Back to Journals » OncoTargets and Therapy » Volume 9
Synergism between PGC-1α and estrogen in the survival of endometrial cancer cells via the mitochondrial pathway
Authors Yang H, Yang R, Liu H, Ren Z, Kong F, Li D, Ma X
Received 1 January 2016
Accepted for publication 9 March 2016
Published 30 June 2016 Volume 2016:9 Pages 3963—3973
DOI https://doi.org/10.2147/OTT.S103482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Hui Yang, Rui Yang, Hao Liu, Zhongqian Ren, Fanfei Kong, Da Li, Xiaoxin Ma
Department of Obstetrics and Gynecology, Shengjing Hospital Affiliated to China Medical University, Shenyang, Liaoning, People’s Republic of China
Abstract: Accumulating evidence shows that peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) is involved in the progression of hormone-related cancers, and there may exist an association between estrogen and PGC-1α. Notably, emerging evidence has led to considerable interest in the role of PGC-1α in endometrial cancer development. However, whether the synergism exists between PGC-1α and estrogen for regulating mitochondrial function to promote the development of endometrial cancer remains largely unknown. Here, we show that: 1) knockdown of PGC-1α attenuates the survival of endometrial cancer cells by inducing cell apoptosis through the mitochondrial pathway; 2) estrogen remedies the PGC-1α efficiency-induced decline of endometrial cancer cell viability; and 3) estrogen modulates the mitochondrial function to inhibit the PGC-1α deficiency-induced apoptosis in endometrial cancer cells. Collectively, these results demonstrated that the synergism between PGC-1α and estrogen was required for the survival of endometrial cancer cells, which was dependent on the mitochondrial pathway.
Keywords: PGC-1α, estrogen, endometrial cancer, survival, mitochondrial pathway
Introduction
Endometrial cancer is the most common gynecological malignancy. Approximately 80% of endometrial cancer cases are type I tumors, which are associated with a history of unopposed estrogen exposure or other hyperoestrogenic risk factors. Cell proliferation and apoptosis resistance might be mechanisms by which estrogens promote the progression of endometrial cancer.1 However, a series of reports also documented an association between antiestrogen therapy and the development of endometrial carcinoma.2,3 Therefore, an improved understanding of molecular pathogenesis of endometrial cancers may identify biomarkers for successful therapeutic strategies.
Estrogens have a broad spectrum of physiological functions and mediate their biological effects by binding to specific intracellular receptors, estrogen receptor-α (ERα) and ERβ. Recently, some interesting studies have indicated that ERs could localize to and play important roles in mitochondria.4,5 Estrogen protects the mitochondrial function to improve the function of heart.6 Furthermore, estrogen activates the mitochondrial biogenesis through the regulation of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) in cardiomyocytes.7 PGC-1α is a multifunctional transcription coactivator that cooperates with nuclear receptors to regulate genes involved in mitochondrial function. It has been reported that PGC-1α could regulate ERα transcriptional activity in vitro and in vivo.8,9 Moreover, PGC-1α enhanced abnormal local estrogen production by stimulating aromatase activity in endometriosis.10 Therefore, these data provide a clue that there might exist an association between estrogen and PGC-1α in the regulation of mitochondrial function.
The critical role of PGC-1α in the regulation of cell metabolism and mitochondrial biogenesis has led to speculation that reduced PGC-1 function could be involved in the metabolic reprogramming undergone by cancer cells. Accumulated evidence indicated that PGC-1α was also involved in the progression of hormone-related cancer. Indeed, it was overexpressed in various estrogen-dependent diseases such as breast and ovarian cancers.11 Silencing of PGC-1α in breast cancer cells suspended their invasive potential and attenuated metastasis.12 PGC-1α could induce apoptosis in human epithelial ovarian cancer cells through a PPAR-γ-dependent pathway.13 Our previous study suggested that upregulation of PGC-1α and estrogen-related receptor γ (ERRγ) in endometrial cancer might be a requirement for cancer cell energy metabolism, which contributes to the development of endometrial cancer.14 Therefore, the similar effect of PGC-1α and estrogen on the mitochondrial function offered a potential mechanism that the synergism between PGC-1α and estrogen can be used for regulating mitochondrial function to promote the development of endometrial cancer. But the potential mechanism remained to be unknown.
