Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Swollen lymph nodes may not be clinical manifestations of chronic myeloid leukemia: case report and revision of literature
Received 23 July 2017
Accepted for publication 19 August 2017
Published 5 September 2017 Volume 2017:13 Pages 1159—1162
DOI https://doi.org/10.2147/TCRM.S147056
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Zhihe Liu,1 Siyun Li,2 Ou Bai1
1Department of Hematology, The First Hospital of Jilin University, Changchun, 2Department of Pediatrics, Women and Children’s hospital of Qingdao university, Qingdao, People’s Republic of China
Abstract: We present here the case of a 33-year-old Chinese female patient with synchronous double primary malignant tumors (chronic myeloid leukemia [CML] and classic Hodgkin lymphoma). This patient was admitted to our hospital because of bilateral cervical lymph node enlargement and recurrent fever for 2 weeks. The complete blood cell count revealed white blood cell counts of 18.2×109/L, hemoglobin of 9.6 g/dL, and platelet counts of 1,547×109/L. Chromosome karyotype analysis demonstrated that t(9;22)(q34;q11) was positive in all 20 cells examined. Reverse transcription polymerase chain reaction showed that the ratio of BCR/ABL1 to ABL was 45.3%. This patient was diagnosed with CML. After definite diagnosis, this patient regularly received imatinib therapy. Three months later, although complete blood count was normal, swollen lymph nodes further increased. Swollen lymph node biopsy was performed to evaluate the nature of these swollen lymph nodes, and results displayed that Hodgkin and Reed–Sternberg cells, CD30, CD15, and Epstein–Barr virus-encoded RNA was positive. In conclusion, this patient was diagnosed with synchronous double primary malignant tumors. This case report suggests that swollen lymph nodes may be due to lymphoma, rather than as a clinical manifestation of CML.
Keywords: imatinib, lymphoma, BCR/ABL1, fluorescence in situ hybridization
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that is defined by the presence of Philadelphia chromosome and/or the chimeric gene BCR/ABL1.1 The t(9;22)(q34;q11) translocation is present in 95% of cases with CML, and this gives rise to the BCR/ABL1 gene.2 The development of CML is a multiple-step process initiated by significantly proliferation of myeloid cells.3 According to the criterion of National Comprehensive Cancer Network for CML, it is classified into chronic phase (CML-CP), accelerated phase, and blast phase.4 It is reported that approximately 3%–7.9% CML patients in blast phase may suffer from extramedullary recurrence, and this event may occur or precede concurrently with CML.1 Lymph nodes, the central or peripheral nervous system, and the bone marrow are common extramedullary sites.5 Although tumor cells in extramedullary sites are all derived from a clone of CML, it is very difficult to distinguish between extramedullary recurrence of CML and true de novo lymphoma based on morphology and clinical features alone.
In this study, we present the case of a CML-CP patient with swollen lymph node, evaluating the nature of swollen lymph node by immunohistochemistry technology and fluorescence in situ hybridization (FISH) on formalin-fixed and paraffin-embedded lymph node biopsy tissue, and the results indicated that the nature of swollen lymph nodes was Hodgkin lymphoma (HL), rather than extramedullary recurrence of CML.
Case report
Written informed consent was obtained from the patient’s next of kin for publication of this case report and the associated images. A 33-year-old woman was admitted to our hospital because of bilateral cervical lymph node enlargement and recurrent fever for 2 weeks. On admission, the patient presented with white blood cell count of 18.2×109/L, hemoglobin of 9.6 g/dL, and platelet counts of 1,547×109/L. Peripheral blood smear revealed 79% neutrophils, 2% eosinophils, 7% basophils, 4% monocytes, and 8% lymphocytes. Bone marrow smear demonstrated 1% myeloblasts, 74.5% neutrophils, 5.5% eosinophils, 1% basophils, 0.5% lymphocytes, 3% plasmocytes, and 14.5% erythrocytes. Cytogenetic analysis was performed by conventional technique with G-banding, and results showed that t(9;22)(q34;q11) was positive in all 20 analyzed mitoses. Reverse transcriptase polymerase chain reaction (RT-PCR) displayed that the ratio of BCR/ABL1 to ABL was 45.3%. Physical examination showed that spleen was 6.5 cm below the costal margin. These data allowed us to make the diagnosis of CML in chronic phase.
