Back to Journals » Journal of Inflammation Research » Volume 15
Sympathectomy Ameliorates CFA-Induced Mechanical Allodynia via Modulating Phenotype of Macrophages in Sensory Ganglion in Mice
Authors Mai L, Jia S, Liu Q, Chu Y, Liu J, Yang S, Huang F , Fan W
Received 1 September 2022
Accepted for publication 3 November 2022
Published 11 November 2022 Volume 2022:15 Pages 6263—6274
DOI https://doi.org/10.2147/JIR.S388322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Lijia Mai,1,2,* Shilin Jia,1,2,* Qing Liu,3 Yanhao Chu,2 Jinyue Liu,2 Shengyan Yang,3 Fang Huang,1,2 Wenguo Fan1,2
1Hospital of Stomatology, Guanghua School of Stomatology, Sun Yat-sen University, Guangzhou, People’s Republic of China; 2Institute of Stomatological Research, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Stomatology, Guangzhou, People’s Republic of China; 3Faculty of Dentistry, The University of Hong Kong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenguo Fan; Fang Huang, Guanghua School of Stomatology, Sun Yat-sen University, No. 74 Zhongshan Rd 2, Guangzhou, 510080, People’s Republic of China, Tel +86 20 87330570, Fax +86 20 87330709, Email [email protected]; [email protected]
Background: The sympathetic nervous system (SNS) is suggested to be involved in some forms of pain, but the mechanisms of which are incompletely known. Moreover, there is a lack of information on the regulatory role of the SNS on macrophages in sensory ganglion, which plays an important role in pain development. The present study aims to investigate the effects of the SNS on orofacial inflammatory pain and examine, if any, how the SNS influences trigeminal ganglion (TG) macrophage responses.
Methods: Sympathectomy was performed on male C57BL/6 mice before receiving a local injection of Complete Freund’s adjuvant (CFA) to induce inflammatory pain. Effects of sympathectomy on orofacial pain were examined by Von Frey test and c-Fos expression. Polarization of TG macrophage was evaluated by immunohistochemistry and the level of norepinephrine (NE) in the TG were determined by liquid chromatography. Sympathetic signaling to TG macrophages were predicted based on single-cell analysis.
Results: CFA injection induced a significant increase in mechanical allodynia, the number of c-Fos-positive neuron, and the level of NE in TG, which were largely reduced by sympathectomy. The number of M1 macrophages was markedly increased by CFA and was largely reduced by sympathectomy from 1 to 14 days post-injection. Single-cell RNA sequencing analysis and immunofluorescence staining showed that TG macrophages mainly express β 2 adrenergic receptors for NE. Cell–cell communication analysis predicted sympathetic signaling that may modulate macrophage phenotypes, including Colony-stimulating factor-1, Migration inhibitory factor, Pleiotrophin and Nicotinamide phosphoribosyl transferase.
Conclusion: The SNS may involve in CFA-induced mechanical allodynia via modulating macrophage phenotypes in the TG. Targeting sympathetic activation might be useful in treating some painful conditions in the orofacial region.
Keywords: trigeminal ganglion, orofacial pain, sympathectomy, macrophages phenotype, neuroinflammation
Introduction
Pain is a hallmark of inflammation that can be either protective or detrimental during the acute or chronic stage.1 The cell bodies of nociceptors are located in the dorsal root ganglion (DRG) and the trigeminal ganglion (TG) for the body and orofacial region, respectively. They are responsible for transmission of nociceptive information from their target organ to the central nervous system.2 Interactions between neurons and non-neuronal cells in the primary sensory ganglion is believed to be involved in pain mechanisms.3 Recent works have emphasized the importance of macrophage of two different phenotypes infiltrated in the DRG in the development and resolution of chronic pain.4,5 As macrophages are highly heterogeneous, the activated macrophage may exhibit a pro-inflammatory (M1 phenotype) or an anti-inflammatory (M2 phenotype) profile depending on the micro-environment.6 However, few studies have explored the mechanisms that regulate the polarization of ganglionic macrophages in a chronic pain models of inflammatory pain.
The sympathetic nervous system (SNS) is part of the autonomic nervous system, playing an important role in maintaining many painful conditions.7 For example, chemical or surgical sympathectomy can effectively reduce neuropathic pain,8,9 postoperative pain,10 visceral pain,11 cancer-related pain,12 limb ischemic pain, complex regional pain syndrome (CRPS), pain related to postherpetic neuralgia13,14 and peripheral inflammation.15 Previous studies suggested that the SNS might involve in the pathophysiology of pain via regulating the nociceptors and the immune cells in the peripheral.16 In vitro studies have indicated that norepinephrine (NE), the primary neurotransmitter mediating sympathetic firing, can maintain macrophages in either an anti-inflammatory or a pro-inflammatory state depending on the concentration17,18 and the activation of its receptors.19–23 However, whether the SNS mediates the phenotype of TG macrophage through noradrenergic signaling, in a painful condition has not been determined.