In the present study, we evaluated the functional interaction between PGC-1α and estrogen and their impact on mitochondrial function in endometrial cancer cells. Our results suggested that the synergism between PGC-1α and estrogen was required for the survival of endometrial cancer cells, which was dependent on the mitochondrial pathway.
Materials and methods
Cell lines and culture
Human endometrial cancer (HEC-1-A) and Ishikawa cell lines were purchased from the Shanghai Institute of Cell Biology of Chinese Academy of Sciences (Shanghai, People’s Republic of China). The HEC-1-A cells were respectively cultured as adherent monolayers in McCoy’s 5A medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (HyClone, Logan, UT, USA) at 37°C and 5% CO2 in a saturated humidified incubator. The Ishikawa cells were cultured as adherent monolayers in Roswell Park Memorial Institute-1640 medium (HyClone) containing 10% fetal bovine serum at 37°C in a 5% CO2 humidified incubator. The study protocols were approved by the Ethics Committee of China Medical University, Shenyang, People’s Republic of China.
Transfection
Two small interfering RNAs (siRNAs) targeting different sites of human PGC-1α mRNA (GenBank accession no NM_013261.3) were designed and synthesized by the Genepharmacy Inc. (Jiangsu, People’s Republic of China) to knock down the PGC-1α expression, and a no-silence siRNA was synthesized as a negative control to eliminate the possibility of nonspecific silencing effects. All the siRNAs mentioned previously are shown in Table 1.
  | Table 1 siRNA sequence used in this study |
The cells were seeded into six-well plates and incubated overnight to allow for full extension and adherence before transfection. The cells were transfected by using DharmaFECT transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s instruction. In all, 10 μL of 5 μM siRNA was added to 190 μL of serum-free medium. At the same time, 5 μL of DharmaFECT transfection reagent was added to 195 μL of serum-free medium. Then the contents of each tube were mixed and incubated for 5 minutes at room temperature. The content of siRNA was added to the DharmaFECT transfection reagent, and then 1,600 μL of antibiotic-free complete medium was added. Finally, the culture medium was removed from the wells of the six-well plate and 2 mL of the appropriate transfection medium was added to each well. The cells were harvested 72 hours after transfection for future experiments. For estrogen treatment, 48 hours after transfection, the cells were treated with 10 nmol/L 17β-estradiol for the indicated time followed by the individual experiments.
Cell proliferation assay
Cell proliferation was determined by Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan). A total of 2×103 cells/well were seeded into the 96-well plates and treated with siRNAs with or without estrogen. In all, 10 μL of CCK-8 solution was added to each well and incubated for 2 hours. The absorbance of each well was measured at 450 nm using a microplate reader.
Annexin V–fluorescein isothiocyanate/propidium iodide double staining assay
Cell apoptosis of endometrial cancer cells was analyzed by flow cytometry using an Annexin V-fluorescein isothiocyanate (AV-FITC) apoptosis detection kit (Beyotime Biotechnology, Shanghai, People’s Republic of China). After treatment with estrogen for 24 hours, different groups of cells were stained with the apoptosis detection kit according to the manufacturer’s instructions. The cells from each sample were suspended and incubated in 195 μL of AV-FITC binding buffer and 5 μL of AV-FITC for 20 minutes at room temperature in the dark. Then the samples were centrifuged at 1,000× g for 5 minutes and resuspended in 190 μL of binding buffer and 10 μL of propidium iodide (PI). The samples were analyzed using FACScan flow cytometry (BD Biosciences, San Jose, CA, USA) with at least 10,000 events recorded for each condition.
Measurement of mitochondrial membrane potential
After treatment with estrogen for 24 hours, the changes in mitochondrial membrane potential were assessed by Mitochondrial Membrane Potential Assay Kit with JC-1 (Beyotime Biotechnology). After treatment, the cells were washed with phosphate-buffered saline (PBS) and incubated with the medium containing 20 μg/mL JC-1 at 37°C for 20 minutes. The cells were collected and washed twice with PBS and then analyzed by flow cytometry.
