Back to Journals » Lung Cancer: Targets and Therapy » Volume 11
Symptomatic CNS Radiation Necrosis Requiring Neurosurgical Resection During Treatment with Lorlatinib in ALK-Rearranged NSCLC: A Report of Two Cases
Authors Zhu VW , Nagasaka M , Kubota T , Raval K, Robinette N , Armas O, Al-Holou W, Ou SI
Received 27 July 2019
Accepted for publication 20 December 2019
Published 14 January 2020 Volume 2020:11 Pages 13—18
DOI https://doi.org/10.2147/LCTT.S224991
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Joseph Locker
Viola W Zhu, 1,* Misako Nagasaka, 2, 3,* Takafumi Kubota, 4 Kunil Raval, 5 Natasha Robinette, 6 Octavio Armas, 7 Wajd Al-Holou, 8 Sai-Hong Ignatius Ou 1
1Chao Family Comprehensive Cancer Center, Division of Hematology/Oncology, Department of Medicine, University of California Irvine, Orange, CA, USA; 2Department of Oncology, Karmanos Cancer Institute/Wayne State University, Detroit, MI, USA; 3Department of Advanced Medical Innovations, St. Marianna University Graduate School of Medicine, Kawasaki, Japan; 4Department of Neurology, Sapporo Nishimaruyama Hospital, Sapporo, Japan; 5Department of Pathology, Wayne State University, Detroit, MI, USA; 6Department of Oncology, Imaging Division, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA; 7Department of Pathology, Sharp Grossmont Hospital, La Mesa, CA, USA; 8Department of Neurosurgery, Karmanos Cancer Institute/Wayne State University, Detroit, MI, USA
*These authors contributed equally to this work
Correspondence: Sai-Hong Ignatius Ou
Chao Family Comprehensive Cancer Center, Department of Medicine, Division of Hematology/Oncology, University of California Irvine, 200 Manchester Dr, Suite 410, Orange, CA 92868, USA
Tel +1714-456-8104
Fax +1714-456-2242
Email [email protected]
Abstract: Central nervous system (CNS) metastasis carries a significant morbidity and mortality in anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC). Next-generation ALK tyrosine kinase inhibitors (TKIs) are highly CNS-penetrant and have demonstrated remarkable intracranial activity across clinical studies, and yet radiation remains the mainstay of treatment modality against CNS metastasis. We have previously reported alectinib can induce CNS radiation necrosis even after a remote history of radiation (7 years post-radiation). Lorlatinib is another potent next-generation ALK TKI that can overcome many ALK resistance mutations and has been shown to have excellent activity in patients with baseline CNS metastasis. Here we report two ALK-rearranged NSCLC patients who developed radiation necrosis shortly after initiating lorlatinib following progression on the sequential treatment of crizotinib, alectinib, and brigatinib. In both cases, radiation necrosis is evidenced by serial MRI images and histological examination of the resected CNS metastasis that had previously been radiated. Our cases highlight the importance of recognizing CNS radiation necrosis that may mimic disease progression in ALK-rearranged NSCLC treated with and potentially precipated by next-generation ALK TKIs.
Keywords: CNS, radiation necrosis, lorlatinib, ALK-rearranged NSCLC, alectinib
Introduction
Central nervous system (CNS) metastasis carries a significant morbidity and mortality in anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC). Among the three most common receptor tyrosine kinase (RTK) fusions in NSCLC (ALK, ROS1, RET), ALK-rearranged NSCLC has the highest cumulative incidence of CNS metastasis over the course of the disease process with a projected cumulative incidence of 65% at 6 years from diagnosis.1 Lorlatinib is a highly CNS-penetrant and potent third-generation ALK tyrosine kinase inhibitor (TKI) that can overcome almost all of the crizotinib-resistant ALK mutations including C1156Y, I1171N/S/T, F1174C, L1196M, D1203N, G1269A, as well as the solvent front mutation G1202R.2,3 Given its excellent CNS penetration, the efficacy of lorlatinib has been observed even in patients with CNS metastasis after disease progression on other second-generation ALK TKIs.4,5 Lorlatinib has been approved by the US FDA in November 2018 for the treatment of advanced ALK-rearranged NSCLC after progression on first-line alectinib or ceritinib or after crizotinib and at least one other ALK TKI.6 While oligometastatic CNS metastasis could be considered for surgical resection when anatomically feasible, lung cancer patients are oftentimes found with multiple CNS metastases that are typically treated with radiation (whole brain or stereotactic radiation). Thus, lorlatinib is frequently used in ALK-rearranged NSCLC patients with previously radiated CNS metastasis. Here we report two cases of radiation necrosis requiring neurosurgical resection during lorlatinib treatment following disease progression on second-generation ALK TKIs.
