Back to Journals » Cancer Management and Research » Volume 12
Synchronous Metastatic Nasopharyngeal Carcinoma: Characteristics and Survival of Patients Adding Definitive Nasopharyngeal-Neck Radiotherapy to Systematic Chemotherapy
Authors Liao W, He J, Gou Q, Duan B, Liu L, Ai P, Li Y , Ren K, Chen N
Received 9 August 2020
Accepted for publication 22 September 2020
Published 15 October 2020 Volume 2020:12 Pages 10211—10219
DOI https://doi.org/10.2147/CMAR.S276286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Wenjun Liao,* Jinlan He,* Qiheng Gou, Baofeng Duan, Lei Liu, Ping Ai, Yanchu Li, Kexing Ren, Nianyong Chen
Department of Radiation Oncology, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nianyong Chen Department of Radiation Oncology
Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, Pepole’s Republic of China
Tel/Fax +86 28 8542 3278
Email [email protected]
Purpose: To determine the M1 sub-staging in synchronous metastatic nasopharyngeal carcinoma (smNPC) and to examine the effect of nasopharyngeal-neck radiotherapy (RT) and local treatment of metastases on overall survival (OS) of smNPC patients.
Patients and Methods: A total of 150 patients with smNPC were included. Metastatic characteristics associated with their potential prognostic significance were analyzed. Then, a stratification system of the M1 sub-staging in smNPC was provided according to metastatic features. Moreover, the OS of patients with or without nasopharyngeal-neck RT was compared by Log rank test. The OS of patients who received or did not receive local treatment of metastases was also analyzed.
Results: We successfully divided the M1 stage into three sub-staging: M1a (a single site with a single lesion), M1b (a single site with multiple lesions), and M1c (multiple sites with multiple lesions). The median OS was 53.2, 25.8, and 18.9 months for M1a, M1b, and M1c, respectively (p < 0.001). Nasopharyngeal-neck RT plus systematic chemotherapy (CT) significantly improved OS compared to systematic CT (median OS, 34.0 vs 15.2 months, p = 0.002). However, incorporation of local treatment of metastases did not bring survival benefit to smNPC patients who received nasopharyngeal-neck RT plus systematic CT (median OS, 25.8 vs 35.1 months, p = 0.374).
Conclusion: The sub-staging of the M1 stage in smNPC had promising prognostic value. Adding nasopharyngeal-neck RT on the basis of systematic CT markedly improved the survival of smNPC patients, while addition of local treatment of metastases to nasopharyngeal-neck RT plus systematic CT for smNPC needed further exploration.
Keywords: nasopharyngeal carcinoma, chemotherapy, local therapy, immunotherapy, metastasis
Introduction
Nasopharyngeal carcinoma (NPC), originating from the nasopharynx mucosal lining, occurs commonly in southern China, accounting for 47.7% of all cases diagnosed worldwide in 2018.1 The local control rate of NPC was quite satisfactory with the introduction of intensive modulated radiation therapy (IMRT) and concurrent chemo-radiotherapy (CCRT).2 However, development of distant metastasis remains now the most common failure pattern in patients with NPC.3,4 While over 20% locally advanced NPC developed post-treatment distant metastases,5 4–10% of newly diagnosed NPC patients exhibit distant metastasis, so-called “synchronous metastatic” NPC (smNPC).6–9
Survival outcomes of smNPC patients were divergent, which are greatly affected by the metastatic site, the number of metastatic sites, and the number of metastatic lesions.10 At present, the tumor, node, metastasis (TNM) classification system for smNPC is a “catch-all” M1 classification, ignoring the striking differences between each smNPC patient.11 Therefore, a M1 sub-staging of smNPC should be further determined to accurately predict prognosis.