TUNEL assay
Cell apoptosis was also examined using TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay. The one-step TUNEL Apoptosis Assay Kit (Beyotime Biotechnology) was used to stain cells according to manufacturer’s instructions. Cells were seeded into six-well plates containing disinfected and sterilized glass coverslips. After 72 hours of transfection, cells were fixed with 4% paraformaldehyde for 30 minutes, rinsed with PBS, and permeabilized with 0.1% Triton X-100 for 2 minutes on ice. The cells were incubated with TUNEL reaction mixture for 1 hour at 37°C. Then the cells were washed twice with PBS and incubated with 4′,6-diamidino-2-phenylindole for 5 minutes at room temperature. After that, cells were washed thrice with PBS. A fluorescent microscopy was used to image the TUNEL positive cells.
Western blot
After treatment with estrogen for 24 hours, cells were collected, washed twice with ice-cold PBS, and incubated in radioimmunoprecipitation assay buffer for 30 minutes on ice. Then, the samples were centrifuged at 12,000× g for 15 minutes at 4°C. The BCA Protein Assay kit (Beyotime Biotechnology) was used to determine the protein concentrations. Total proteins were resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes using a semidry transfer instrument. The membranes were blocked with Tris-buffered saline plus Tween-20 containing 5% nonfat milk for 2 hours. Immunodetection was performed with a rabbit anti-PGC-1α antibody (dilution 1:500; Santa Cruz Biotechnology Inc., Dallas, TX, USA), a mouse anti-Bcl-2 antibody (dilution 1:500; Zhongshan Biotechnology, Beijing, People’s Republic of China), and a mouse anti-Bax antibody (dilution 1:500; Zhongshan Biotechnology), followed by a horseradish peroxidase-conjugated goat anti-rabbit IgG antiserum (dilution 1:5,000; Zhongshan Biotechnology) or a goat anti-mouse IgG antiserum (dilution 1:5,000; Zhongshan Biotechnology). The same membrane was reprobed with a tublin antibody as a loading control. Proteins were visualized using enhanced chemiluminescence.
Statistical analysis
Each experiment was repeated at least three times, and data were expressed as the mean ± standard deviation values. SPSS Version 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. χ2 analysis and Student’s t-test were appropriately used for different categories of data. P<0.05 was considered statistically significant.
Results
Knockdown of PGC-1α impaired the viability of endometrial cancer cells
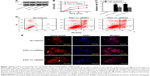
Our previous study suggested that PGC-1α could actually support cell proliferation by the regulation of energy metabolism in endometrial carcinoma tissues.14 To further ascertain the role of PGC-1α in the progress of endometrial cancer, the expression of Ishikawa cell, an ER-positive endometrial cancer cell line, was downregulated by using two different PGC-1α siRNAs. The levels of PGC-1α were detected by Western blot to determine the effect of RNA interference. As shown in Figure 1A, two different PGC-1α siRNAs effectively reduced the endogenous expression of PGC-1α. Next, the effect of PGC-1α knockdown on cell viability was measured by CCK-8. The results showed that there was a significant reduction in cell viability of PGC-1α knockdown cells (Figure 1B). Furthermore, the apoptosis in the Ishikawa cell was analyzed by flow cytometry with AV-FITC/PI double staining (Figure 1C and D). There was a significant difference in the apoptotic cell population between control and PGC-1α siRNAs groups. Compared with the control, knockdown of PGC-1α led to an increase in apoptotic rate in the early apoptotic phase as well as later apoptotic phase, which could be further confirmed by the detection of TUNEL apoptosis (Figure 1E). These results suggested that knockdown of PGC-1α attenuates the survival of endometrial cancer cells by inducing cell apoptosis.
Lack of PGC-1α induced the apoptosis of endometrial cancer cells through the mitochondrial pathway
Alteration in mitochondrial membrane permeability is also regarded as one of the crucial events in cellular apoptosis.15 Therefore, we first measured the potential using a method of staining with JC-1, and then the cells were subsequently analyzed by flow cytometry (Figure 2A). The aggregate JC-1 was dramatically decreased, while the green fluorescent JC-1 monomer was increased (Figure 2B). These data suggested that PGC-1α deficiency induced an obvious alteration in mitochondrial membrane permeabilization.