Case Presentation
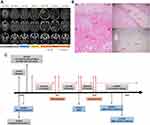
Case 1 patient is a 45-year-old Caucasian woman with a former light-smoking history who presented with stage IV lung adenocarcinoma including CNS metastasis, bilateral lung involvement, and left iliac bony metastasis. Stereotactic radiosurgery (SRS) was given to the right frontal lobe, right occipital lobe, and right cerebellar lesions (2000 cGy in a single fraction to each lesion; Figure 1A). Molecular profiling of the lung mass was positive for ALK by FISH (62% split signal) and negative for activating EGFR mutations and ROS1 fusion. Crizotinib at 250 mg twice daily was started but it was switched to alectinib 9 months later due to CNS progression. However, after being on alectinib for 6 months, CNS progression continued with worsening known brain lesions (Figure 1A) and a new left frontal lobe lesion for which whole-brain radiation (WBRT) was given (3750 cGy in fifteen fractions). Alectinib was held during WBRT and then switched to brigatinib which achieved stable CNS control for 4 months until a new left cerebellar lesion appeared. This lesion subsequently resolved 2 months after switching to lorlatinib, but 3 additional months later the patient presented with headaches, nausea, and vomiting and was found to have worsening edema around the right frontal lobe lesion (Figure 1A). She underwent neurosurgical resection of this lesion and pathology was consistent with radiation necrosis (Figure 1B). Lorlatinib was held for 1 week peri-operatively. Six months after resuming lorlatinib, the patient again developed headaches due to worsening edema around the right occipital lobe lesion (Figure 1A). Pathology from the second resection was again consistent with radiation necrosis (Figure 1B). The patient has remained on lorlatinib since. Throughout the treatment course, her extracranial disease was responding to various ALK TKIs with a confirmed partial response (PR).
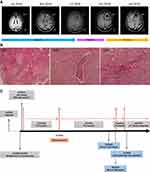
Case 2 patient is a 75-year-old never-smoking African American woman who was diagnosed with stage IV lung adenocarcinoma in October 2006. She had received multiple lines of chemotherapy until July 2014 when a biopsy of a liver lesion revealed an EML4-ALK variant 1 fusion. She achieved a PR on crizotinib for 16 months. Due to CNS progression, crizotinib was switched to alectinib and she achieved a PR for another 27 months until further progression of a left frontal lobe lesion (Figure 2A). SRS was given to this lesion (1800 cGy in single fraction) and three other small lesions while continuing alectinib. Three months later, she received additional SRS (2000 cGy in single fraction each) to six new asymptomatic small CNS lesions. Subsequently, alectinib was switched to brigatinib but the first surveillance scan after 7 weeks of treatment with brigatinib revealed an increase in the size of the left frontal lobe lesion with increased surrounding edema (Figure 2A), along with three additional punctate enhancing lesions. Within 2 weeks of switching from brigatinib to lorlatinib, the patient developed altered mental status due to further increased edema around the left frontal lobe lesion (Figure 2A). Pathology from resection of this lesion revealed radiation necrosis (Figure 2B). Currently, she is on a reduced dose of lorlatinib at 50 mg once daily. The schematic summary of the treatment course of each case is shown in Figures 1C and 2C respectively. Both patients have provided a written informed consent to have the case details published and institutional approval was not required.
Discussion
Case 1 illustrates a relatively common clinical scenario with RTK fusion-driven lung cancer where there may be continuous intracranial progression despite extracranial disease response with various ALK TKIs. Repeated radiation is often delivered to treat new and/or progressing CNS metastasis. In the randomized ALEX trial comparing the first-line crizotinib with alectinib, the cumulative incidence of CNS metastasis at 12 months was 8.6% for alectinib and 50.4% for crizotinib among patients who had received prior radiation for CNS metastasis.7 Lorlatinib has demonstrated significant intracranial activity even after disease progression on alectinib or ceritinib with an intracranial response rate of 63.0% and a median duration of intracranial response of 14.5 months in ALK-rearranged NSCLC with exposure to at least one prior ALK TKI.5 We have previously reported that radiation necrosis necessitating neurosurgical resection could occur with alectinib.8,9 In one case, radiation necrosis occurred after 7 years from the last radiation treatment and 12 months into alectinib treatment.9 While radiation necrosis did not occur during alectinib treatment in our case 1 patient, symptomatic radiation necrosis occurred twice both requiring neurosurgical resection during lorlatinib treatment with the second episode of radiation necrosis occurring after 11 months into lorlatinib treatment. Radiation necrosis is a known side effect of radiation treatment.10,11 While in case 1 both radiation necrosis could have been attributed to radiation itself, we hypothesize that lorlatinib may have conceivably accelerated the development of radiation necrosis. Due to its potent ALK inhibitory activity, any residual tumor growth in previously radiated CNS metastasis could have been inhibited by lorlatinib potentially even triggering further tumor necrosis. In case 1, we could not rule out the possibility of radiation necrosis starting at the time of switching from alectinib to brigatinib, but the appearance of three new punctate lesions necessitated the transition from brigatinib to lorlatinib due to limited clinical efficacy data on sequencing from alectinib to brigatinib.12 Radiation necrosis rapidly developed 2 weeks after initiating lorlatinib. As such, there remains a possibility of lorlatinib accelerating the occurrence of radiation necrosis potentially initiated during brigatinib treatment.