In addition, there is little consensus among oncologists regarding the optimal treatment modalities for patients with smNPC. The well-established therapeutic strategy has been systematic chemotherapy (CT), with a relatively high objective response rate (40–65%),12,13 but the overall prognosis of smNPC patients was still dismal. Recently studies have demonstrated that addition of nasopharyngeal-neck radiotherapy (RT) to systematic CT considerably prolonged survival in smNPC patients.8,14 However, due to the low incidence of this disease, some researches were only single-arm studies comprising a limited sample size.15–17 Therefore, more robust evidence is warranted to confirm this conclusion. Moreover, the effect of local treatment to metastases on overall survival (OS) of smNPC patients has not been explored adequately.
In this study, we therefore reviewed the metastatic characteristics associated with their prognostic significance, treatment modalities, and survival of smNPC patients, in an attempt to further determine the M1 sub-staging and examine the role of nasopharyngeal-neck RT and local treatment of metastases in smNPC.
Materials and Methods
Patients Population
All records of NPC patients (n = 2106) treated at the West China Hospital of Sichuan University between Jan 2010 and Dec 2017 were reviewed. Eligibility criteria were as follows: (1) Patients pathologically diagnosed as NPC; (2) Patients confirmed with distant metastasis at initial diagnosis according to evaluation as follows: A biopsy of the metastatic lesions; or computed tomography (CT)/chest-x ray for chest, ultrasonic/CT/MRI of the abdomen, whole-body bone scan, or positron emission tomography/computed tomography (PET/CT); (3) Patients received at least two cycles of systematic CT with or without nasopharyngeal-neck RT. Patients with insufficient clinical data or patients with other malignancies were excluded. In total, 150 smNPC were included in this study.
All patients were restaged according to the eighth edition of the American Joint Committee on Cancer (AJCC/UICC). The ethics institutional board of the West China Hospital of Sichuan University approved the study. All patients provided written informed consent, and all procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki.
Treatments
The treatment modalities are shown in Table 1. All patients (n = 150) underwent systematic CT, among which 117 (78.0%) received nasopharyngeal-neck RT additionally. The predominant cisplatin-based CT regimens in this study included cisplatin (80 mg/m2) plus docetaxel (60–80 mg/m2), paclitaxel (135–175 mg/m2) with or without 5-fluorouracil (600–1000 mg/m2) (TPF/TP), and cisplatin (80 mg/m2) plus gemcitabine (1000 mg/m2) (GP). The decision of regimen and cycles of CT was at the discretion of the treating physicians. Generally, in this study, the systemic CT regimen was mainly TPF (n = 75, 50.0%), followed by TP (n = 25, 16.7%) (Table 1).
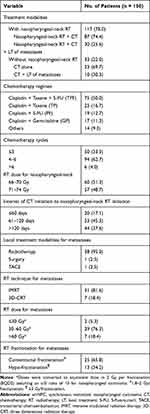 |
Table 1 Treatment Characteristics of smNPC Patients |
Of the 117 smNPC patients who underwent nasopharyngeal-neck RT, 60 (51.3%) received a radiation dose of 66–70 Gy, and 57 (48.7%) received 71–74 Gy. The median interval of systematic CT initiation to nasopharyngeal-neck RT initiation was 99 days (range 0 to 240 days), and the median number of cycles of CT before the initiation of nasopharyngeal-neck RT was four (range 1 to 8). Moreover, all patients received nasopharyngeal-neck RT using IMRT with conventional fractionations.
In addition, 40 (26.7%) patients received local treatment of metastatic lesions, with 38 cases receiving local RT alone, one receiving surgery resection, and one receiving transarterial chemoembolization (TACE) (Table 1). Among the 38 patients who underwent RT for metastases, the median RT dose converted to equivalent dose in 2 Gy per fractionation (EQD2) was 50 Gy (18–88 Gy). Moreover, regarding patients with less than three metastatic lesions (n = 19), all metastatic lesions were treated with RT (n = 18) or surgery (n = 1). For those with more than three metastatic lesions (n = 21), local treatment was administrated only to the lesions that were resistant to systematic CT or threatened important functions of body.
