. Later on, it was found that exogenous alanine and glucose can restore the susceptibility of multidrug-resistant bacteria to aminoglycosides by increasing PMF and stimulating aminoglycoside uptake.7 More recently, Deng et al8 reported that exogenous L-lysine sensitizes Gram-negative and Gram-positive bacterium to aminoglycosides and the combination of L-lysine with aminoglycosides killed clinically isolated multidrug-resistant Acinetobacter baumannii and persister cells via regulation of proton motive force and antibiotics uptake. Herbal drugs and phytochemicals also exhibited resistance modification when used in combination with antibiotics through different strategies such as inhibition of modifying and drug degrading enzymes or efflux pumps.9 However, their effect to enhance aminoglycosides uptake in CRE was not described. In the present study, by screening a 280 Chinese herbal compounds collection using a CRKp with induced gentamicin resistance as model, we identified plumbagin as a potent potentiator of gentamicin via stimulating PMF and antibiotics uptake.
Materials and Methods
Antibiotics, Compounds, and Reagents
Gentamicin and kanamycin (Solarbio, Beijing, China) were prepared in sterilized Milli-Q water (Millipore, Milford, MA, USA). Plumbagin (Yuanye, Shanghai, China) was dissolved in DMSO (Sigma Aldrich, St Louis, MO, USA). The stock solutions were freshly prepared, filtered, and used at indicated concentrations.
Bacterial Strains and Culture Conditions
The antibiotics-susceptible strain of Klebsiella pneumoniae CCTCC AB 2010163 was obtained from China Center for Type Culture Collection (CCTCC), Wuhan, China. The CRKp strain ATCC BAA-1705 was purchased from the American Type Culture Collection (ATCC), Manassas, USA. They were grown in Luria-Bertani (LB) broth containing 10 g/L tryptone (AOBOX, Beijing, China), 5 g/L yeast extract (AOBOX, Beijing, China), and 10 g/L NaCl (Solarbio, Beijing, China). To obtain gentamicin-resistant CRKp, the ATCC BAA-1705 strain was selected by sequential propagations in LB broth with gentamicin until MIC >16 μg/mL. The susceptibility to gentamicin and imipenem was evaluated by agar dilution method as described previously.10 The strain was stored at −80°C and incubated in LB broth in the shaker incubator at 37°C until OD600nm reaches the desired value for experimental use.
Screening of Herbal Compounds
A collection of 280 Chinese herbal compounds including 23 flavones, 30 alkaloids, eight quinones, 41 glycosides, 26 terpenoids, five carbohydrates, seven coumarins, and 140 other compounds (Yuanye, Shanghai, China) was screened against the gentamicin-resistant CRKp. Synergistic effect of the herbal compounds with gentamicin was determined by broth microdilution method in the 96-well microtiter plates referring to M07-A9 document of CLSI guidelines. The final concentrations of herbal compounds and gentamicin were 50 μM and 4 μg/mL, respectively. The 96-well microtiter plates were cultured in the incubator (Thermo Fisher Scientific, Waltham, USA) at 37°C for 24 hours and then OD600nm was measured by microplate reader (PerkinElmer, USA).
Synergistic Effect of Plumbagin with Gentamicin
The synergistic effect of plumbagin (0, 25, 50, and 100 μM) with gentamicin (2 μg/mL and 4 μg/mL for ATCC BAA-1705, 0.2 μg/mL and 0.4 μg/mL for CCTCC AB 2010163) was determined by broth microdilution method as described above. The growth dynamic of bacteria was measured by the following methods. Four groups; the control group, plumbagin (100 μM) monotherapy, gentamicin (16 μg/mL) monotherapy, and combination therapy, were established with the initial OD600nm of 0.2–0.3 and shaken at 37°C after treatment. OD600nm value was measured every 20 minutes for 6 hours.