Therefore, the present study aims to investigate the effects of the SNS on chronic pain and examine the role, if any, of the SNS in regulating the phenotype of macrophage in the sensory ganglion. We demonstrated the effects of sympathectomy on Complete Freund’s adjuvant (CFA)-induced pain-like behaviors and neuronal activation in the central nervous system. We also described changes of macrophage phenotype in the TG in the context of orofacial pain and the potential role and mechanisms of sympathetic nerves in this regulation.
Methods
Animals
A total of 98 adult male C5BL/6 mice (aged 6–8 weeks, weighed 20–30 g) were used in our experiments and were randomly assigned to each group (control, CFA and sympathectomy or SYM group, Supplemental Table S1). The animals were housed in cages in a 12:12 light/dark cycle with food and water provided ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University and carried out in accordance with approved guidelines (No. 2020000245).
Sympathectomy and Inflammatory Pain Models
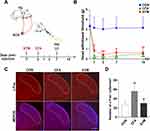
To perform superior ganglion sympathectomy, mice assigned to the SYM group were anesthetized with 1% pentobarbital sodium (50 mg/kg). A vertical incision was made on the ventral surface of the neck adjacent to the midline. The right superior cervical ganglion (SCG) was exposed and carefully removed. Mice from the other two groups received sham surgery involving anesthesia and SCG exposure but not dissection. The incision site was thereafter sutured. Analgesics and antibiotics were administered for 3 days post-operatively. After post-operative recovery for 7 days, mice from the control group received a subcutaneous injection of 20 μL of 0.9% NaCl solution while the CFA and SYM groups were injected with 20 μL of CFA (Sigma Aldrich, USA) into the right whisker pad (Figure 1A).
Behavioral Testing
For evaluating mechanical allodynia, 8 mice were chosen randomly at each time point per group. Mechanical allodynia was assessed using the von Frey hairs stimulation before CFA or saline injection as a baseline and at 1-, 3-, 7- and 14-days post-injection (dpi). After a period of acclimation, a series of von Frey hairs (North Coast, USA) was applied to the right whisker pad for a maximum of 5 seconds. A positive response was defined as an acute head withdrawal or shaking. Testing began with the application of the 0.6 g hair and progressed according to an up-down method.24
Sample Preparation
Mice were deeply anesthetized with 1% pentobarbital sodium (50 mg/kg) and transcardially perfused with 30 mL ice-cold phosphate-buffered saline (PBS) followed by 30 mL of fixative (4% paraformaldehyde) at 0, 1, 3, 7 and 14 dpi. The right TG and the spinal trigeminal nucleus caudalis (SpVc, 7.5–8.5 mm posterior to the bregma)25 were collected and post-fixed in the same fixative for 12 hours. The post-fixed TGs were dehydrated in graded ethanol, embedded in paraffin, and stored at 4°C. TG and SpVc were cryoprotected in 30% sucrose for 48 hours before embedded in Tissue-Tek® OCT compound (Sakura, USA).
Immunohistochemistry
To investigate macrophage polarization in the TG, immunohistochemistry was done to detect CD163- and CD86-positive cells using the Streptavidin-HRP kit (Cwbio, China) according to the manufacturer’s instructions. In brief, paraffin-embedded TG sections (4.5 μm thick) were deparaffinized, heat retrieved, blocked, and thereafter incubated with primary antibody (Table 1) at 4°C overnight. Then, the sections were incubated with HRP-conjugated secondary antibodies. Signals were visualized using diaminobenzidine. The distribution of c-Fos positive neurons in the SpVc was detected by immunofluorescence. To validate the distribution of β2-AR (adrenoceptor) expressed on TG macrophage, double-staining was performed for CD68 and ADRB2 in the control group. The frozen samples were prepared into 40-μm (for SpVc) or 12-μm (for TG) thick, blocked with 10% goat serum for 1 hour at room temperature. Slices were incubated with primary antibody for 24 hours at 4°C followed by incubation with the secondary antibody for 1 hour. Z series stacks were captured using a confocal laser scanning microscope (FV3000, Olympus, Japan). Images were analyzed by using NIH ImageJ software (version 1.60).
 |
Table 1 Antibodies for Immunochemical Staining |
The region of interest was located in the superficial layer (laminae I/II) of SpVc and the maxillary division of the TG ipsilateral to the injection side. The area of immunoreactive cells was counted manually from five fields of view at ×40 magnification in 4 sections for each animal (n = 4).
Norepinephrine Measurements
NE concentration in TG was measured using liquid chromatography (Agilent, USA) as previously described.26 Briefly, mice (n = 6 per group) were anesthetized and transcardially perfused with 30 mL ice-cold PBS at 7 dpi. The TGs were isolated, weighed, and homogenized at 4°C by a Vibra-Cell ultrasonic processor supplemented with 500 μL 0.14% sodium heptane sulfonate solution (pH 3.0 ± 0.1) five times for 10s with intervals of 10s at 60 Hz. The mixture was centrifuged at 4°C, 12,000 rpm for 15 minutes. The supernatant was collected and sonicated before being injected onto the liquid chromatography system employing the fluorescence method. Quantification was achieved by a series of standard solutions.