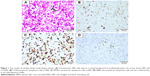
After definite diagnosis, this patient regularly received imatinib therapy. Three months later after therapy, complete blood count was normal; however, swollen lymph nodes further increased. Therefore, we considered that swollen lymph nodes may not be clinical manifestations of CML. For the purpose of evaluating the nature of swollen lymph nodes, lymph node biopsy was performed. Hodgkin and Reed–Sternberg cells were seen in this swollen lymph node biopsy section, and immunohistochemical staining showed that lymphoma cells were positive for CD30, CD15, EMA, and MUM-1 and negative for LCA, MPO, CD117, CD68, CD20, CD3, and TdT; Epstein–Barr virus-encoded RNA was positive for lymphoma cells and was confirmed by in situ hybridization (Figure 1). FISH analysis using BCR and ABL cosmid probes showed that there were no BCR/ABL1 fusion genes in lymph node biopsy section (Figure 2). Based on the abovementioned results, the patient was diagnosed as having classic HL.
This patient was finally diagnosed with synchronous double primary malignant tumors (CML and HL), and then she received imatinib combined with doxorubicin, bleomycin, vinblastine, and dacarbazine therapy. Unfortunately, the patient passed away after CML progression to acute leukemia.
Discussion
Extramedullary neoplasm is regarded as an early sign of the recurrence in CML patients. It is reported that the incidence of extramedullary neoplasm in CML patients ranges from 4% to 7.9%.3,6 In these studies, the frequency of lymph node swelling was as high as 50% in CML patients with extramedullary neoplasm. Out of these CML patients with extramedullary neoplasm, 37.5%–50.0% cases are in the hematological chronic phase.
As far as we know, there are very few articles on CML-CP patients with extramedullary neoplasm. Kobayashi et al7 reported a case of CML-CP patient with lymph node swelling, which represented extramedullary involvement composed of cells at different stages of maturation. Kumar et al8 provided a case of CML-CP patient with multiple skin chloromas in 2013. Ganessan et al9 presented a unique case of childhood CML with extramedullary biphenotypic blast crisis (myeloid/T-cell type) at initial presentation with bone marrow remaining in chronic phase. Panikar et al10 provided a rare case with concurrent CML and tuberculous lymphadenitis. In 2004, Sakakura et al11 reported a 59-year-old man with CML in chronic phase who presented with a large abdominal tumor, and the results of abdominal tumor biopsy indicated that it was an extramedullary hematopoietic tumor of CML origin. In other articles, an intra-atrial mass, skin and pericardial effusion, and the endometrium were also seen in CML-CP patients with extramedullary involvement.12–14 Different from these rare reports mentioned, we presented herein the case of a CML-CP patient with lymph node swelling, and based on the results of morphology, immunohistochemistry, and FISH in lymph node biopsy section, the nature of swollen lymph nodes was confirmed to be de novo lymphoma, rather than clinical manifestations of CML.
Lymphoma coexisting with CML is a rare disease entity. Martoïa et al15 reported the case of a female CML patient developing a malignant follicular lymphoma. Rodler et al16 described the case of a CML patient developing mantle cell lymphoma after 3 years of imatinib therapy. According to our clinical experience on multiple primary malignant tumors, we considered that environmental exposures, specific chemotherapy drugs, radiotherapy, and genetic instability may be associated with the pathogenesis of multiple primary malignant tumors.17 But the etiology of this rare disease entity remains unclear to date.
Currently, it is still very difficult to make a definite diagnosis between extramedullary tumors of CML and true de novo lymphoma based on morphology and clinical features alone. Lymphoma is rare in CML patients and is easy to be misdiagnosed as an extramedullary transformation. For patients with synchronous double primary malignant tumors, like CML and HL, conventional cytogenetic or RT-PCR cannot clarify the nature of extramedullary neoplasm.16 Therefore, a complex investigation including conventional cytogenetic analysis, immunohistochemistry, FISH, and RT-PCR is necessary for making a definite diagnosis.