Follow-Up and Statistical Analysis
Patients were routinely evaluated every two cycles of systematic CT. Follow up occurred every three months in the first two years; thereafter, every six months until death or loss to follow-up (the last follow-up was December 31, 2019). OS was defined as the duration from the date of diagnosis to the date of death from any reason.
Statistical analysis was performed using the SPSS 20.0 software package (IBM SPSS Inc). Survival analysis was calculated via the Kaplan–Meier method, and the survival curves of different groups were compared using the Log rank test. The Cox regression model was used to determine multiple prognostic factors associated with survival, and the Chi-square test was performed to compare categorical variables. A two-tailed p-value <0.05 was considered statistically significant.
Results
Overall Survival of smNPC Patients
The median follow-up was 23.7 months (Range, 1.0 to 107.9 months). At last follow-up, three (2.0%) patents experienced loco-regional recurrence, and 101 (67.3%) patients were dead. The 1-year, 3-year, and 5-year OS of the entire cohort were 79.9%, 40.0%, and 29.2%, respectively. In addition, we found that there were 23 (15.3%) patients achieved an OS of more than five years, of whom 17 (11.3%) cases were still alive with an OS ranged from 63.0 to 107.9 months.
Prognostic Value of M1 Sub-Staging in smNPC Patients
In patients with smNPC, the most frequently involved metastatic sites were bone (n = 85, 56.7%), lungs (n = 52, 34.7%), liver (n = 32, 21.3%), and distant lymph nodes (n = 24, 16.0%); Single metastatic site (n = 114, 76.0%) was most common and multiple metastatic lesions were detected more frequently compared to single metastatic lesion for each involved metastatic site (Table 2).
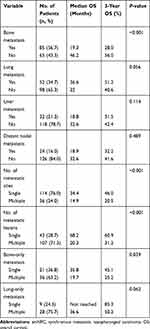 |
Table 2 Univariate Analysis of OS According to Metastatic Characteristics of smNPC |
The prognostic value of each specific metastatic site, and the number of metastatic sites and metastatic lesions were examined to determine the optimal M1 sub-staging. Patients who had bone metastasis, multiple metastatic sites, or multiple metastatic lesions had significantly shorter median OS than those who had no bone metastasis (19.3 vs 46.2 months, 3-year OS 28.0% vs 56.0%, p < 0.001), single metastatic sites (14.9 vs 34.4 months, 3-year OS 20.5% vs 46.0%, p < 0.001), or single metastatic lesions (20.3 vs 68.2 months, 3-year OS 31.2% vs 60.9%, p < 0.001), respectively (Table 2). The median OS of patients with single metastatic lesions only in the bone was significantly longer than those with multiple metastatic lesions only in the bone (35.8 vs 19.7 months, 3-year OS 45.1% vs 25.2%, p = 0.039). The 3-year OS of patients with single metastatic lesion in the lung was obviously higher compared to those with multiple metastatic lesions in the lung (85.3% vs 50.2%), although the difference was not statistically significant (p = 0.062) (Table 2).
According to the number of metastatic sites and metastatic lesions, M1 sub-staging was generally organized as follows: M1a (a single site with a single lesion) (n = 43, 28.7%), M1b (a single site with multiple lesions) (n = 71, 49.3%), and M1c (multiple sites with multiple lesions) (n = 36, 24.0%). The Kaplan–Meier analysis showed that the median OS of patients in M1a, M1b, and M1c group was 53.2, 25.8, and 18.9 months, and the 3-year OS was 60.2%, 35.3%, and 20.1%, respectively (p < 0.001, Figure 1). After adjusting for age, gender, T stage, N stage, bone metastasis, lung metastasis, liver metastasis, and distant lymph node metastasis, multivariate analysis indicated that M1 sub-staging significantly differed in terms of OS (M1b vs M1a: hazard ratio (HR) = 2.152,95% confidence inter (CI) = 1.253–3.696, p = 0.005; M1c vs M1a: HR = 4.169,95% CI = 1.464–11.871, p = 0.007) (Table 3).
