Back to Journals » Infection and Drug Resistance » Volume 13
Synergistic and Antagonistic Effects of Metal Nanoparticles in Combination with Antibiotics Against Some Reference Strains of Pathogenic Microorganisms
Authors Abo-Shama UH , El-Gendy H, Mousa WS, Hamouda RA, Yousuf WE, Hetta HF , Abdeen EE
Received 12 October 2019
Accepted for publication 23 January 2020
Published 7 February 2020 Volume 2020:13 Pages 351—362
DOI https://doi.org/10.2147/IDR.S234425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Usama H Abo-Shama,1 Hanem El-Gendy,2 Walid S Mousa,3 Ragaa A Hamouda,4,5 Wesam E Yousuf,4 Helal F Hetta,6,7 Eman E Abdeen8
1Department of Microbiology and Immunology, Faculty of Veterinary Medicine, Sohag University, Sohag 82524, Egypt; 2Department of Pharmacology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt; 3Department of Animal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt; 4Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Sadat City, Egypt; 5Department of Biology, Faculty of Sciences and Arts Khulais, University of Jeddah, Jeddah, Kingdom of Saudi Arabia; 6Department of Medical Microbiology & Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt; 7Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, USA; 8Department of Bacteriology, Mycology and Immunology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt
Correspondence: Usama H Abo-Shama Email [email protected]; [email protected]
Background and Aim: Nanosized inorganic antibacterial materials have received increasing attention in recent years. The present study aimed to determine the antimicrobial activity of silver (Ag) and zinc oxide (ZnO) nanoparticles alone and in combination with antibiotics against reference strains of pathogenic microorganisms as Staphylococcus aureus (Staph. aureus), Salmonella enterica subsp. Bukuru, Escherichia coli (E.coli) and Candida albicans ( C. albicans).
Methods: The antimicrobial effect of metal-nanoparticles (AgNPs and ZnONPS) and in combination with antibiotics was studied using the normal disc-diffusion method.
Results: Both AgNPs and ZnONPs had increased antibacterial activity with an increase in their concentration against Gram-positive bacterium (Staph. aureus), Gram-negative bacteria (E. coli and Salmonella spp) and no effect on C. albicans. The synergistic effect of antibiotics (azithromycin, cefotaxime, cefuroxime, fosfomycin and chloramphenicol) against E. coli was significantly increased in the presence of AgNPs compared to antibiotic only. However, all antibiotics had a synergistic effect in the presence of AgNps against Salmonella spp. On the other hand, the antibacterial action of AgNPs with oxacillin and neomycin antibiotics against Staph. aureus was significantly decreased in comparison with antibiotics only. The synergistic effect of antibiotics (azithromycin, oxacillin, cefotaxime, cefuroxime, fosfomycin and oxytetracycline) against E. coli was significantly increased in presence of ZnONPs compared to antibiotic only and also the synergistic effect of antibiotics (azithromycin, cefotaxime, cefuroxime, fosfomycin, chloramphenicol and oxytetracycline) against Staph. aureus was significantly increased in the presence of ZnONPs compared to antibiotics only. On the other hand, most antibiotics had an antagonistic effect in presence of ZnONps against Salmonella spp.
Conclusion: AgNPs and ZnONPs demonstrate a good synergistic effect with antibiotics and this may open the door for a future combination therapy against pathogenic bacteria.
Keywords: AgNPs, ZnONPs, antimicrobial activity E. coli Staph. aureus, Salmonella spp, Candida albicans
Introduction
Nano drug delivery system includes design, synthesis, production, characterization, application of structures, devices and systems of nanoparticles with size ranging from 1 to 100 nm.1–4 Wide use of antimicrobial agents contributes to the improvement and rapid spread of bacterial resistance which was identified in commensals bacteria as Escherichia coli (E. coli), zoonotic enteropathogens (eg Salmonella spp.) as well as animal pathogens (eg Pasteurella multocida or Actinobacillus spp.).5–7
Recently, mutli-drug-resistant pathogenic bacterial strains appear where most of the available antibiotics are not effective against these pathogens.8–10 In both human and veterinary medicine, bacterial resistance involves a decrease in antibiotic efficiency which may lead to further health issues.8,11–14 This problem encourages researchers to study the new advanced techniques for characterizing antimicrobial agents which can effectively avoid bacterial growth.8,11 Due to the increasing bacterial resistance to standard antibiotics, the studies on the antibacterial activity of nanoparticles have improved.15,16