Measurement of Intracellular Gentamicin C-Complex
The concentrations of intracellular gentamicin C-complex were analyzed by LC-MS/MS as described previously.11 The monotherapy group (gentamicin 16 μg/mL) and combination group (plumbagin 100 μM and gentamicin 16 μg/mL) were established with the initial OD600nm of 0.5–0.6 and shaken for 2 hours at 37°C. Samples were collected and washed three times with 0.9% NaCl (4°C) to remove medium components. Bacterial cells were normalized to OD600nm of 0.90±0.02 and centrifuged at 4000 g at 4°C for 15 minutes. The pellets were subject to freeze–thaw cycles and ultrasonication to release gentamicin. After centrifugation, 450 μL supernatant was collected and mixed with 50 μL kanamycin (10 μg/mL) as an internal standard. The gentamicin C-complex was extracted as described previously followed by a clean-up step by solid phase extraction (SPE) using Oasis HLB extraction cartridges (Waters, Milford, MA, USA).12 The elutes were dried by rotary evaporation and redissolved in 100 μL solution of 0.1% formic acid:acetonitrile (1:1, v/v).
The measurements of gentamicin C-complex were performed on an ultimate 3000 UHPLC system (Thermo Fisher, San Jose, CA, USA) coupled with a BEH amide column (100 mm x 2.1 mm, 1.7 μm, Waters, Milford, MA, USA) maintained at 40°C. The mobile phase consisted of 95% 2 mM aqueous ammonium acetate, 5% acetonitrile, and 0.2% formic acid (Solvent A) and 95% acetonitrile, 5% 2 mM aqueous ammonium acetate and 0.2% formic acid (Solvent B) and the flow rate of 0.3 mL/min. The gradient elution program is shown in Table 1 and the injection volume was 3 μL. Mass spectrometry detection was operated on a TSQ Vantage triple-stage quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source while the quantification of the gentamicin C-complex and internal standard was performed using a selective reaction monitor (SRM) in the positive ionization mode (Table 2). Spray voltage, sheath gas pressure, auxiliary gas pressure, vaporizer temperature, and capillary temperature were set as 3.5 kV, 45 arb, 25 arb, 450°C, and 350°C, respectively.
 |
Table 1 Gradient Elution Program of Mobile Phase |
 |
Table 2 The Analytical Conditions for the LC-MS/MS Analysis of Gentamicin C1, C1a, C2, and Kanamycin |
Measurement of Membrane Potential
Membrane potential of control and plumbagin-treated bacterial samples was measured using BacLightTM Bacterial Membrane Potential Kit (Life Technologies, USA) according to manufacturer’s instruction.
Preparation of Intracellular Metabolites Extracts
Intracellular metabolites of CRKp were extracted according to previously reported methods with modifications.13 Bacterial cell pellets were harvested and resuspended in 600 μL extraction solvent (methanol/water, 9:1, v/v, −80°C) containing 1,2–13C myristic acid. The samples were frozen in liquid nitrogen for three freeze–thaw cycles and disrupted using an ultrasonic cell crusher for 10 minutes to lyse cells and release cellular metabolites. After centrifugation at 8000 g for 10 minutes at 4°C, 450 μL of supernatant was collected for derivatization process and metabolomics analysis described previously.14 Aliquots of 50 μL from each sample were pooled and mixed to form the quality control (QC) sample. The QC samples were injected at intervals of six study samples in order to assess system suitability and system stability.
Data Processing for Metabolomics
Raw data files obtained from GC-MS were converted to ABF format using Abf Converter and then imported into MS-DIAL (v.2.7.2) software for peak detection, identification, and alignment. Detected peaks were matched with FiehnLib database according to RI and fragments,15 while the similarity of fragments above 80% were confirmed using National Institute of Standards and Technology (NIST). The alignment results of peak height intensity were normalized by sum and then exported into MetaboAnalyst 4.0 (http://www.metaboanalyst.ca/) for multivariate analysis, such as principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), and heatmap analysis. Moreover, statistical analysis was also performed using Mann–Whitney test for identifying biomarker candidates.
Measurement of NADH
Bacteria culture was diluted to OD600nm of 0.5–0.6 and incubated with plumbagin (100 μM) at 37°C for 2 hours. Then cells were washed three times with 0.9% NaCl by centrifugation at 4000 g for 15 minutes at 4°C. Bacterial cells were normalized to OD600nm of 0.90±0.02 and centrifuged at 4000 g at 4°C for 15 minutes. Cellular NADH concentrations were detected via EnzyChrom NAD/NADH assay kit (BioAssay Systems) according to manufacturer’s instruction.