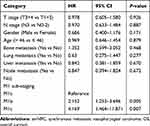 |
Table 3 Multivariate Analysis on Assessing the Impact of M1 Sub-Staging on OS in smNPC |
 |
Figure 1 Kaplan–Meier curves of overall survival according to the M1 sub-staging in patients. |
Role of Nasopharyngeal-Neck RT in smNPC
To examine the effect of nasopharyngeal-neck RT on survival of smNPC patients, we compared OS of patients with or without nasopharyngeal-neck RT on the basis of systematic CT. The clinical characteristics were well balanced between the two treatment groups (Table 4). The analysis showed that smNPC patients treated with nasopharyngeal-neck RT plus systematic CT experienced markedly increased median OS compared to those receiving systematic CT (34.0 vs 15.2 months, 3-year OS 44.9% vs 24.8%, p = 0.002, Figure 2). Furthermore, in the multivariate model adjusting for age, gender, T stage, N stage, bone metastasis, lung metastasis, liver metastasis, distant lymph node metastasis, and M1 sub-staging, nasopharyngeal-neck RT plus systematic CT was still an independent factor for predicting a better OS (HR = 0.510,95% CI = 0.320–0.813, p = 0.005) (Table 5).
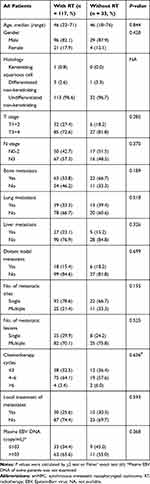 |
Table 4 Clinical Characteristics of smNPC Patients with or Without Nasopharyngeal-Neck RT |
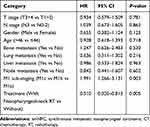 |
Table 5 Multivariate Analysis on Assessing the Effect of Nasopharyngeal-Neck on OS in smNPC |
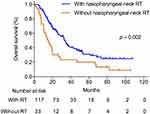 |
Figure 2 Overall survival for patients with and without nasopharyngeal-neck RT. RT, Radiotherapy. |
Additionally, we examined the impact of interval of systematic CT initiation to nasopharyngeal-neck initiation on OS of smNPC patients. We found that there were no significant differences in OS among patients with nasopharyngeal-neck RT initiation within 60 days, 60–120 days, or >120 days of systematic CT start (p = 0.532, Supplementary Figure 1).
Role of Local Treatment of Metastases in smNPC
To explore the influence of local treatment of metastases on survival of smNPC patients who had already received nasopharyngeal-neck RT plus systematic CT, we compared the OS of patients with or without local treatment of metastases. In this study, there were 30 patients who received local treatment combined with systematic CT plus nasopharyngeal-neck RT. Similarly, the clinical characteristics were well balanced between the two treatment groups (Table 6). The result suggested that there was no significant difference in median OS between patients with or without local treatment of metastases (25.8 vs 35.1 months, 3-year OS 35.2% vs 50.0%, p = 0.374, Figure 3).
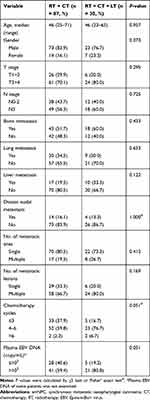 |
Table 6 Clinical Characteristics of smNPC Patients with or Without Local Treatment of Metastases on the Basis of Nasopharyngeal-Neck RT Plus Systematic CT |
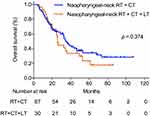 |
Figure 3 Overall survival for patients treated with nasopharyngeal-neck RT plus systematic CT with and without local treatment for metastases. |
Discussion
In this study, we analyzed the effect of metastatic features on OS of patients with smNPC, finding that patients with multiple metastatic sites or multiple metastatic lesions had poorer OS than those with single metastatic site or single metastatic lesion. Hence, a novel M1 sub-staging (M1a, M1b and M1c) was presented according to the number of metastatic sites and lesions, which provided important prognostic significance. Moreover, in terms of treatment modalities, we demonstrated that nasopharyngeal-neck RT plus systematic CT significantly improved OS of smNPC patients. However, local treatment of metastases was not associated with an improved OS in patients who underwent nasopharyngeal-neck RT plus systematic CT.