Transcriptomic Analysis of TG Macrophages and SCG Neurons
Online single-cell RNA-sequencing (scRNA-seq) data (No. GSE175421 and GSE186421) processing was processed by the Seurat R package (version 4.0.2). Low-quality cells were filtered before normalization by performing Seurat’s “NormalizeData” function. The top 3000 highly variable genes were identified and used for principal component (PC) analysis. The top 15 PCs were selected for nonlinear dimensionality reduction using Uniform Manifold Approximation and Projection (UMAP), which generated unsupervised identification of clusters based on shared nearest neighbor clustering algorithm.
Cluster-specific marker genes was obtained using “FindAllMarkers” function with parameters average log2 fold change (avg_log2FC) > 2, expressed ratio > 0.6 and P < 0.001 (Wilcoxon Rank Sum test). Cell types were manually annotated based on marker genes of each cluster and the automatic cell annotation tool SingleR (version 1.4.1) and Celldex R package (version 1.0.0).
Cell-Cell Communication Analysis
Signaling from SCG neurons to TG macrophages was inferred from scRNA-seq data using CellChat R package (version 1.1.3). In brief, the average expression levels of ligand-receptor pairs between two cell types were analyzed based on the CellChat database that takes into account known composition of the ligand-receptor complexes. Genes expressed in less than 10% of cells in one cell type were excluded, and only P value less than 0.01 are considered significant.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0. Data are presented as mean ± standard deviation. Two-group differences were determined using Student’s t-test. Multigroup comparisons were analyzed using one-way ANOVA with Dunnett t’s post hoc test. Ratios were compared using the Chi squared test. P values of less than 0.05 were of statistical significance.
Results
Sympathectomy Improves CFA-Induced Orofacial Pain Hypersensitivity in Male Mice
Successful removal of SCG was confirmed by Horner’s syndrome ipsilateral to the operation side in mice. To assess the involvement of sympathectomy in the pathogenesis of orofacial pain, a pain model was induced by subcutaneous injection of CFA 7 days after sympathectomy. Behavioral assays were performed at 0, 1, 3, 7 and 14 dpi to assess withdrawal responses in mice (one-way ANOVA, n = 8 per group).
Before implementing the CFA-induced animal model, no statistically significant difference was observed in baseline mechanical withdrawal threshold between sham surgery and the sympathectomy group. Sham control animals injected with CFA showed a significant reduction in the withdrawal threshold to mechanical stimuli as early as 1 dpi, which persisted for at least 14 days (P < 0.001). In comparison, mice receiving sympathectomy followed by CFA injection showed marked reduction of mechanical allodynia at 1, 3 and 7 dpi (P < 0.05 at 1 and 7 dpi, P < 0.01 at 3 dpi), but no obvious difference was observed at 14 dpi (P = 0.65, Figure 1B).
The nociceptive information released from the TG was relayed to the second station of the pain pathway located in SpVc, whose activation is associated with central sensitization. Thus, we examined the number of c-Fos positive neurons in SpVc by immunostaining (one-way ANOVA, n = 4 per group). The results showed a higher abundance of c-Fos neurons after CFA injection compared to the saline-injected group (56.6 ± 6.4 versus 27.2 ± 4.3, P < 0.01). In contrast, sympathectomy resulted in a significant reduction in the number of c-Fos positive neuron (30.4 ±3.1), compared to the sham control (P < 0.05, Figure 1C and D).
Polarization Patterns of Ganglionic Macrophages in Response to Orofacial Inflammation and Sympathectomy Over Time
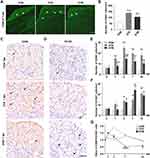
To assess the effects of the SNS on macrophage activation in a chronic pain condition, we detected changes in macrophage immunoreactive for CD68 in TG at 7 dpi (one-way ANOVA, n = 4). CD68 has been indicated as a marker of all the activated macrophages in the sensory ganglion.27 The results showed that CFA administration significantly increase the number of macrophages (compared to the saline control, P < 0.0001). Sympathectomy markedly reduced the number of macrophages infiltrated in the TG after the induction of orofacial inflammation (105.7 ± 7.4 in SYM group versus 138.1 ± 5.7 in CFA group, P < 0.01, Figure 2A and B).
To further investigate the change of the macrophage phenotype in the TG driven by sympathetic activity in an inflammatory pain condition, immunohistochemistry was performed to detect M1 or M2 macrophage using antibodies against CD86 and CD163, respectively (one-way ANOVA, n = 4 per time point). As reported previously, polarized macrophages in the sensory ganglion can be detected by CD86 (marks M1 phenotype) and CD163 (marks M2 phenotype).28,29
Sham surgery or surgical sympathectomy had no effect on macrophage phenotype since no statistically significant difference was observed before CFA or saline injection (P > 0.05). The number of M1 macrophage increased from day 1, peaked at day 3, and did not recover to baseline within 2 weeks (P < 0.0001, compared to the saline-injected control). Macrophage M1 polarization resulting from CFA injection was markedly inhibited by sympathectomy from 1 to 14 dpi (P < 0.01, Figure 2C and E).