Silver nanoparticles (AgNPs) and zinc oxide nanoparticles (ZnONPs) are known to affect bacterial membranes.17 AgNPs are effective as antimicrobial agents at low concentrations (mg/L) and are not cytotoxic to eukaryotic cells, including human erythrocytes.18 AgNPs have proven to be active as antimicrobial agents at a very low concentration and they may prevent the growth of antibiotic-resistant bacteria. AgNPs interact with membrane proteins and bacterial DNA which contain phosphorous and sulphur complex that have a high attraction to AgNPs.19 AgNPs demonstrate potent bactericidal and antibacterial properties not only against Gram-positive and Gram-negative bacteria but also against methicillin-resistant strains.20
Challenges and needs have controlled to a reappearance in the use of silver nanoparticles as antiseptics that may be related to their broad-spectrum activity and far lower tendency to encourage microbial resistance than antibiotics.21
The interactions of antibiotics with silver nanoparticles are the most common among studies committed to the challenging of the mutual action of nanoparticles with antibiotics and studying the effectiveness of antimicrobial agents can be developed by the combination between them with nanoparticles against various pathogens, including Staphylococcus aureus (Staph. aureus) and E. coli.22,23 Some metal nanoparticles have been appreciated for increasing the antibacterial activities of different antibiotics such as zinc oxide nanoparticles which have therapeutic roles in different diseases have been recognized in recent years.24
The considerable antimicrobial properties of inorganic metal nanoparticles, such as ZnO, along with their selective toxicity to biological systems encourage their potential use as diagnostics, therapeutics, surgical devices and antimicrobial methods.25,26 Additionally, Zinc oxide nanoparticles yield better results when used in combination with beta-lactams, cephalosporins and aminoglycosides against different pathogenic microorganisms.27,28
Furthermore, ZnONPs demonstrate antibacterial activity and may reduce the attachment of microbes on biomedical surfaces.29,30 Several mechanisms have been described for the antibacterial activity of ZnONPs, for example, ZnONPs can interact with membrane lipids and alter the membrane structure, which may lead to damage to the membrane integrity, malfunction, and may cause bacterial death.31 ZnONPs can also inhibit bacterial growth through the entrance into bacterial cells at a nanoscale with subsequent production of toxic oxygen radicals, which damage the DNA structure and cell membranes.32,33
Synergism is associated with the generation of hydroxyl radicals, alteration of protective cellular functions and an anti-biofilm potential. The combination of antibiotics with nanoparticles is more effective for enhancing antibiotic efficacy in comparison with the action of antibiotics when used in clinical practice. The combination involves reduced development of bacterial resistance, reduce the duration of treatment and reduce antibiotic dose requirements.23 The objective of this study was to detect the antimicrobial activity of silver (Ag) and zinc oxide (ZnO) nanoparticles alone and in combination with antibiotics against gram-positive and gram-negative bacteria.
Materials and Methods
Test Organisms
Reference strains of pathogenic microorganisms as Staph. aureus (MF359584) Salmonella enterica subsp. Bukuru (KY315943) E. coli (KY797673) and C. albicans (KU852509) were obtained from Bacteriology, Mycology and Immunology department, Faculty of Veterinary Medicine, University of Sadat City.
Preparation of the Test Organisms
A loopful of each test organism was taken from the stock culture of these organisms then streaked on slant nutrient agar. The obtained bacterial culture was emulsified in sterile saline and the microbial suspension was adjusted to 105–106 CFU/mL by using MacFarland standards.34
Antibiotics
For observing the combination effect of metal-nanoparticles (AgNPs and ZnONPS) with antibiotics; the antibiotics applied in our study were chosen to cover nearly all the different antibiotic classes appropriate for these microorganisms (Staph. aureus, E. coli, Salmonella spp. and C. albicans). The antibiotics used with their antimicrobial subclasses along with the discs concentration are tabulated in Table 1.
 |
Table 1 The Antibiotics Used with Their Antimicrobial Subclasses Along with the Discs Concentration Against E. coli, Staph. aureus, Salmonella spp and C. albicans |
Biological Synthesis of Metal Nanoparticles
Ulva fasciata alga was collected from shallow water beside the shore of Abu-qir coast, Alexandria, Egypt and was identified as previously described.35,36 The aqueous extracts were prepared by the addition of one gm of dry powder Ulva fasciata to 100 mL DD water boiled for 1 hr then filtrated to obtain an algal aqueous extract.