Concurrent use of EGFR TKIs with upfront WBRT to SRS has been identified as a significant risk factor for developing radiation necrosis.13 Interestingly, ALK-rearranged NSCLC has also been identified as a risk factor for developing radiation necrosis (hazard ratio of 6.36).14 Large-scale multi-institutional analyses will be required to determine whether the increased risk of radiation necrosis in ALK-rearranged NSCLC is the result of inherent tumor biology, a high incidence of CNS metastasis, repeated CNS radiation, systemic treatment with highly CNS-penetrant ALK TKIs, or a combination of these factors. When feasible, upfront use of CNS-penetrant ALK TKIs prior to radiation even in patients with untreated large or symptomatic CNS metastasis should be explored.15
Conclusion
With the advent of multiple ALK TKIs, the median overall survival of ALK-rearranged NSCLC patients treated with sequential ALK TKIs can be up to 7.5 years;16 and the median overall survival from stage IV disease can be at least 6.8 years.17 ALK-rearranged NSCLC patients are likely to develop CNS metastasis during their disease course requiring radiation treatment. Acknowledging the fact that radiation necrosis could occur during a later line of lorlatinib treatment is critical as it often mimics disease progression which may lead to premature discontinuation of lorlatinib.
Disclosure
VWZ has received honoraria from AstraZeneca, Biocept, Roche-Foundation Medicine, Roche/Genentech, Takeda, and consulting fees from TP Therapeutics, outside the submitted work. MN has received honoraria from AstraZeneca and Tempus, outside the submitted work. SIO has previously served on the Scientific Advisory Board of Turning Point Therapeutics with stock ownership; has received honoraria from AstraZeneca, Pfizer, Merck, Novartis, Roche-Foundation Medicine, Roche/Genentech, Spectrum, Takeda, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13:1595–1601. doi:10.1016/j.jtho.2018.07.004
2. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi:10.1158/2159-8290.CD-16-0596
3. Basit S, Ashraf Z, Lee K, et al. First macrocyclic 3rd-generation ALK inhibitor for treatment of ALK/ROD1 cancer: clinical and designing strategy update of lorlatinib. Eur J Med Chem. 2017;134:348–356. doi:10.1016/j.ejmech.2017.04.032
4. Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28:70–81. doi:10.1016/j.ccell.2015.05.010
5. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global Phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi:10.1016/S1470-2045(18)30649-1
6. Syed YY. Lorlatinib: first global approval. Drugs. 2019;79:93–98. doi:10.1007/s40265-018-1041-0
7. Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naïve anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29:2214–2222. doi:10.1093/annonc/mdy405
8. Ou SH, Klempner SJ, Azada MC, et al. Radiation necrosis presenting as pseudoprogression (PsP) during alectinib treatment of previously radiated brain metastases in ALK-positive NSCLC: implications for disease assessment and management. Lung Cancer. 2015;88:355–359. doi:10.1016/j.lungcan.2015.03.022
9. Ou SH, Weitz M, Jalas JR, et al. Alectinib induced CNS radiation necrosis in an ALK + NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer. 2016;96:15–18. doi:10.1016/j.lungcan.2016.03.008
10. Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8:395. doi:10.3389/fonc.2018.00395
11. Sneed PK, Mendez J, Vemer-van den Hoek JM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123:373–386. doi:10.3171/2014.10.JNS141610
12. Lin JJ, Zhu VW, Schoenfeld AJ, et al. Brigatinib in patients with alectinib-refractory ALK-positive NSCLC. J Thorac Oncol. 2018;13:1530–1538. doi:10.1016/j.jtho.2018.06.005
13. Kim JM, Miller JA, Kotecha R, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol. 2017;133:357–368. doi:10.1007/s11060-017-2442-8
14. Miller JA, Bennett EE, Xiao R, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96:1060–1069. doi:10.1016/j.ijrobp.2016.08.039
15. Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of alectinib in patients with ALK-positive NSCLC and symptomatic or large CNS metastases. J Thorac Oncol. 2019;14:683–690. doi:10.1016/j.jtho.2018.12.002
16. Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;83:21903–21917.
17. Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14:691–700. doi:10.1016/j.jtho.2018.12.014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.