The number of M2 macrophage showed a steady increase trend from 1 to 14 dpi after the induction of inflammatory pain, as a noticeable increase of CD163 was observed in TG followed by CFA injection from 1 dpi (P < 0.01, compared to the saline-injected control).
The sympathectomy group showed a much higher accumulation of M2 macrophages compared to the CFA group at 7 dpi (14.7 ± 1.8 versus 23.7 ± 1.6, P < 0.05), but no statistically significant between sympathectomy and CFA group at 14 dpi (19.4 ± 1.0 versus 16.2 ± 1.7, P > 0.05, Figure 2D and F).
The ratio of M1/M2 in TG was evaluated from 1dpi to 14 dpi (Chi squared test). We observed a trend toward an increased M1 proportion in TG at the early stage of peripheral inflammation. The ratio of M1/M2 was markedly increased by CFA, and this was partly mitigated by prior sympathectomy from day 3 to day 14 post-injection (P < 0.05). Importantly, the CFA group showed a much higher ratio than the sympathectomy group at 7 dpi (P < 0.0001, Figure 2G). The longitudinal data gave us an overview of the changes of macrophage phenotypes in the TG in response to CFA subcutaneous injection in the orofacial region, and how macrophages respond to the inflammatory stimuli in a sympathetic-dependent manner over time.
Sympathetic-Mediated Mechanism of TG Macrophage Polarization During Peripheral Inflammation
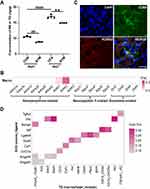
Sympathetic hyperactivity is usually accompanied by increased NE concentration.7 Therefore, to determine whether sympathetic activity acts on sensory ganglion either in a normal or an inflammatory condition, we obtained TG at 0 dpi before injection and at 7 dpi for NE measurements (n = 6 per time point). The level of NE was greatly reduced by surgical sympathectomy before CFA treatment (P < 0.0001), from a baseline mean of 10.54 ± 0.25 to 8.82 ± 0.14 ng/g. CFA administration led to a significant increase in NE level compared to the control group, reaching to a maximal mean level of 16.90 ± 0.28 ng/g while the sympathectomy group revealed a marked decrease in the NE level at 7 dpi, declined to a mean level of 14.91 ± 0.06 ng/g, compared to the CFA group (P < 0.01, Figure 3A).
We next performed transcriptional analysis to gain better insight into genes expression on SCG neurons and TG macrophages based on scRNA-seq datasets. All SCG cells were unbiasedly grouped into nine clusters, among which cluster with high expression of Dbh (encodes dopamine β-hydroxylase), Slc6a2 (encodes sodium-dependent noradrenaline transporter), Th (encodes tyrosine 3-monooxygenase), Slc18a2 (encodes Synaptic vesicular amine transporte), Npy (encodes pro-neuropeptide Y) was defined as SCG neurons (GSE175421). These highly expressed genes associated with the synthesis of NE, Epinephrine, Dopamine and Neuropeptide Y (Supplemental Table S2). We then explored the gene expression of TG macrophages. After excluding low-quality cells, we performed cluster analysis of TG cells and identified eighteen clusters (GSE186421). Cluster was labeled macrophage for the high expression of H2-Aa that encodes H-2 class II histocompatibility antigen and Ctss that encodes cathepsin (Supplemental Table S3). Receptors for neurotransmitters released from SCG neurons expressed on TG macrophages are listed in Figure 3B. Our results showed that macrophages highly and exclusively expressed Adrb2 (encodes β2-AR), with less expression of Adrb1, Npy1r and Drd1. The other receptors indicated no expression. The distribution of β2-AR on ganglionic macrophages was validated by double immunostaining for ADRB2 and CD68 (Figure 3C).
To infer signaling from SCG neurons to TG macrophages, we analyzed all the ligand-receptor pairs between two cell types based on the single-cell datasets. There are ten ligand-receptor pairs showing a statistically significant (P < 0.01) (Figure 3D, Supplemental Table S4). These unique sympathetic neuron-macrophage interactions included signaling related to Tgfb3, Ptn, Nampt, Mif, Lgals9, Gas6, Csf1, Ccl27a, Angptl2 and Angptl4. Highlighting the utility of scRNA-seq data, our analysis provided sympathetic signaling mechanisms that can be hypothesized to mediate ganglionic macrophage during orofacial inflammation.
Discussion
In the present study, we used inflammatory pain models induced by CFA and demonstrated that the SNS contributed to orofacial inflammatory pain at the sensory ganglion level. The peripheral mechanisms involved in orofacial pain might be attributed to the changes of macrophage phenotype in the TG where sympathetic outflow mediated.