Biosynthesis of Silver Nanoparticles (AgNPs)
Ten mL of previous prepared Ulva fasciata aqueous extract was added slowly to 90 mL of freshly prepared 0.1 mm of AgNO3 with stirring and heating at 40°C for 30 mins until the color change to brown.37
Biosynthesis of Zinc Oxide Nanoparticles (ZnO-NPs)
0.02M aqueous Zinc acetate dehydrates was added to 40 mL distilled water in constant stirring. Then, 10 mL was added of algal aqueous extract after 10 min, stirring then 2.0M NaOH was added, stirring for 2hrs. The pale white precipitate was filtered and washed two times with purified water then by ethanol. Then, pale white powder of zinc oxide nanoparticles was obtained after drying at 60° C in a vacuum oven overnight.37,38
Characterization of the Biologically Synthesized Nanoparticles
UV–Visible Spectroscopy Analysis
The metal ion reduction was examined by measuring UV Spectrum of Ag-NPs and ZnO-NPs treated supernatant periodically. The aliquots of this solution were monitored for UV spectra. The UV–Vis spectroscopy measurements were recorded at Genetic Engineering and Biotechnology Research Institute (GEBRI), Egypt from 300 to 600 nm. The Ag-NPs and ZnO-NPs dispersed in deionized water were observed for their surface plasmon resonance at 420 and 280 nm, respectively.39
Transmission Electron Microscopy
Description of the dimension, figure and the nanoparticles formal of the association was observed via consuming Transmission Electron Microscopy (TEM) examination (JEOL JEM-2100) at the National Research Center (NRC), Egypt. Samples for TEM investigation remained organized by assigning two droplets of nanoparticle solutions on carbon-coated TEM grids.
Scanning Electron Microscopy
External morphology, scope and distribution of nanoparticles in the solution remained examined at NRC using JEOL JSM-6100 Scanning Electron Microscopy with EDAX recognized scheme worked at a practical prospective of 15 kV and existed adjusted previous to investigate.
X-Ray Diffraction Pattern Analysis
The biosynthesized Ag-NPs and ZnO-NPs were freeze-dehydrated and crushed so as to examine XRD design.40 The timely creation and pureness of metal nanoparticles existed, checked through XRD designs which were noted expending precipitate X-ray diffractometer.
Assessment of Antibacterial Activities of Ag-NPs and ZnO-NPs Alone and with Their Combination with the Selected Antibiotics
The antibacterial activities of the synthesized (Ag-NPs) and zinc oxide (ZnO-NPs) alone and with their combination with the selected antibiotics were determined against the tested isolates using the disc-diffusion method.41 One milliliter of the prepared microbial suspensions was added to 100 mL of nutrient agar medium which adjusted at PH 7.0 after sterilization and before solidification.42,43 The media were shacked well till the complete mixing of the test organisms with the nutrient agar. Then, 25 mL of the prepared medium was poured into each petri-dish (20×120 mm) using a sterile cylinder (25 mL capacity). The plates were completely solidified through left on a horizontal surface at room temperature. To determine the effect of antibiotic discs only, the antibacterial effect of silver and zinc oxide nanoparticles only with concentrations (170, 85, 42.5, 21.25, 10.625, 5.31, 2.65, 1.33 and 0.66 µg/mL) and (10, 5, 2.5, 1.25 and 0.6 mg), respectively. To study the combined effects of antibiotics with nanoparticles, each standard paper disc was further impregnated with AgNPs with concentrations 170, 85, 42.5 µg/mL and also each standard paper disc was further impregnated with ZnO nanoparticles with a concentration of 10 mg.44,45 These media were put in incubation and the interactions between nanoparticles and antibiotics were measured by measuring inhibition zones.
Statistical Analysis
Antimicrobial experiments were conducted in triplicate. Data points were expressed as the mean ± standard deviation. Data were analyzed using analysis of variance from SPSS version 16 software by using one-way ANOVA (Independent t-test). A p < 0.05 is used for significant responses.
Results
Biosynthesis of Nanoparticles
Two totally different metals nanoparticles inclusive Ag-NPs and ZnO-NPs in our study were biologically synthesized and analyzed by many techniques to insure the completion of the nano formation and detection of their characteristics.
Characterization of Nanoparticles
Optical examination
The synthesis of Ag-NPs determined by optical examination of the color of the biogenesis silver nanoparticles which turned to chromatic brown. The pale white precipitate was appeared in biogenesis ZnO-NPs by victimisation genus Ulva fasicata.
UV–Visible Spectroscopy
The formation and stability of the reduced metal nanoparticles within the colloidal suspension were monitored by victimization UV absorption spectra. A powerful and broad peak was discovered between 420 and 430 nm confirming the formation of Ag-NPs. An absorption peak discovered at 280 nm as shown in Figure 1 indicates the made biogenesis of ZnO-NPs.
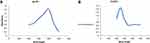 |
Figure 1 UV-Vis absorption spectra of metal nanoparticles (A) Ag-NPs (B) ZnO-NPs. |
Transmission Electron Microscopy
The morphology and therefore the average size of the synthesized nanoparticles were analyzed by transmission electron microscopy. The particles of Ag-NPs and ZnO-NPs biosynthesized are well separated single spherical particles in Ag-NPs, whereas rod-shape in ZnO-NPs without aggregation (Figure 2). The presence of the particles while not aggregation clearly insured the potency of the techniques utilized in the synthesizing processes. The common size of the particles was calculated and located to be 15 ± 0.55 and 187 ± 0.5 nm for Ag-NPs and ZnO-NPs, respectively (Figure 2).