It is well recognized that mitochondrial membrane permeability is controlled by the balance between antiapoptotic protein Bcl-2 and proapoptotic protein Bax. Furthermore, the mitochondrial pathway of apoptosis is initiated by mitochondrial membrane permeabilization, leading to the release of apoptogenic factors such as Smac.16–18 Hence, the changes in these proteins were determined by Western blot in the process. The results showed that knockdown of PGC-1α significantly induced a reduction in the levels of Bcl-2 and an increase in the levels of Bax and Smac (Figure 2C and D). These data indicated that the depletion of PGC-1α triggered the mitochondrial pathway of apoptosis to attenuate the survival of endometrial cancer cells.
Estrogen remedied the PGC-1α efficiency-induced decline of endometrial cancer cells’ viability
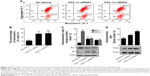
Some reports suggested that there might exist an association between estrogen and PGC-1α in the regulation of mitochondrial function.5 Therefore, the two endometrial cancer cell lines, Ishikawa cells (ER positive) and HEC-1-A cells (ER negative), were selected to further investigate the adaptation of PGC-1α-deficiency cells under estrogen stimulation. First, the results from the CCK-8 analysis showed that estrogen could counteract the effects of PGC-1α deficiency on the cells viability of Ishikawa cells (Figure 3A). However, there were no alterations in HEC-1-A cells’ viability in the presence or absence of estrogen (P>0.05; Figure 3B). Subsequent results from the analysis of flow cytometry with Annexin V-FITC/PI double staining displayed similar results in Ishikawa cells (P<0.05; Figure 3C and D) and HEC-1-A cells (P>0.05; Figure 3E and F). Notably, the downregulation of PGC-1α could augmente estrogen-induced apoptosis resistance in endometrial cancer cells. Meanwhile, estrogen primarily inhibited the early cellular apoptosis induced by PGC-1α deficiency (Figure 3C and D). Collectively, these data suggested that there was a compensatory effect between PGC-1α deficiency and estrogen treatment.
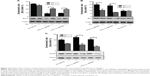
Estrogen modulated the mitochondrial function to inhibit the PGC-1α deficiency-induced apoptosis in endometrial cancer cells
The previous results had indicated the involvement of the mitochondrial pathway in PGC-1α deficiency-induced cellular apoptosis in endometrial cancer cells. To further verify the interaction between PGC-1α and estrogen, the alterations in mitochondrial membrane permeability were measured under estrogen stimulation. As shown in Figure 4, estrogen could remarkably abolish PGC-1α deficiency-induced increase in mitochondrial membrane permeability of Ishikawa cells (P<0.05; Figure 4A and B), whereas PGC-1α deficiency-induced increase remained unchanged in the HEC-1-A cells (P>0.05; Figure 4C and D) after treatment with estrogen. Further results from Western blot also showed that the decreased levels of antiapoptotic protein Bcl-2 were elevated in PGC-1α-deficiency Ishikawa cells after treatment with estrogen (Figure 5A). On the contrary, the increased levels of proapoptotic proteins Bax and Smac were obviously lessening (Figure 5B and C). Taken together, these data suggested that estrogen modulated the mitochondrial function to inhibit the PGC-1α deficiency-induced apoptosis in endometrial cancer cells.
Discussion
The PGC-1α-dependent pathway of mitochondrial biogenesis has been reported in human endometrial tissue.14 Our previous clinical study indicated that the upregulation of PGC-1α and ERRγ in the endometrial cancer tissue was related to tumor energy metabolism, which contributed to the development of endometrial cancer.14 Here, we have identified a novel synergism mechanism between PGC-1α and estrogen in endometrial cancer cells: PGC-1α was required for the survival of endometrial cancer cells, depletion of PGC-1α could induce the mitochondrial-dependent apoptosis in endometrial cancer cells, and the addition of estrogen protected the mitochondrial function to counteract the effect of PGC-1α deficiency in endometrial cancer cells.