Clinically, there are two main types of chronic pain, including inflammatory pain and neuropathic pain, which are initiated by tissue inflammation and nerve injury, respectively.30 As sensory ganglion are a target of the SNS neuron that has intra-ganglionic distribution,31 some orofacial pain conditions could be exacerbated by sympathetic activities, such as neuropathic pain,32 cancer pain,33 post-operative pain,10 atypical facial pain,34 CRPS,35 neurovascular pain,36 postherpetic neuralgia,37 etc. However, Bongenhielm et al found that sympathetic blocks had no effect on neuronal activation after peripheral nerve injury.38 Indeed, studies on the role of sympathetic activity regarding orofacial inflammatory pain are sporadic, and the mechanism whereby the SNS may drive the development of chronic pain has not been fully established.
In our experiments, male mice were used considering sex-related differences in nociceptor translatomes39 and infiltration of macrophage.40 Superior cervical sympathectomy was performed on mice to block sympathetic activity in the orofacial region,41 which is evidenced by Horner’s sign.42,43 The inflammatory agent CFA was capable of inducing a long-lasting inflammation locally, as previously reported.44 We observed that CFA injection caused a mechanical allodynia in mice and remained unresolved by 14 dpi. We also observed that sympathectomy markedly reduced mechanical pain-related hypersensitivity in CFA-injected mouse at 1, 3 and 7 dpi, which corresponds to pain from an acute phase to a chronic stage.45 In a rat model of local DRG inflammation, the effect of sympathectomy was evident as early as 1 dpi, and lasted throughout the experiment.15 However, the analgesic effect of sympathectomy was mitigated at 14 dpi. This may due to anti-inflammatory responses to noxious stimuli within the body. C-Fos is one of reliable molecular markers for neuronal activation in the SpVc during orofacial inflammation.46,47 Our immunofluorescence staining studies showed an increased number of c-Fos-positive neurons in the SpVc after CFA treatment, which was significantly reduced by prior sympathectomy. This result provides additional evidence of the role of sympathetic activity on pain modulation. In this study, we focused on mechanical allodynia with von Frey hairs, which is mostly used in all the pain-associated measurements,48,49 but other measures such as mechanical and thermal hyperalgesia cannot be ignored. Further studies are needed to elucidate the effect of SNS in other pain-related behaviors.
Prior evidence suggests a pivotal role of sensory ganglion, especially the activation of immune cells and the production of pro-inflammatory cytokines within the ganglion, in many painful states and nociception.3 Expansion of macrophages in the DRG has been implicated in pain development.4,50 It has been reported that sympathetic denervation by chemical or surgical sympathectomy alleviates inflammatory pain, which potentially interfere with macrophage accumulation in the DRG.15 However, the mechanisms by which sympathectomy alleviates inflammatory pain involving macrophages in the ganglion is not entirely clear.
To fill this gap in knowledge, we first measured macrophage infiltration by immunohistochemical staining for CD68, and found that CFA-induced change in the infiltration of CD68-labeled macrophages was markedly reduced by prior sympathectomy. As previously studies have found that macrophage with distinct functional infiltrated in the sensory ganglion is important for the maintenance and resolution of pain,51–53 we thereafter detected two genes, CD86 and CD163—two annotated markers that represent distinct functional outcomes in the course of inflammatory pain.54 We found that orofacial inflammation caused a significant increase in both M1 and M2 infiltration in the TG at early days, but the proportion of M2 was significantly lower than that of M1 for 2 weeks in the CFA model. We observed the ratio of M1/M2 was relatively high during the early phases, whereas macrophage transit from a M1-like phenotype to M2 at later time points. This result is consistent with those observed in previous studies.5,55,56 Minimal changes were observed between sympathectomy and sham surgery group before CFA treatment, suggesting sympathectomy has no significant effect on macrophage phenotypic transition. Prior sympathectomy significantly reduced M1 macrophage infiltrated in TG from 1 to 14 dpi, suggesting that the SNS may promote M1 polarization in the TG during orofacial inflammation. Notably, the ratio of M1/M2 in sympathectomized group was much lower than that in CFA group at 7 dpi, suggesting that sympathetic regulation on the phenotypic change of TG macrophage was evident at this time. Meanwhile, during the chronic phase of inflammation, we observed an increased proportion of M2 macrophage at 14 dpi, which may reduce the anti-inflammatory effect of sympathectomy.
As one of the main neurotransmitters released by the SNS, NE is involved in autonomic regulation of body that keeps physically active.7 Catecholamine receptors mediating the effects of NE and epinephrine are classically divided into two main categories, α- and β-ARs. α-Adrenoceptors are classified into subtypes α1A, α1B, α1D, α2A, α2B, and α2C, and β-ARs into subtypes β1, β2, and β3.57 It was recently reported that NE was a key neurotransmitter necessary for DRG cluster firing and spontaneous pain following peripheral nerve injury.58 In our study, we observed that sympathectomy resulted in a significant reduction in the NE level in TG, which was upregulated after CFA injection. This observation can be a result of an enhanced sympathetic activity in TG after the induction of orofacial inflammatory pain. However, CFA injection also upregulated the NE concentration in TG of sympathectomized mice. We speculated that CFA injection may increase excitability of the SNS systemically, which stimulates the secretion of NE from adrenal medulla into blood flow,59 resulting in local retention of NE in TG.