Statistical Analysis
FlowJo V10 was used for processing flow cytometric data. GraphPad Prism 7.0 software was used for statistical analysis. Student’s t-test was performed for determination of differences between groups and statistical significance was considered at P<0.05.
Results
Plumbagin Potentiates Antibacterial Activity of Gentamicin Against CRKp
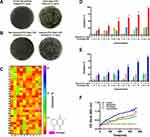
ATCC BAA-1705 is resistant to imipenem (Figure 1A, right), but susceptible to gentamicin (Figure 1B, left). To obtain gentamicin-resistant CRKp, we established a gentamicin-resistant CRKp strain by incremental induction of ATCC BAA-1705 until it could resist >16 μg/mL gentamicin (Figure 1B, right). By screening a 280 Chinese herbal compounds collection, a naphthoquinone compound plumbagin was identified to remarkably potentiate the antibacterial activity of gentamicin against the gentamicin-resistant CRKp (Figure 1C). Plumbagin at 100 μM could bring the MIC of gentamicin from >16 μg/mL to ~4 μg/mL despite its minimal inhibitory effect on CRKp in monotherapy (Figure 1D). A similar potentiating effect was observed in antibiotics-susceptible strain of Klebsiella pneumoniae CCTCC AB 201016 (Figure 1E). Figure 1F shows the time course of anti-CRKp activities by monotherapy or a combination of plumbagin and gentamicin.
Plumbagin Enhances PMF and Promotes Gentamicin Uptake by CRKp
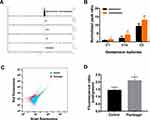
A LC-MS/MS based analysis was employed to access the effect of plumbagin on gentamicin uptake by the CRKp. The elution times of gentamicin C1, C1a, C2, and kanamycin were at 5.58, 5.71, 5.66, and 5.71 minutes, respectively (Figure 2A). Quantification of intracellular gentamicin C-complex C1, C1a, and C2 indicated more uptake of all three forms by CRKp treated by combination therapy (Figure 2B). To determine if the increased gentamicin uptake is related to PMF, we measured the membrane potential in plumbagin-treated CRKp using a BacLightTM Bacterial Membrane Potential Kit. The BacLightTM Bacterial Membrane Potential Kit provides solution of the carbocyanine DiOC2(3). DiOC2(3) exhibits green fluorescence in all bacterial cells, but the fluorescence shifts toward red emission as the dye molecules self-associate at the higher cytosolic concentrations caused by larger membrane potentials. The red-versus-green fluorescence dot plot showed CRKp incubated in the presence or absence of plumbagin (Figure 2C), which was quantified in Figure 2D. Plumbagin induced a significant increase of membrane potential in CRKp.
Plumbagin Increased TCA Efflux and NADH Production
To further characterize the mechanism of plumbagin-induced PMF increase in CRKp, we utilized a GC-MS based metabolomics approach to determine the changes of TCA efflux. The typical ion chromatogram (TIC) of intracellular metabolites is shown in Figure 3A. Principle component analysis (PCA) score plots and heatmap (Figure 3B and C) revealed global metabolic changes in CRKp after plumbagin treatment. According to Mann–Whitney test, significant metabolites were selected based on P<0.05 and FC≧1.2. We observed that the abundance of malate and α-ketoglutarate in the TCA cycle were increased significantly upon plumbagin treatment (Figure 3D and E). Intracellular NADH concentration in plumbagin-treated CRKp was also significantly increased higher than untreated control (Figure 3F).