 |
Figure 2 Transmission electron microscopy of metal nanoparticles (A) Ag-NPs (B) ZnO-NPs. |
Scanning Electron Microscopy
The results obtained by scanning electron microscopy showed that the silver nanoparticles have a spherical shape, low-density dispersion, while the morphology of ZnONPs synthesis by the biological method was flake shape with a narrow size distribution (Figure 3).
 |
Figure 3 Scanning electron microscopy of metal nanoparticles (A) Ag-NPs (B) ZnO-NPs. |
X-Ray Diffraction Pattern Analysis
The XRD pattern of nitrate treated sample shows 5 intense peaks in the whole spectrum 2 hrs starting from 10 to 60. The characteristic XRD peaks were focused at 28°, 38° and 45° that can be made by the subsequent crystalline planes of silver (111), (200) and (220), respectively. XRD analysis showed the diffraction peaks at that the indexed planes were concerning 111, 200 and 220 of the cubic face-centered silver. Whereas in ZnO-NPs the strong and narrow diffraction peaks indicate that the merchandise has a well crystalline structure. The XRD peaks at 32°, 35°, 38°, 48°, and 58° were known as (100), (110), (111), (200) and (310) reflections, respectively.
Antimicrobial Activity of Silver Nanoparticles
Our results showed that AgNPs had antimicrobial activity against Gram-positive bacterium (Staph. aureus), Gram-negative bacteria (E. coli and Salmonella spp) and no effect on C. albicans as illustrated in Table 2.
 |
Table 2 Antimicrobial Activity of AgNPs Against E. coli, Staphylococcus aureus, Salmonella spp and C. albicans |
The Efficacy of AgNPs on Enhancing the Activity of Different Antibiotics
According to Table 3, it was demonstrated that AgNPs act synergistically with all tested antibiotics against Salmonella spp. The antibacterial action of AgNPs with antibiotics (azithromycin, cefotaxime, cefuroxime, fosfomycin, and chloramphenicol) against E. coli had a significant synergistic effect compared to antibiotic only, while the antibacterial action of AgNPs with gentamicin was significantly decreased in comparison with gentamicin only. Referring to oxacillin, neomycin, ampicillin/sulbactam, and oxytetracycline were none significantly effect when compared to AgNPs with this antibiotic.
 |
Table 3 Synergistic and Antagonistic Effect of AgNPs in Combination with Various Antibiotics Against E. coli, Staph. aureus, Salmonella spp |
For Staph. aureus, the antibacterial action of AgNPs with antibiotics (azithromycin, cefotaxime, ampicillin/sulbactam, cefuroxime, fosfomycin, chloramphenicol, and oxytetracycline) were having a significant synergistic effect compared to antibiotic only, in contrary, the antibacterial action of AgNPs with oxacillin and neomycin antibiotics was significantly decreased on comparison with antibiotics only, while for gentamicin, the antibacterial effect was non significantly when compared to AgNPs with gentamicin.
Antimicrobial Activity of Zinc Oxide Nanoparticles
ZnONPs had antibacterial activity against gram-positive bacterium (Staphylococcus aureus), gram-negative bacteria (E. coli and Salmonella spp) and no effect on C. albicans as that illustrated in Table 4.
 |
Table 4 Antimicrobial Activity of ZnONPs Against E. coli, Staph. aureus, Salmonella spp and C. albicans |
The Efficacy of ZnONPs on Enhancing the Activity of Different Antibiotics
Our results show that the antibacterial action of ZnONPs with antibiotics (azithromycin, oxacillin, cefotaxime, cefuroxime, fosfomycin, and oxytetracycline) against E. coli was significantly increased compared to antibiotic only while the antibacterial action of ZnONPs with ampicillin/sulbactam antibiotic against E. coli was significantly decreased in comparison with antibiotic only.
The antibacterial action of ZnONPs with antibiotics (azithromycin, cefotaxime, cefuroxime, fosfomycin, chloramphenicol, and oxytetracycline) against Staph. aureus was significantly increased compared to antibiotics only. Furthermore, the antibacterial action of ZnONPs with oxacillin, neomycin, and ampicillin/sulbactam antibiotics against Staph. aureus was significantly decreased in comparison with antibiotics only.