To further expand our knowledge about the mechanisms of sympathetic modulation on the phenotype of TG macrophage, we first performed clustering analysis on TG and SCG based on single-cell RNA sequencing datasets. Cluster analysis of SCG identified the subpopulation that highly expressed marker gene for sympathetic neuron (Th) and genes related to the synthesis of sympathetic neurotransmitters, including catecholamines and neuropeptides.60 Macrophages were identified from the TG cells for the high expression of H2-Aa, Ctss and Cd74 related to antigen presentation.61 We found Adrb2 (encoded β2-AR) was highly expressed on Cluster 8, consistent with those reported previously that most myeloid cells mainly express β-AR.62 Double immunofluorescence provided further evidence for the single-cell analysis at the protein level. Our findings indicated that NE/β2-AR signaling may involve in regulating TG macrophage in response to orofacial inflammation, considering that macrophage phenotype and cytokine expression can be modified by NE concentration.18 The activation of β2-AR signaling could help to mitigate immune suppression63 and promote M2 polarization.64
As mentioned above, many studies focus on NE/ARs signal, ignoring other sympathetic cotransmitters that may be released from postganglionic sympathetic terminals into the TG and can potentially modulate phenotype of macrophages. The present study found that ten ligand-receptor pairs in SCG neurons and TG macrophages, among which Pleiotrophin or PTN expression has been implicated in promoting immune responses by inducing leukocyte migration and inflammatory cytokines expression, but not in macrophages.65 The binding of migration inhibitory factor (MIF)-CD74 leads to an increase in the expression of pro-inflammatory cytokines from macrophages.66 Colony-stimulating factor-1 (CSF1) signaling has been indicated in neuropathic pain, whose activation is involved in macrophage proliferation and pro-inflammatory responses.67 Increased expression of nicotinamide phosphoribosyl transferase (NAMPT) promotes M1 polarization after inflammatory activation.68,69 Transforming growth factor β3 (Tgfb3)70 and angiopoietin-related protein 4 (Angptl4)71 both play an inhibitory role in the polarization and proinflammatory effects of macrophage. Our study has provided further evidence that the sympathetic signaling such as CSF1, MIF, PTN and NAMPT may act on receptors on the TG macrophages and modulate their phenotypes, in addition to NE. Future studies will be required to better understand the pain mechanism contributed by sympathetic firing.
Conclusions
The present study suggests that the SNS may involve in orofacial inflammatory pain and the regulation of TG macrophage. Our study also indicates that the phenotypic transition of TG macrophages during the initiation and development of pain, is, at least partly, driven by sympathetic nerves. The data merit new attention to the role of the SNS in the pathogenesis of inflammatory pain conditions.
Data Sharing Statement
The single-cell RNA sequencing (scRNA-seq) datasets were downloaded from https://www.ncbi.nlm.nih.gov/geo/ (No. GSE175421 and GSE186421).
Funding
This research was supported in part by the National Natural Science Foundation of China (No. 81771098 and 81870737).
Disclosure
The authors declare no competing interests in this work.
References
1. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982.
2. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284.
3. Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577.
4. Raoof R, Martin Gil C, Lafeber F, et al. Dorsal root ganglia macrophages maintain osteoarthritis pain. J Neurosci. 2021;41(39):8249–8261.
5. van der Vlist M, Raoof R, Willemen H, et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron. 2022;110(4):613–626.e619.
6. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs functional differentiation. Front Immunol. 2014;5:514.
7. Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol. 2018;36:783–812.
8. Zhu X, Xie W, Zhang J, Strong JA, Zhang JM. Sympathectomy decreases pain behaviors and nerve regeneration by downregulating monocyte chemokine CCL2 in dorsal root ganglia in the rat tibial nerve crush model. Pain. 2022;163(1):e106–e120.
9. Xie W, Strong JA, Zhang JM. Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain. 2020;161(8):1925–1936.
10. Kawabata K, Sago T, Oowatari T, Shiiba S. Prolonged blockade of the cervical sympathetic nerve by stellate ganglion block accelerates therapeutic efficacy in trigeminal neuropathy. J Oral Sci. 2022;64(1):6–10.
11. Gil DW, Wang J, Gu C, Donello JE, Cabrera S, Al-Chaer ED. Role of sympathetic nervous system in rat model of chronic visceral pain. Neurogastroenterol Motil. 2016;28(3):423–431.
12. Spiegel MA, Hingula L, Chen GH, Legler A, Puttanniah V, Gulati A. The use of L2 and L3 lumbar sympathetic blockade for cancer-related pain, an experience and recommendation in the oncologic population. Pain Med. 2020;21(1):176–184.
13. Duong S, Bravo D, Todd KJ, Finlayson RJ, Tran Q. Treatment of complex regional pain syndrome: an updated systematic review and narrative synthesis. Can j Anaesth. 2018;65(6):658–684.