Conclusion
Given the results of morphology, immunohistochemistry, FISH, and RT-PCR, this patient was finally diagnosed with synchronous double primary malignant tumors, rather than extramedullary transformation of CML. This case strongly reminds us that we should distinguish between extramedullary transformation of CML and true de novo lymphoma as soon as possible, so as to avoid misdiagnosis.
Disclosure
The authors report no conflicts of interest in this work.
References
Yashima-Abo A, Satoh T, Abo T, et al. Distinguishing between proliferating nodal lymphoid blasts in chronic myelogenous leukemia and non-Hodgkin lymphoma: Report of three cases and detection of a bcr/abl fusion signal by single-cell analysis. Pathol Int. 2005;55(5):273–279. | ||
Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1–positive chronic myeloid leukemia. Blood. 2009;113(8):1619–1630. | ||
Inverardi D, Lazzarino M, Morra E, et al. Extramedullary disease in Ph’-positive chronic myelogenous leukemia: frequency, clinical features and prognostic significance. Haematologica. 1990;75(2):146–148. | ||
O’Brien S, Radich JP, Abboud CN, et al. Chronic myelogenous leukemia, version 1. 2015. J Natl Compr Canc Netw. 2014;12(11):1590–1610. | ||
Ichinohasama R, Miura I, Takahashi N, et al. Ph-negative non-Hodgkin’s lymphoma occurring in chronic phase of Ph-positive chronic myelogenous leukemia is defined as a genetically different neoplasm from extramedullary localized blast crisis: report of two cases and review of the literature. Leukemia. 2000;14(1):169–182. | ||
Kantarjian HM, Keating MJ, Talpaz M, et al. Chronic myelogenous leukemia in blast crisis: analysis of 242 patients. Am J Med. 1987;83(3):445–454. | ||
Kobayashi Y, Tanaka T, Kawata E, et al. Chronic myelogenous leukemia in the chronic phase with lymph node swelling which represented extramedullary involvement composed of cells at different stages of maturation. Rinsho Byori. 2011;59(4):360–363. | ||
Kumar V, Jain N, Chaudhary SC, et al. Multiple skin chloromas: a rare presentation of chronic myelogenous leukaemia in chronic stable phase. BMJ Case Reports. 2013;2013:bcr2013008626. | ||
Ganessan K, Goel R, Kumar K, et al. Biphenotypic extramedullary blast crisis as a presenting manifestation of Philadelphia chromosome-positive CML in a child. Pediatr Hematol Oncol. 2007;24(3):195–198. | ||
Panikar N, Sikka M, Singh N. Concurrent chronic myelogenous leukemia and tuberculous lymphadenitis: a case report. Acta Cytol. 2005;49(6):650–652. | ||
Sakakura M, Ohishi K, Nomura K, et al. Case of chronic-phase chronic myelogenous leukemia with an abdominal hematopoietic tumor of leukemic clone origin. Am J Hematol. 2004;77(2):167–170. | ||
Creagh TM, Bain BJ, Evans DJ, et al. Endometrial extramedullary haemopoiesis. J Pathol. 1995;176(1):99–104. | ||
Freeman RK, Howden FM, Gable PS, et al. Extramedullary hematopoiesis presenting as an intraatrial mass in a patient with chronic myelogenous leukemia. J Thorac Cardiovasc Surg. 1995;110(2):552–554. | ||
Shih LY, Lin FC, Kuo TT. Cutaneous and pericardial extramedullary hematopoiesis with cardiac tamponade in chronic myeloid leukemia. Am J Clin Pathol. 1988;89(5):693–697. | ||
Martoïa R, Lamy T, Delmaire P, et al. Occurrence of non-Hodgkin’s lymphoma in chronic myeloid leukemia. Rev Med Interne. 1987;8(5):471–474. | ||
Rodler E, Welborn J, Hatcher S, et al. Blastic mantle cell lymphoma developing concurrently in a patient with chronic myelogenous leukemia and a review of the literature. Am J Hematol. 2004;75(4):231–238. | ||
Liu Z, Liu C, Guo W, et al. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10(5):e0125754. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.