For Salmonella, the antibacterial action of ZnONPs with oxacillin, cefuroxime and fosfomycin against was significantly increased when compared to antibiotics only. Contrarily, the antibacterial action of ZnONPs with antibiotics (azithromycin, gentamicin, cefotaxime, neomycin, ampicillin/sulbactam, chloramphenicol, and oxytetracycline) was significantly decreased in comparison with antibiotics only as shown in Table 5.
 |
Table 5 Synergistic and Antagonistic Effect of ZnONPs in Combination with Various Antibiotics Against E. coli, Staph. aureus, Salmonella spp |
Discussion
The occurrence of antibiotic-resistant microorganisms has led to serious health problems globally. With the development of silver and zinc oxide nanoparticles show a promising and far-ranging chance for the biomedical field, especially for antibacterial, anticancer drug/gene delivery, cell imaging, biosensing.46 Although the combination of antibiotics with nanoparticle metals has been shown to increase some antibiotics efficacy against some pathogens, its influence on the multidrug-resistant against Gram-positive bacterium (Staph. aureus), Gram-negative bacteria (E. coli and Salmonella spp) the results are still controversial. So that we aimed to detect the antimicrobial activity of silver (Ag) and zinc oxide (ZnO) nanoparticles alone and in combination with antibiotics against gram-positive and gram-negative bacteria.
In our study, we observed that the antimicrobial effect of AgNPs against Salmonella spp., Staph. aureus, and E. coli. was dose-dependent which came in concordance with the previous work done by Sondi and Salopeck-Sondi.47
The antibacterial effect of AgNPs could be explained by the fact that AgNPs can adhere easily to the bacterial cell surface due to the electrostatic attraction between the positive surface charge of the AgNPs and the negatively charged cell membrane of microorganisms and also due to the interaction between Ag+ ions and the sulfur contain proteins in the bacterial cell wall leading to disruption of cell wall.48,49
The antimicrobial potential of AgNPs depends on the thickness and structure of bacterial cell walls.48 AgNPs exerts a higher antimicrobial activity against Gram-negative bacteria regardless of their resistance level as compared to Gram-positive bacteria.48 This could be explained based on the difference in cell wall structure between Gram-positive and Gram-negative bacteria. The cell wall in Gram-positive bacteria such as Staphylococcus is mainly composed of a thick layer of peptidoglycan while in Gram-negative bacteria such as E. coli and Salmonella spp, it consists mainly of lipopolysaccharide, followed by a thin layer of peptidoglycan.49 The extremely small size of metallic nanoparticles allows them to have a large surface area in contact with the bacterial surface leading to a high chance of bacterial elimination.50
Recently, many researchers investigated the antimicrobial action of silver and its compounds and it had been reported that silver is non-toxic to human cells in low concentrations.51–53 The bactericidal effect of silver nanoparticles may be due to the catalytic oxidation by metallic silver and reaction with dissolved monovalent silver ion. Microbial resistance to sliver is less likely to develop, compared to conventional and narrow-target antibiotics, because the metal attacks many targets in the organism simultaneously so the organism must develop several mutations to be resistant.54
Our study clearly shows that the synergistic effect of antibiotics (azithromycin, cefotaxime, cefuroxime, fosfomycin and chloramphenicol) against E. coli was significantly increased in the presence of AgNPs compared to antibiotic only. However, all antibiotics had a synergistic effect in the presence of AgNps against Salmonella spp. On the other hand, the antibacterial action of AgNPs with oxacillin and neomycin antibiotics against Staph. aureus was significantly decreased in comparison with antibiotics only. The exact mechanism of the action of AgNPs is still unclear; however, several theories have been proposed. One theory suggests that AgNPs react with oxygen so disrupt the cellular respiratory chain. Also, AgNPs interact with cell membrane leading to cell death. Another theory proposed that AgNPs could exert antibacterial action through inhibition of unwinding of DNA.55 Moreover, the antibacterial activity of Ag-NPs may be due to the oxidative damage caused by reactive oxygen species (ROS) which might be accountable for the antibacterial activity of AgNPs.56
McShan et al showed tetracycline-AgNPs and neomycin-AgNPs both acted synergistically to inhibit Salmonella typhimurium growth with half-maximal inhibitory concentrations (IC50) of 0.07 μg/mL and 0.43 μg/mL, respectively.57