14. Doroshenko M, Turkot O, Horn DB. Sympathetic nerve block. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
15. Xie W, Chen S, Strong JA, Li AL, Lewkowich IP, Zhang JM. Localized sympathectomy reduces mechanical hypersensitivity by restoring normal immune homeostasis in rat models of inflammatory pain. J Neurosci. 2016;36(33):8712–8725.
16. Fan W, Zhu X, He Y, et al. Peripheral sympathetic mechanisms in orofacial pain. J Pain Res. 2018;11:2425–2431.
17. Cong Z, Li D, Lv X, et al. α2A-adrenoceptor deficiency attenuates lipopolysaccharide-induced lung injury by increasing norepinephrine levels and inhibiting alveolar macrophage activation in acute respiratory distress syndrome. Clin Sci. 2020;134(14):1957–1971.
18. Willemze RA, Welting O, van Hamersveld P, et al. Loss of intestinal sympathetic innervation elicits an innate immune driven colitis. Mol Med. 2019;25(1):1.
19. Ağaç D, Estrada LD, Maples R, Hooper LV, Farrar JD. The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun. 2018;74:176–185.
20. Arora V, Morado-Urbina CE, Gwak YS, et al. Systemic administration of a β2-adrenergic receptor agonist reduces mechanical allodynia and suppresses the immune response to surgery in a rat model of persistent post-incisional hypersensitivity. Mol Pain. 2021;17:1744806921997206.
21. Xin JZ, Wu JM, Hu GM, et al. α(1)-AR overactivation induces cardiac inflammation through NLRP3 inflammasome activation. Acta Pharmacol Sin. 2020;41(3):311–318.
22. Huang JL, Zhang YL, Wang CC, et al. Enhanced phosphorylation of MAPKs by NE promotes TNF-α production by macrophage through α adrenergic receptor. Inflammation. 2012;35(2):527–534.
23. Liu D, Xu X, Dai Y, et al. Blockade of AIM2 inflammasome or α1-AR ameliorates IL-1β release and macrophage-mediated immunosuppression induced by CAR-T treatment. J Immunother Cancer. 2021;9(1):e001466.
24. Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26.
25. Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates, Compact. Academic Press; 1997.
26. Jung-Klawitter S, Kuseyri Hübschmann O. Analysis of catecholamines and pterins in inborn errors of monoamine neurotransmitter metabolism-from past to future. Cells. 2019;8(8):897.
27. Austah ON, Lillis KV, Akopian AN, Harris SE, Grinceviciute R, Diogenes A. Trigeminal neurons control immune-bone cell interaction and metabolism in apical periodontitis. Cell Mol Life Sci. 2022;79(6):330.
28. Iwai H, Ataka K, Suzuki H, et al. Tissue-resident M2 macrophages directly contact primary sensory neurons in the sensory ganglia after nerve injury. J Neuroinflammation. 2021;18(1):227.
29. Yuan K, Zheng J, Shen X, et al. Sensory nerves promote corneal inflammation resolution via CGRP mediated transformation of macrophages to the M2 phenotype through the PI3K/AKT signaling pathway. Int Immunopharmacol. 2022;102:108426.
30. Jiang BC, Liu T, Gao YJ. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 2020;212:107581.
31. Kummer W. Sensory ganglia as a target of autonomic and sensory nerve fibres in the Guinea-pig. Neuroscience. 1994;59(3):739–754.
32. Kohjitani A, Miyawaki T, Kasuya K, Shimada M. Sympathetic activity-mediated neuropathic facial pain following simple tooth extraction: a case report. Cranio. 2002;20(2):135–138.
33. Darabad RR, Kalangara JP, Woodbury A. Case series: cancer-related facial pain treated with stellate ganglion block. Palliat Med Rep. 2020;1(1):290–295.
34. Jeon Y, Kim D. The effect of stellate ganglion block on the atypical facial pain. J Dental Anesth Pain Med. 2015;15(1):35–37.
35. Lee YH, Lee KM, Kim HG, et al. Orofacial complex regional pain syndrome: pathophysiologic mechanisms and functional MRI. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(2):e164–e170.
36. Noma N, Kamo H, Nakaya Y, et al. Stellate ganglion block as an early intervention in sympathetically maintained headache and orofacial pain caused by temporal arteritis. Pain Med. 2013;14(3):392–397.
37. Sinofsky A, Sharma T, Wright T. Stellate ganglion block for debilitating photophobia secondary to trigeminal, postherpetic neuralgia. Pain Pract. 2016;16(7):e99–e102.
38. Bongenhielm U, Yates JM, Fried K, Robinson PP. Sympathectomy does not affect the early ectopic discharge from myelinated fibres in ferret inferior alveolar nerve neuromas. Neurosci Lett. 1998;245(2):89–92.
39. Tavares-Ferreira D, Ray PR, Sankaranarayanan I, et al. Sex differences in nociceptor translatomes contribute to divergent prostaglandin signaling in male and female mice. Biol Psychiatry. 2022;91(1):129–140.