Compared with other head and neck squamous malignancies, NPC is more likely to present with synchronous metastasis.18 The outcomes of these patients are very poor, with a median OS of around 14 months under palliative CT treatment.19 Although great achievements have been achieved in locally advanced NPC, little progress has been made in metastatic NPC in last decades. Therefore, how to further understand biological manner of smNPC and improve survival of smNPC patients pose a great challenge.
Patients with smNPC do not behave in a uniform manner. However, in the current TNM stage of metastatic NPC, there was no hierarchical staging of distant metastasis, and a uniform M1 stage was utilized to cover all metastatic statuses. This simple classification incorporated some metastatic patients with certain metastatic characteristics and significantly different prognoses into the M1 stage. Hence, we divided the M1 stage of smNPC into three groups (M1a, M1b, and M1c) based on metastatic features of imaging detection. Survival analysis showed that patients in these three sub-stages had significantly different OS, with patients in M1c having the highest risk of mortality and worse survival.
In this sub-staging system, we did not exclusively incorporate the metastatic status of liver (patients with single or multiple liver metastasis were classified as an independent prognostic subgroup), which was inconsistent with several studies.11,20,21 The reason was that in our analysis, liver metastasis was not found to be significantly associated with OS. Additionally, one study indicated that patients with limited liver metastatic lesions also had a relatively good prognosis, with reported 3-year OS rate of more than 70%.22 Therefore, it might be more reasonable to consider the number of metastatic lesions, rather than just considering whether the liver is involved or not. Overall, together with other studies,20,21,23 our results suggested that the M1 stage of smNPC should be further stratified to better reflect prognostic information and inform personalized treatment.
The necessity of RT to nasopharyngeal-neck in smNPC has been debated for years. Recently, however, a number of studies have proved that nasopharyngeal-neck RT could bring survival benefit to smNPC patients.24 A multicenter randomized clinical trial including 126 smNPC showed that the 2-year OS of patients receiving nasopharyngeal-neck RT plus systematic CT was 76.4%, while the 2-year OS of patients receiving systematic CT was only 54.5% (HR = 0.42, 95% CI = 0.42, p = 0.004).25 In our study, we also confirmed that the addition of nasopharyngeal-neck RT considerably increased the median OS of smNPC patients by 18.8 months compared to those with systematic CT, and reduced the risk of mortality by 51% in our multivariate analysis.
Additionally, data from our analysis also provided an insight into the pattern of combination of systematic CT and nasopharyngeal-neck RT. It was indicated that the interval between systematic CT and nasopharyngeal-neck RT had no significant effect on OS of smNPC patients. Consistently, Rusthoven et al also found that no matter nasopharyngeal-neck RT initiation was within 10 days, 30 days, 60 days, or even within 120 days of systematic CT start, all the OS curves were similar.14 Together with those results, both earlier nasopharyngeal-neck RT and later nasopharyngeal-neck RT are optional in the treatment of smNPC with respect to the timing of systematic CT initiation.