Although a growing body of evidence points toward an important role for PGC-1α in cancer, there exists a contradiction about the roles of PGC-1α in different cancer types.19–21 An improved understanding of the role of PGC-1α in different tumor types will be important in determining whether this pathway will be amenable to therapeutic intervention. In the present study, our findings revealed that PGC-1α had protumorigenic function in the development of endometrial cancer, that is, PGC-1α was required for the survival of endometrial cancer. Our results indicated that the endometrial cancer cells’ knockdown of PGC-1α was inclined to apoptosis. Given its role as a “master regulator” of mitochondria, a question was raised whether PGC-1α was involved in the progress of the mitochondrial-mediated apoptotic pathway. Subsequently, this hypothesis was verified by the enhancement in mitochondrial membrane permeabilization, the reduction in antiapoptotic protein Bcl-2, and the increase in the proapoptotic proteins Bax and Smac in PGC-1α-deficiency endometrial cancer cells. It had been reported that PGC-1α could interact with different tissue-specific transcription factors, thus driving distinct genetic programs in different cancer types.22 The heterogeneous response of PGC-1α in various tumors was likely dictated at least in part by tumor microenvironment. Therefore, we postulated that the roles of PGC-1α in endometrial cancer were likely affected by estrogen stimulation, and then, whether the estrogen response overlaid the effects of PGC-1 to enhance the mitochondrial function and then led to the outcome of apoptotic resistance. Given this, we further investigated the effects of estrogen on the mitochondrial function under PGC-1α-deficiency condition.
Accumulating studies have shown that estrogen could rescue the mitochondrial function.23,24 For example, estrogen amelioration of Aβ-induced defects in mitochondria is mediated by mitochondrial signaling pathway.23 Estrogen could modulate metabolic adaptations by mitochondrial pathways in breast cancer cells.24 Some studies previously reported that estrogen acted through ERs to upregulate expression of nuclear-encoded mitochondrial proteins to modulate the mitochondrial function.5 In Ishikawa cells, ER-positive endometrial cancer cells, the addition of estrogen restored the effects of PGC-1α deficiency on the mitochondrial function, including enhanced mitochondrial membrane permeabilization, decreased antiapoptotic protein Bcl-2, and increased proapoptotic proteins Bax and Smac. However, in the HEC-1-A cells, ER-negative endometrial cancer cells, there were no effects on these alterations in the presence of estrogen. These data demonstrated that the rescue effects of estrogen in the process were ER-dependent pathways. More interestingly, upon only estrogen stimulation, lack of PGC-1α yet induced the apoptosis of all endometrial cancer cells, which was not limited with the existence of ERs. This disconnection suggests that estrogen protected the mitochondria function to inhibit cell apoptosis by a pathway distinct from PGC-1α. Based on this, our findings suggested that PGC-1α did not mediate effects of estrogen on ERs and mitochondrial function in endometrial cancer cells, while PGC-1α coactivates both ERs. A recent study has indicated that estrogen modulates several master transcriptional regulators of mitochondrial function, NRF-1, TFAM, and PGC-1 isoforms, in the blood vessels of brain.25 The pattern of a decrease in PGC-1α together with an increase in PGC-1β, PRC, NRF-1, and TFAM appeared to underlie protective effects of estrogen on cerebral blood vessel mitochondria. Conversely, another study indicated that the increased mitochondrial biogenesis in type I endometrial cancer was associated with the upregulation of PGC-1α signaling pathway, which might transactivate NRF-1 leading to TFAM content increase and increase in mitochondrial biogenesis.26 These contradictory results may reflect context-specific actions of estrogen and the particular role of mitochondria in supporting the different metabolic requirements of tissue function. In the present paper, we provided experimental evidence to demonstrate that PGC-1α signaling-mediated mitochondrial apoptosis resistance paralleled with the mitochondrial effects of estrogen in endometrial cancer cells, but there was a synergistic effect on the protection of mitochondrial function to resist mitochondria-dependent apoptosis.
Conclusion
Our study revealed that PGC-1α was required for the survival of endometrial cancer cells and downregulation of PGC-1α is one of the major contributors of apoptosis in endometrial cancer cells. Additionally, there was a synergism between PGC-1α and estrogen, that is, estrogen stimulation from tumor microenvironment could exaggerate the effects of PGC-1α, which was separate from the PGC-1α signaling. Our study supported a crucial and potential role for PGC-1α in endometrial cancer and suggested that it might be a potential target for therapeutic strategy in endometrial cancer.