Discussion
The rapid worldwide spread of carbapenem-resistant Enterobacteriaceae (CRE), such as CRKp, has become a substantial public health problem with few therapeutic options remaining.16 Although aminoglycosides (mostly gentamicin) are historically susceptible antibiotics for CRE, they are not commonly used as monotherapy for severe infections due to the lower clinical efficacy, potential toxicity, and extensive drug resistance.4 Synergistic interaction of natural products, such as herbal drugs and phytochemicals, with antibiotics is an effective tool to counter multidrug resistance and to lower the dosages.9 In the present study, we first established a gentamicin-resistant strain of CRKp by incremental induction of the gentamicin-susceptible strain ATCC BAA-1705. Using this strain, we screened a collection of 280 Chinese herbal compounds co-administered with gentamicin and identified a bicyclic naphthoquinone compound plumbagin [5-hydroxy-2-methyl-1,4-naphthoquinone] that effectively reversed its resistance to gentamicin. Plumbagin was recently reported to have relatively potent antibacterial activity (MIC range of 4–8 μg/mL or 21.26 μM-42.52 μM) against methicillin-resistant Staphylococcus aureus (MRSA), which was not impacted by multi-drug resistance.17 At much higher concentration (>30 μg/mL) plumbagin can potentiate the antibiofilm effect of gentamicin against Pseudomonas aeruginosa although its direct antibacterial activity is very low (MIC~400 μg/mL).18
We found that at 100 μM concentration that has a minimal inhibitory effect on the gentamicin-resistant CRKp, plumbagin could bring the MIC of gentamicin from >16 μg/mL to ~4 μg/mL. Because plumbagin also exhibited a remarkable synergistic effect with gentamicin in an antibiotics-susceptible standard strain of Klebsiella pneumoniae CCTCC AB 201016, its potentiating activity is unlikely due to interference of particular drug resistance mechanisms such as enzymatic degradation of drugs, inactivation of drug targets, and drug efflux pump-induced multidrug resistance that only exists in CRKp. Aminoglycosides uptake driven by the bacterial PMF is a mechanism affecting their bactericidal activity in both drug-resistant and drug-susceptible bacteria.8,19 To test the hypothesis that plumbagin regulates PMF and gentamicin uptake, we analyzed the intracellular concentrations of gentamicin isoforms by LC-MS/MS. Interestingly, we found that plumbagin indeed significantly promoted the uptake of gentamicin C-complex C1, C1a, and C2 by the CRKp. Consistently, plumbagin also induced a significant increase of PMF as indicated by higher membrane potential in the CRKp. Therefore, we identified a novel mechanism of plumbagin to potentiate the antibacterial activity of gentamicin against Klebsiella pneumoniae.
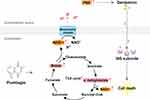
The electrical potential across bacterial membrane (ΔΨ) driven by TCA cycle control is responsible for generation of PMF by promoting NADH production, which facilitates the uptake of aminoglycosides.19 To further determine whether plumbagin induced an increase of the PMF is associated with TCA cycle, we performed metabolomics analysis of the plumbagin-treated CRKp and found that the abundance of malate and α-ketoglutarate in the TCA cycle increased significantly. Intracellular NADH concentration in plumbagin-treated CRKp was also significantly higher than in untreated control CRKp, suggesting increased NADH production from malate and α-ketoglutarate in the TCA cycle. Based on these results, a proposed model for a possible mechanism of the plumbagin synergy with gentamicin against CRKp is summarized in Figure 4. However, we cannot rule out other possible mechanisms of the synergistic anti-CRKp effect because the plumbagin-induced increase of gentamicin uptake is not dramatic. Future studies are warranted to figure out new mechanisms and to determine if plumbagin has synergistic effects with aminoglycosides on other multidrug resistant gram-negative and gram-positive bacterial pathogens via similar mechanisms.
Plumbagin is a plant naphthoquinone compound with various important pharmaceutical activities including anticancer, antibacterial, antifungal, neuroprotective, and hypolipidemic activities.20 It possesses high therapeutic efficacy and minimal side-effects and therefore shows potential for treatments of various diseases.21 Due to the increasing worldwide commercial demand, biotechnological approaches for large scale production of plumbagin and its controlled drug release or drug delivery systems are under development.22,23 Therefore, we have reason to expect that plumbagin has great potential in development of combination therapy with aminoglycosides antibiotics for severe infections caused by CRKp.