40. Liu L, Karagoz H, Herneisey M, et al. Sex differences revealed in a mouse CFA inflammation model with macrophage targeted nanotheranostics. Theranostics. 2020;10(4):1694–1707.
41. Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6(3):1239–1278.
42. Kubota K, Sunada K. Changes in blood flow at the mandibular angle and Horner syndrome in a rat model of superior cervical ganglion block. J Dental Anesth Pain Med. 2018;18(2):105–110.
43. Khan Z, Bollu PC. Horner syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
44. Araújo-Filho HG, Pereira EWM, Campos AR, Quintans-Júnior LJ, Quintans JSS. Chronic orofacial pain animal models - progress and challenges. Expert Opin Drug Discov. 2018;13(10):949–964.
45. Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248.
46. Romero-Reyes M, Akerman S, Nguyen E, et al. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache. 2013;53(1):137–151.
47. Imbe H, Dubner R, Ren K. Masseteric inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845(2):165–175.
48. Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009;10(7):767–773.
49. Sadler KE, Mogil JS, Stucky CL. Innovations and advances in modelling and measuring pain in animals. Nat Rev Neurosci. 2022;23(2):70–85.
50. Yu X, Liu H, Hamel KA. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11(1):264.
51. Domoto R, Sekiguchi F, Tsubota M, Kawabata A. Macrophage as a Peripheral Pain Regulator. Cells. 2021;10(8):1881.
52. Luo X, Chen O, Wang Z, et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109(17):2691–2706.e5.
53. Jean-Toussaint R, Lin Z, Tian Y, et al. Therapeutic and prophylactic effects of macrophage-derived small extracellular vesicles in the attenuation of inflammatory pain. Brain Behav Immun. 2021;94:210–224.
54. Cutolo M, Campitiello R, Gotelli E, Soldano S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front Immunol. 2022;13:867260.
55. Chen H, Jiang L, Zhang D, et al. Exploring the correlation between the regulation of macrophages by regulatory T cells and peripheral neuropathic pain. Front Neurosci. 2022;16:813751.
56. Gao L, Fan F, Wang L, et al. Polarization of macrophages in the trigeminal ganglion of rats with pulpitis. J Oral Rehabil. 2022;49(2):228–236.
57. Ruffolo RR, Hieble JP. Alpha-adrenoceptors. Pharmacol Ther. 1994;61(1–2):1–64.
58. Zheng Q, Xie W, Lückemeyer DD, et al. Synchronized cluster firing, a distinct form of sensory neuron activation, drives spontaneous pain. Neuron. 2022;110(2):209–220.e206.
59. Jin Y, Sato J, Yamazaki M, et al. Changes in cardiovascular parameters and plasma norepinephrine level in rats after chronic constriction injury on the sciatic nerve. Pain. 2008;135(3):221–231.
60. Klimaschewski L, Kummer W, Heym C. Localization, regulation and functions of neurotransmitters and neuromodulators in cervical sympathetic ganglia. Microsc Res Tech. 1996;35(1):44–68.
61. Huggins DN, LaRue RS, Wang Y, et al. Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 2021;81(20):5284–5295.
62. Barnes PJ. Beta-adrenoceptors on smooth muscle, nerves and inflammatory cells. Life Sci. 1993;52(26):2101–2109.
63. Mohammadpour H, MacDonald CR, Qiao G, et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129(12):5537–5552.
64. Koda S, Zhang B, Zhou QY, et al. β2-adrenergic receptor enhances the alternatively activated macrophages and promotes biliary injuries caused by helminth infection. Front Immunol. 2021;12:754208.
65. Shen D, Podolnikova NP, Yakubenko VP, et al. Pleiotrophin, a multifunctional cytokine and growth factor, induces leukocyte responses through the integrin Mac-1. J Biol Chem. 2017;292(46):18848–18861.
66. Tilstam PV, Schulte W, Holowka T, et al. MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model. J Clin Invest. 2021;131(23):e127171.
67. Lee S, Shi XQ, Fan A, West B, Zhang J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol Pain. 2018;14:1744806918764979.
68. He Y, Dai J, Niu M, et al. Inhibition of nicotinamide phosphoribosyltransferase protects against acute pancreatitis via modulating macrophage polarization and its related metabolites. Pancreatology. 2021;21(5):870–883.
69. Cameron AM, Castoldi A, Sanin DE, et al. Inflammatory macrophage dependence on NAD(+) salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat Immunol. 2019;20(4):420–432.
70. Zhao G, Miao H, Li X, et al. TGF-β3-induced miR-494 inhibits macrophage polarization via suppressing PGE2 secretion in mesenchymal stem cells. FEBS Lett. 2016;590(11):1602–1613.
71. Zhang X, Yuan S, Zhang X, et al. ANGPTL4 regulates CD163 expression and Kuppfer cell polarization induced cirrhosis via TLR4/NF-κB pathway. Exp Cell Res. 2021;405(2):112706.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.