In our study, we found that ZnONPs had antibacterial activity against gram-positive bacterium (Staph. aureus) and Gram-negative bacteria (E. coli and Salmonella spp). Reddy et al (2007)58 have reported the same results emphasizing on the higher susceptibility of Gram-positive bacteria compared to Gram-negative bacteria. Previously, several researchers found that nano-sized ZnO displays varying morphologies that show significant antibacterial activity over a wide spectrum of bacterial species.59 Concentration, size, and stability are major factors affecting the antimicrobial properties of ZnO NP.60,61 Moreover, ZnO nanoparticles are characterized by chemical and physical stability, effective antibacterial activity, intensive ultraviolet, and infrared adsorption and a wide range of applications.62
The exact mechanism of the antibacterial activity of ZnO-NPs is still unclear. However, previous studies demonstrated that their bactericidal and bacteriostatic actions may be due to the generation of ROS as hydrogen peroxide (H2O2) which causes damage to the cell wall and cell membrane and facilitate the entrance of ZnO-NPs due to loss of proton stimulus force.63–66 Another mechanism of the antibacterial activity of ZnO-NPs may be due to the binding of ZnO-NPs on the bacterial surface due to the electrostatic forces.67
Our results revealed that the antibiotic activity was significantly increased when combined with ZnO nanoparticles except for Salmonella. It was reported that Imipenem had elevated antibacterial activity against K. pneumoniae when combined with ZnO NPs.62,68 In our study, E. coli developed resistance against ZnONPs and its combined effect with antibiotics and this came in concordance with Joshi et al69 who reported that E. coli developed resistance against Ag-NPs and the reason for that bacteria protected itself through the overproduction of extracellular polymeric substances called colanic acid.70 Additionally, our results revealed that the antioxidant activity of nanoparticles is dose-dependent. Azizi et al71 reported the same result where the scavenging of Diphenyl-2-picrylhydrazyl radicals was dose-dependent and found to be increasing as the concentration of ZnONPs increased.72
Conclusion
The current study showed that AgNPs with tested antibiotics give a good synergistic effect than ZnONPs with the same antibiotics. Moreover, AgNPs and ZnONPs compounds are safe and non-toxic and may be considered for future combination therapies against pathogenic bacteria due to its potential synergistic effect with important antibiotics.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Brosset E. The law of the European U nion on nanotechnologies: comments on a paradox. Rev Eur Comp Int Environ Law. 2013;22(2):155–162. doi:10.1111/reel.12030
2. Madhuri S, Maheshwar S, Sunil P, Oza G. Nanotechnology: Concepts and Applications. USA: CRC Press; 2012.
3. Abd Ellah NH, Tawfeek HM, John J, Hetta HF. Nanomedicine as a future therapeutic approach for Hepatitis C virus. Nanomedicine. 2019;14(11):1471–1491. doi:10.2217/nnm-2018-0348
4. Abd Ellah NH, Ahmed EA, Abd-Ellatief RB, Ali MF, Zahran AM, Hetta HF. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: association with regulatory T cells and proinflammatory cytokines. Int J Nanomedicine. 2019;14:2383–2395. doi:10.2147/IJN.S196842
5. Schwarz S, Kehrenberg C, Walsh T. Use of antimicrobial agents in veterinary medicine and food animal production. Int J Antimicrob Agents. 2001;17(6):431–437. doi:10.1016/S0924-8579(01)00297-7
6. Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. 2013;69(3):827–834. doi:10.1093/jac/dkt443
7. Dayao DAE, Gibson JS, Blackall PJ, Turni C. Antimicrobial resistance in bacteria associated with porcine respiratory disease in Australia. Vet Microbiol. 2014;171(1–2):232–235. doi:10.1016/j.vetmic.2014.03.014
8. Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Controlled Release. 2011;156(2):128–145. doi:10.1016/j.jconrel.2011.07.002
9. Al-Kadmy IM, Ibrahim SA, Al-Saryi N, Aziz SN, Besinis A, Hetta HF. Prevalence of genes involved in colistin resistance in acinetobacter baumannii: first report from Iraq. Microbial Drug Resist. 2019. doi:10.1089/mdr.2019.0243
10. Farhan SM, Ibrahim RA, Mahran KM, Hetta HF, El-Baky RMA. Antimicrobial resistance pattern and molecular genetic distribution of metallo-β-lactamases producing Pseudomonas aeruginosa isolated from hospitals in Minia, Egypt. Infect Drug Resist. 2019;12:2125. doi:10.2147/IDR.S198373
11. Dizaj SM, Mennati A, Jafari S, Khezri K, Adibkia K. Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bulletin. 2015;5(1):19.
12. El-Mokhtar MA, Hetta HF. Ambulance vehicles as a source of multidrug-resistant infections: a multicenter study in Assiut City, Egypt. Infect Drug Resist. 2018;11:587. doi:10.2147/IDR.S151783
13. El-Baky RMA, Sandle T, John J, Abuo-Rahma GE-DA, Hetta HF. A novel mechanism of action of ketoconazole: inhibition of the NorA efflux pump system and biofilm formation in multidrug-resistant Staphylococcus aureus. Infect Drug Resist. 2019;12:1703–1718. doi:10.2147/IDR.S201124
14. Ahmed S, Ahmed S, Mohamed W, et al. Nosocomial vancomycin and methicillin resistant staphylococcal infections in intensive care units in Assiut University Hospitals. Egypt J Med Microbiol. 2011;20:2.