Acknowledgments
We thank Dr. Jinjun Shan of the Medical Metabolomics Center of Nanjing University of Chinese Medicine for technical assistance. This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Integration of Chinese and Western Medicine). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Zhang Y, Jin L, Ouyang P, et al. Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J Antimicrob Chemother. 2020;75(2):327–336. doi:10.1093/jac/dkz446
2. Guo X, Cao Z, Dai Z, et al. Antimicrobial susceptibility and molecular epidemiology of multidrug-resistant klebsiella pneumoniae in central China. Jpn J Infect Dis. 2017;70(3):229–234. doi:10.7883/yoken.JJID.2016.049
3. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
4. Zavascki AP, Klee BO, Bulitta JB. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther. 2017;15(6):519–526. doi:10.1080/14787210.2017.1316193
5. McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med. 2019;380(8):791–793. doi:10.1056/NEJMc1807634
6. Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi:10.1038/nature10069
7. Peng B, Su YB, Li H, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015;21(2):249–262. doi:10.1016/j.cmet.2015.01.008
8. Deng W, Fu T, Zhang Z, et al. L-lysine potentiates aminoglycosides against via regulation of proton motive force and antibiotics uptake. Emerg Microbes Infect. 2020;9(1):639–650. doi:10.1080/22221751.2020.1740611
9. Ayaz M, Ullah F, Sadiq A, et al. Synergistic interactions of phytochemicals with antimicrobial agents: potential strategy to counteract drug resistance. Chem Biol Interact. 2019;308:294–303. doi:10.1016/j.cbi.2019.05.050
10. Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi:10.1038/nprot.2007.521
11. Díez C, Guillarme D, Staub Spörri A, et al. Aminoglycoside analysis in food of animal origin with a zwitterionic stationary phase and liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2015;882:127–139. doi:10.1016/j.aca.2015.03.050
12. Yao L, Zhou F, Cai M, et al. Development and validation of a sensitive LC-MS/MS method without derivatization/ion-pairing agents for etimicin quantification in rat plasma, internal ear and kidney. J Pharm Biomed Anal. 2017;146:96–102.
13. Maifiah MH, Creek DJ, Nation RL, et al. Untargeted metabolomics analysis reveals key pathways responsible for the synergistic killing of colistin and doripenem combination against Acinetobacter baumannii. Sci Rep. 2017;7:45527. doi:10.1038/srep45527
14. Qian W, Kang A, Peng L, et al. Gas chromatography-mass spectrometry based plasma metabolomics of H1N1-induced inflammation in mice and intervention with Flos Lonicerae Japonica-Fructus Forsythiae herb pair. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1092:122–130. doi:10.1016/j.jchromb.2018.05.047
15. Kind T, Wohlgemuth G, Lee DY, et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81(24):10038–10048. doi:10.1021/ac9019522
16. Sheu CC, Chang YT, Lin SY, et al. Infections caused by carbapenem-resistant: an update on therapeutic options. Front Microbiol. 2019;10:80. doi:10.3389/fmicb.2019.00080
17. Periasamy H, Iswarya S, Pavithra N, et al. In vitro antibacterial activity of plumbagin isolated from Plumbago zeylanica L. against methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol. 2019;69(1):41–49. doi:10.1111/lam.13160
18. Gupta P, Sarkar A, Sandhu P, et al. Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: a study with plumbagin and gentamicin. J Appl Microbiol. 2017;123(1):246–261. doi:10.1111/jam.13476
19. Taber HW, Mueller JP, Miller PF, et al. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987;51(4):439–457. doi:10.1128/MMBR.51.4.439-457.1987
20. Padhye S, Dandawate P, Yusufi M, et al. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012;32(6):1131–1158. doi:10.1002/med.20235
21. Sumsakul W, Plengsuriyakarn T, Na-Bangchang K. Pharmacokinetics, toxicity, and cytochrome P450 modulatory activity of plumbagin. BMC Pharmacol Toxicol. 2016;17(1):50. doi:10.1186/s40360-016-0094-5
22. Roy A, Bharadvaja N. Biotechnological approaches for the production of pharmaceutically important compound: plumbagin. Curr Pharm Biotechnol. 2018;19(5):372–381. doi:10.2174/1389201019666180629143842
23. Rajalakshmi S, Vyawahare N, Pawar A, et al. Current development in novel drug delivery systems of bioactive molecule plumbagin. Artif Cells Nanomed Biotechnol. 2018;46(sup1):209–218. doi:10.1080/21691401.2017.1417865
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.