Acknowledgments
Our project was supported by National Natural Science Foundation of China (Nos 82172874 and 81472438), Department of Science and Technology of Liaoning Province (No 2013225079), and Shenyang City Science and Technology Bureau (Nos F12-227-1-62 and F14-158-9-47).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6(5):360–368. | ||
Di Benedetto L, Giovanale V, Caserta D. Endometrial tubal metaplasia in a young puerperal woman after breast cancer. Int J Clin Exp Pathol. 2015;8(6):7610–7613. | ||
Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000;78(2):181–186. | ||
Sastre-Serra J, Nadal-Serrano M, Pons DG, Valle A, Oliver J, Roca P. The effects of 17β-estradiol on mitochondrial biogenesis and function in breast cancer cell lines are dependent on the ERα/ERβ ratio. Cell Physiol Biochem. 2012;29(1–2):261–268. | ||
Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105(6):1342–1351. | ||
Chen Y, Zhang Z, Hu F, et al. 17β-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: a preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation. J Steroid Biochem Mol Biol. 2015;147:92–102. | ||
Ruiz M, Courilleau D, Jullian JC, et al. A cardiac-specific robotized cellular assay identified families of human ligands as inducers of PGC-1α expression and mitochondrial biogenesis. PLoS One. 2012;7(10):e46753. | ||
Bourdoncle A, Labesse G, Margueron R, Castet A, Cavaillès V, Royer CA. The nuclear receptor coactivator PGC-1alpha exhibits modes of interaction with the estrogen receptor distinct from those of SRC-1. J Mol Biol. 2005;347(5):921–934. | ||
Morselli E, Fuente-Martin E, Finan B, et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014;9(2):633–645. | ||
Suganuma I, Mori T, Ito F, et al. Peroxisome proliferator-activated receptor gamma, coactivator 1α enhances local estrogen biosynthesis by stimulating aromatase activity in endometriosis. J Clin Endocrinol Metab. 2014;99(7):E1191–E1198. | ||
Chang CY, McDonnell DP. Molecular pathways: the metabolic regulator estrogen-related receptor α as a therapeutic target in cancer. Clin Cancer Res. 2012;18(22):6089–6095. | ||
LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. | ||
Zhang Y, Ba Y, Liu C, et al. PGC-1alpha induces apoptosis in human epithelial ovarian cancer cells through a PPARgamma-dependent pathway. Cell Res. 2007;17(4):363–373. | ||
Ren Z, Yang H, Wang C, Ma X. The effects of PGC-1α on the proliferation and energy metabolism of malignant endometrial cancer cells. Onco Targets Ther. 2015;8:769–774. | ||
Tait SW, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013;5(9):a008706. | ||
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–1374. | ||
Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem. 2014;115(4):632–640. | ||
Cosentino K, García-Sáez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181:62–75. | ||
D’Errico I, Salvatore L, Murzilli S, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci U S A. 2011;108(16):6603–6608. | ||
Haq R, Shoag J, Andreu-Perez P, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013;23(3):302–315. | ||
Vazquez F, Lim JH, Chim H, et al. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23(3):287–301. | ||
Bhalla K, Hwang BJ, Dewi RE, et al. PGC1α promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71(21):6888–6898. | ||
Sarkar S, Jun S, Simpkins JW. Estrogen amelioration of Aβ-induced defects in mitochondria is mediated by mitochondrial signaling pathway involving ERβ, AKAP and Drp1. Brain Res. 2015;1616:101–111. | ||
O’Mahony F, Razandi M, Pedram A, Harvey BJ, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol. 2012;26(12):2058–2070. | ||
Kemper MF, Stirone C, Krause DN, Duckles SP, Procaccio V. Genomic and non-genomic regulation of PGC1 isoforms by estrogen to increase cerebral vascular mitochondrial biogenesis and reactive oxygen species protection. Eur J Pharmacol. 2014;723:322–329. | ||
Cormio A, Guerra F, Cormio G, et al. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem Biophys Res Commun. 2009;390(4):1182–1185. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.