15. Panáček A, Kvitek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110(33):16248–16253. doi:10.1021/jp063826h
16. Kargozar S, Baino F, Hamzehlou S, Hill RG, Mozafari M. Bioactive glasses entering the mainstream. Drug Discov Today. 2018;23(10):1700–1704. doi:10.1016/j.drudis.2018.05.027
17. Gajjar P, Pettee B, Britt DW, Huang W, Johnson WP, Anderson AJ. Antimicrobial activities of commercial nanoparticles against an environmental soil microbe, Pseudomonas putida KT2440. J Biol Eng. 2009;3(1):9. doi:10.1186/1754-1611-3-9
18. Krajewski S, Prucek R, Panacek A, et al. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. 2013;9(7):7460–7468. doi:10.1016/j.actbio.2013.03.016
19. Arokiyaraj S, Arasu MV, Vincent S, et al. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: an in vitro study. Int J Nanomedicine. 2014;9:379. doi:10.2147/IJN
20. Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. 2007;3(2):168–171. doi:10.1016/j.nano.2007.02.001
21. Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver‐containing Hydrofiber® dressing. Wound Repair Regener. 2004;12(3):288–294. doi:10.1111/j.1067-1927.2004.012304.x
22 Brown A, Smith K, Samuels TA, Lu J, Obare S, Scott ME. Nanoparticles functionalized with ampicillin destroy multiple antibiotic resistant isolates of Pseudomonas aeruginosa, Enterobacter aerogenes and Methicillin resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78:2768–2774. AEM. 06513–06511.
23. Hwang I-S, Hwang JH, Choi H, Kim K-J, Lee DG. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol. 2012;61(12):1719–1726. doi:10.1099/jmm.0.047100-0
24. Sharma N, Jandaik S, Kumar S. Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms. Anais Da Academia Brasileira De Ciências. 2016;88(3):1689–1698. doi:10.1590/0001-3765201620150713
25. Jahanshahi M, Babaei Z. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7:25.
26. Emami-Karvani Z, Chehrazi P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr J Microbiol Re. 2011;5(12):1368–1373.
27. Thati V, Roy AS, Ambika Prasad M, Shivannavar C, Gaddad S. Nanostructured zinc oxide enhances the activity of antibiotics against Staphylococcus aureus. J Biosci Tech. 2010;1(2):64–69.
28. Mulfinger L, Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C. Synthesis and study of silver nanoparticles. J Chem Educ. 2007;84(2):322. doi:10.1021/ed084p322
29. Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorganic Mater. 2001;3(7):643–646. doi:10.1016/S1466-6049(01)00197-0
30. Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci Technol Adv Mater. 2008;9(3):035004. doi:10.1088/1468-6996/9/3/035004
31. Krishnamoorthy V, Hiller DB, Ripper R, et al. Epinephrine induces rapid deterioration in pulmonary oxygen exchange in intact, anesthetized RatsA flow and pulmonary capillary pressure-dependent phenomenon. Anesthesiol. 2012;117(4):745–754. doi:10.1097/ALN.0b013e31826a7da7
32. Irzh A, Genish I, Klein L, Solovyov LA, Gedanken A. Synthesis of ZnO and Zn nanoparticles in microwave plasma and their deposition on glass slides. Langmuir. 2010;26(8):5976–5984. doi:10.1021/la904499s
33. Applerot G, Lipovsky A, Dror R, et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS‐mediated cell injury. Adv Funct Mater. 2009;19(6):842–852. doi:10.1002/adfm.v19:6
34. Rao B, Boominathan M. Antibacterial activity of silver nanoparticles of seaweeds. Am J Adv Drug Delivery. 2015;3:296–307.
35. Taylor W. Marine algae of the Smithsonian-Bredin expedition to Yucatán—1960. Bulletin Marine Sci. 1972;22(1):34–44.
36. Devi JS, Bhimba BV, Ratnam K. In vitro anticancer activity of silver nanoparticles synthesized using the extract of Gelidiella sp. Int J Pharm Pharm Sci. 2012;4(4):710–715.
37. Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi:10.1016/S0031-9422(00)00235-1
38. Trease GE and Evans WC. Phenols and Phenolic Glycosides. In: Trease and Evans Pharmacology and Bikere. Tindall, London. 1996:832–836..
39. Jayaseelan C, Rahuman AA, Kirthi AV, et al. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta Part A. 2012;90:78–84. doi:10.1016/j.saa.2012.01.006
40. Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B. 2010;81(1):358–362. doi:10.1016/j.colsurfb.2010.07.036
41. Fattah K, Gamal A, Ibrahim S, Mohamed E, Saleh A. Investigation of the efficacy of synthesized silver and zinc oxide nanoparticles against multi-drug resistant gram negative bacterial clinical isolates. Arch Clin Microbiol. 2017;8(6):67.