When it comes to the RT doses for nasopharyngeal-neck tumor, there was growing evidence that increased doses of RT were positively correlated with improved survival. The study of Karam et al indicated that when RT doses were lower than 50 Gy, addition of nasopharyngeal-neck RT to systematic CT did not bring OS benefit to smNPC patients compared to systematic CT.14 However, when RT doses were increased to 50–69 Gy, OS advantage was observed, and there was a maximal survival benefit in patients undergoing ≥70 Gy. Consistently, another study showed that the median OS of smNPC patients with RT doses greater than 65 Gy was longer than those with RT doses less than it.26 In our study, all patients received nasopharyngeal-neck RT with definitive RT doses (≥66 Gy), and the 3-year OS of smNPC patients was 47.0%, which was comparable to the reported outcome (3-year OS, 45–50%) of patients receiving RT does ≥70 Gy in the study of Karam.14 Hence, it is suggested that definitive RT for nasopharyngeal-neck tumor plus systematic CT is preferable.
The benefits of nasopharyngeal-neck RT plus chemotherapy have been addressed. A number of questions still remain concerning how to optimize the systematic therapy in the treatment of smNPC, since anti-PD-1 or PD-L1 agents have been actively tested in this disease and might be incorporated into the first-line therapy in the future.27
Additionally, the role of local treatment of metastases remains controversial in smNPC. One study has demonstrated that local treatment of metastases showed no survival benefits to smNPC patients on the basis of systematic CT and definitive nasopharyngeal-neck RT. Even in patients with synchronous bone metastasis, aggressive RT with no less than 60 Gy for metastases was not associated with a better OS.20 In another study, consistently, it was indicated that patients with bone-only metastasis at initial diagnosis could not obtain survival benefit from local treatment to metastases.28 In our study, we yielded the similar result that the median OS of patients who received local treatment was not higher compared with those who did not. It seemed that that nasopharyngeal-neck RT combined with systematic CT might be sufficient for smNPC patients. However, further accumulation of cases is warranted to determine the value of local treatment for metastatic lesions. Hence, for those patients, adoption of aggressive local treatment such as stereotactic body radiotherapy (SBRT) for metastases requires further exploration.
Although both baseline characteristics of the two groups with or without nasopharyngeal-neck RT, and of the two groups with or without local treatment of metastases were balanced, the selection biases were inevitable. Due to the small size of patients who received local treatment of metastases in the present study, more cases were warranted to further confirm the effect of local treatment of metastases on survival.
Conclusion
Taken together, we showed that the M1 stage of smNPC could be subdivided into three categories, and the survival curves of the three subgroups were distinctly different. In terms of treatment modalities, nasopharyngeal-neck RT plus systematic CT significantly improved survival of smNPC patients compared to those with systematic CT. However, the role of local treatment of metastases needs to be further explored in patients who had received nasopharyngeal-neck RT plus systematic CT.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi:10.1016/S0140-6736(19)30956-0
2. Sun XS, Liu SL, Luo MJ, et al. The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: a cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys. 2019;105:581–590. doi:10.1016/j.ijrobp.2019.06.2549
3. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi:10.1016/j.radonc.2013.10.020
4. Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi:10.1016/j.ijrobp.2010.03.024
5. Li AC, Xiao WW, Shen GZ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget. 2015;6:24511–24521. doi:10.18632/oncotarget.4312
6. Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–270. doi:10.1016/0360-3016(92)90740-9
7. Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31:2861–2869. doi:10.1200/JCO.2012.46.0816
8. Hu J, Kong L, Gao J, Hu W, Guan X, Lu JJ. Use of radiation therapy in metastatic nasopharyngeal cancer improves survival: a SEER analysis. Sci Rep. 2017;7.
9. Chan OS, Ngan RK. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol. 2014;50:791–797. doi:10.1016/j.oraloncology.2014.01.004
10. Pan CC, Lu JIN, Yu JR, et al. Challenges in the modification of the M1 stage of the TNM staging system for nasopharyngeal carcinoma: a study of 1027 cases and review of the literature. Exp Ther Med. 2012;4:334–338. doi:10.3892/etm.2012.584
11. Shen LJ, Wang SY, Xie GF, et al. Subdivision of M category for nasopharyngeal carcinoma with synchronous metastasis: time to expand the M categorization system. Chin J Cancer. 2015;34.
12. Wang TL, Tan YO. Cisplatin and 5-fluorouracil continuous infusion for metastatic nasopharyngeal carcinoma. Ann Acad Med Singapore. 1991;20:601–603.
13. Jin Y, Shi YX, Cai XY, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138:1717–1725. doi:10.1007/s00432-012-1219-x
14. Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol. 2017;124:139–146. doi:10.1016/j.radonc.2017.03.019
15. Shuang H, Feng J, Caineng C, et al. The value of radical radiotherapy in the primary tumor of newly diagnosed oligo-metastatic nasopharyngeal carcinoma patients. Clin Transl Oncol. 2018;21:213–219. doi:10.1007/s12094-018-1911-7
16. Yin Z, Zhang X, Wang Y, Wang P, Yuan Z. The combination of systemic therapy and locoregional radiotherapy prolongs survival in newly diagnosed metastatic nasopharyngeal carcinoma patients. Onco Targets Ther. 2017;10:5677–5683. doi:10.2147/OTT.S150035
17. Hu SX, He XH, Dong M, et al. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol. 2015;32.
18. Liu JC, Bhayani M, Kuchta K, Galloway T, Fundakowski C. Patterns of distant metastasis in head and neck cancer at presentation: implications for initial evaluation. Oral Oncol. 2019;88:131–136. doi:10.1016/j.oraloncology.2018.11.023
19. Verma V, Allen PK, Simone CB, Gay HA, Lin SH. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J Natl Compr Canc Netw. 2017;15:1383–1391. doi:10.6004/jnccn.2017.7001
20. Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117–126. doi:10.1016/j.ejca.2017.02.029
21. Tian YM, Huang WZ, Lan YH, Zhao C, Bai L, Han F. Prognostic model and optimal treatment for patients with stage IVc nasopharyngeal carcinoma at diagnosis. Sci Rep. 2019;9:19272. doi:10.1038/s41598-019-55586-w
22. Shen L, Li W, Wang S, et al. Image-based multilevel subdivision of M1 category in TNM staging system for metastatic nasopharyngeal carcinoma. Radiology. 2016;280:805–814. doi:10.1148/radiol.2016151344
23. Jiang R, You R, Pei XQ, et al. Development of a ten-signature classifier using a support vector machine integrated approach to subdivide the M1 stage into M1a and M1b stages of nasopharyngeal carcinoma with synchronous metastases to better predict patients’ survival. Oncotarget. 2016;7:3645–3657. doi:10.18632/oncotarget.6436
24. Liao W, Tian M, Chen N. Characteristic and novel therapeutic strategies of nasopharyngeal carcinoma with synchronous metastasis. Cancer Manag Res. 2019;11:8431–8442. doi:10.2147/CMAR.S219994
25. You R, Liu YP, Huang PY, et al. Efficacy and safety of locoregional radiotherapy with chemotherapy vs chemotherapy alone in de novo metastatic nasopharyngeal carcinoma: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2020;6(9):1345. doi:10.1001/jamaoncol.2020.1808
26. Lin S, Tham IWK, Pan J, Han L, Chen Q, Lu JJ. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol. 2012;35:474–479. doi:10.1097/COC.0b013e31821a9452
27. Riaz N, Sherman E, Lee N. The importance of locoregional therapy in metastatic nasopharyngeal cancer. JAMA Oncol. 2020;6(9):1353. doi:10.1001/jamaoncol.2020.1793
28. Sun XS, Liang YJ, Liu SL, et al. Subdivision of nasopharyngeal carcinoma patients with bone-only metastasis at diagnosis for prediction of survival and treatment guidance. Cancer Res Treat. 2019;51(4):1259–1268. doi:10.4143/crt.2018.652
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