42. Boussaada O, Ammar S, Saidana D, et al. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol Res. 2008;163(1):87–95. doi:10.1016/j.micres.2007.02.010
43. Sutton S. Measurement of microbial cells by optical density. J Validation Technol. 2011;17(1):46–49.
44. Seil JT, Webster TJ. Antibacterial effect of zinc oxide nanoparticles combined with ultrasound. Nanotechnology. 2012;23(49):495101. doi:10.1088/0957-4484/23/49/495101
45. Mirhosseini M, Arjmand V. Reducing pathogens by using zinc oxide nanoparticles and acetic acid in sheep meat. J Food Prot. 2014;77(9):1599–1604. doi:10.4315/0362-028X.JFP-13-210
46. Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;22(12):1825–1834. doi:10.1016/j.drudis.2017.08.006
47. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi:10.1016/j.jcis.2004.02.012
48. Abbaszadegan A, Ghahramani Y, Gholami A, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;16(1):53.
49. Rai M, Deshmukh S, Ingle A, Gade A. Silver nanoparticles: the powerful nanoweapon against multidrug‐resistant bacteria. J Appl Microbiol. 2012;112(5):841–852. doi:10.1111/jam.2012.112.issue-5
50. Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720. doi:10.1128/AEM.02218-06
51. Oka H, Tomioka T, Tomita K, Nishino A, Ueda S. Inactivation of enveloped viruses by a silver-thiosulfate complex. Met Based Drugs. 1994;1(5–6):511. doi:10.1155/MBD.1994.511
52. Tokumaru T, Shimizu Y, Fox JC. Antiviral activities of silver sulfadiazine in ocular infection. Res Commun Chem Pathol Pharmacol. 1974;8(1):151–158.
53. Oloffs A, Grosse-Siestrup C, Bisson S, Rinck M, Rudolph R, Gross U. Biocompatibility of silver-coated polyurethane catheters and silvercoated Dacron® material. Biomaterials. 1994;15(10):753–758. doi:10.1016/0142-9612(94)90028-0
54. Herrera M, Carrion P, Baca P, Liebana J, Castillo A. In vitro antibacterial activity of glass-ionomer cements. Microbios. 2001;104(409):141–148.
55. Jamaran S, Zarif BR. Synergistic effect of silver nanoparticles with neomycin or gentamicin antibiotics on mastitis-causing Staphylococcus aureus. Open J Ecol. 2016;6(07):452. doi:10.4236/oje.2016.67043
56. Xu H, Qu F, Xu H, et al. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157: H7. Biometals. 2012;25(1):45–53. doi:10.1007/s10534-011-9482-x
57. McShan D, Zhang Y, Deng H, Ray PC, Yu H. Synergistic antibacterial effect of silver nanoparticles combined with ineffective antibiotics on drug resistant Salmonella typhimurium DT104. J Environ Sci Health Part C. 2015;33(3):369–384. doi:10.1080/10590501.2015.1055165
58. Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett. 2007;90(213902):2139021–2139023. doi:10.1063/1.2742324
59. Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. doi:10.1021/la104825u
60. Al-Holy MA, Lin M, Cavinato AG, Rasco BA. The use of Fourier transform infrared spectroscopy to differentiate Escherichia coli O157: H7 from other bacteria inoculated into apple juice. Food Microbiol. 2006;23(2):162–168. doi:10.1016/j.fm.2005.01.017
61. Zhang L, Jiang Y, Ding Y, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res. 2007;9(3):479–489. doi:10.1007/s11051-006-9150-1
62. Matei A, Cernica I, Cadar O, Roman C, Schiopu V. Synthesis and characterization of ZnO – polymer nanocomposites. Int J Mater Forming. 2008;1(1):767–770. doi:10.1007/s12289-008-0288-5
63. Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77(7):2325–2331. doi:10.1128/AEM.02149-10
64. Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–284. doi:10.1016/j.msec.2014.08.031
65. Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol. 2011;45(5):1977–1983. doi:10.1021/es102624t
66. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters. 2015;7(3):219–242. doi:10.1007/s40820-015-0040-x
67. Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18(17):6679–6686. doi:10.1021/la0202374
68. Iram S, Akbar Khan J, Aman N, Nadhman A, Zulfiqar Z, Arfat Yameen M. Enhancing the anti-enterococci activity of different antibiotics by combining with metal oxide nanoparticles. Jundishapur j Microbiol. 2016;9(3):e31302. doi:10.5812/jjm
69. Ghule K, Ghule AV, Chen B-J, Ling Y-C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006;8(12):1034–1041. doi:10.1039/b605623g
70. Joshi N, Ngwenya BT, French CE. Enhanced resistance to nanoparticle toxicity is conferred by overproduction of extracellular polymeric substances. J Hazard Mater. 2012;241:363–370. doi:10.1016/j.jhazmat.2012.09.057
71. Azizi S, Mohamad R, Mahdavi Shahri M. Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis (L.) Schrad: characterization and biomedical applications. Molecules. 2017;22(2):301. doi:10.3390/molecules22020301
70. Azizi S, Mohamad R, Mahdavi Shahri M. Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis (L.) Schrad: characterization and biomedical applications. Molecules. 2017;22:2. doi:10.3390/molecules22020301
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
