Back to Journals » International Journal of Nanomedicine » Volume 14
Sustained delivery of a camptothecin prodrug – CZ48 by nanosuspensions with improved pharmacokinetics and enhanced anticancer activity
Authors Dong D, Hsiao CH, Giovanella BC, Wang Y, Chow DSL , Li Z
Received 29 November 2018
Accepted for publication 19 March 2019
Published 24 May 2019 Volume 2019:14 Pages 3799—3817
DOI https://doi.org/10.2147/IJN.S196453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Dong Dong,1,2,* Cheng-Hui Hsiao,3,* Beppino C Giovanella,4,† Yifei Wang,2 Diana SL Chow,3 Zhijie Li1
1International Ocular Surface Research Center and Institute of Ophthalmology, Jinan University Medical School, Guangzhou, China; 2GuangZhou (Jinan) Biomedical Research and Development Center Co. Ltd, Guangzhou, China; 3Department of Pharmacological and Pharmaceutical Sciences, College of Pharmacy, University of Houston, Houston, TX, USA; 4CHRISTUS Stehlin Foundation for Cancer Research, Houston, TX, USA
*These authors contributed equally to this work
†Dr Beppino C Giovanella passed away in 2017.
Background and aim: We have synthesized a novel lactone-stabilized camptothecin (CPT) analog named CZ48 and demonstrated its potent anticancer effects via bioconversion to the active CPT in earlier studies. Herein, we aimed to develop, optimize and characterize CZ48 nanosuspensions, for a sustained delivery of this drug in humans with an intravenous (i.v.) administration.
Methods and materials: A three-factor, five-level central composite design (CCD) was employed to establish the impacts of the critical influencing factors (concentrations (wt%) of CZ48, polysorbate 80 (Tween-80), and Pluronic®, F-108 (F-108)) on the responses (particle size and zeta potential). Based on the quantitative influencing factor–response relationships, two optimized CZ48 nanosuspensions of 197.22 ± 7.12 nm (NS-S) and 589.35 ± 23.27 nm (NS-L) were developed with the zeta potential values of –26.5 mV and –27.9 mV, respectively.
Results: CZ48 released from the nanosuspensions in a sustained manner in contrast to the rapid release from cosolvent in both PBS and human plasma. Moreover, NS-S exhibited more favored pharmacokinetic properties than NS-L, with a 31-fold prolonged elimination half-life of CPT, and a 2.4-fold enhanced CPT exposure over cosolvent. In efficacy study, NS-S exhibited significant tumor suppression and an improved survival rate with a higher tolerable dose, compared to CZ48 cosolvent.
Conclusion: We have successfully developed CZ48 nanosuspensions with significantly favorable pharmacokinetics and improved efficacy using CCD approach. The formulation offers potential merits as a preferred candidate for clinical trials with the prolonged CPT exposure, which is known to correlate with the clinical efficacy.
Keywords: sustained drug delivery, CZ48, camptothecin, nanosuspension, anticancer
Introduction
In earlier studies, we have designed and synthesized a prodrug of camptothecin (CPT, Figure 1B) named CZ48, the C20-propionate ester of CPT (Figure 1A).1,2 CPT, as a topoisomerase-Ι inhibitor, shows great potency against various cancers such as pancreatic and colon cancers.3 Clinical development of CZ48 is of considerable interest, since it is probably more effective than other anticancer agents such as adriamycin, Alkeran and 5-fluorouracil.1 More importantly, the toxicities caused by CZ48 may be less severe than those by other CPT analogs, such as the marketed Irinotecan® (CPT-11).1 Clinical use of CPT-11 has been limited by its severe toxicities (resulted from its active metabolite SN-38) of neutropenia and diarrhea.4,5
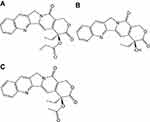 | Figure 1 Chemical structures of CZ48 and its analogs: (A) CZ48; (B) CPT; (C) CZ44.Abbreviation: CPT, camptothecin. |
CZ48 holds great potential as a promising anticancer agent. However, there are two hurdles in conducting clinical trials of CZ48. First, CZ48 is insoluble in aqueous media, which makes it impossible to prepare an intravenous (i.v.) solution with an effective concentration for human application. Second, no drug delivery systems are currently available to deliver CZ48 in a sustained fashion. Such a delivery system is critical because the efficacy of topoisomerase-Ι inhibitors is better with a prolonged exposure at low concentrations than with a short-term exposure at high concentrations.6
Therefore, the aim of this study was to provide a possible solution to the CZ48 delivery challenges using formulation strategy. A nanosuspension-based drug delivery system could achieve a prolonged CPT circulation in vivo by releasing CZ48 in a sustained manner by i.v. administration. Nanosuspensions are submicron colloidal dispersions of pure drug particles in water, which are stabilized by surfactants.7,8 Advantages of nanosuspensions include: 1) higher drug loading (because drug is suspended in solid state) that leads to a lower volume of dose administration; 2) reduced toxicity by requiring a relatively limited quantity of stabilizing surfactants; 3) diverse routes of administration, such as oral, parenteral, pulmonary and ocular pathways, due to the nanorange of the particle size; and 4) potential passive targeting and depot effect, as nanoparticles are taken up by the macrophages in the liver, spleen and lung, and subsequently dissolved slowly in the macrophages and diffuse out of the cells to provide a depot effect.9–13 Recently, there are two basic technologies to prepare nanosuspension: media milling and high-pressure homogenization. Four commercial nanosuspension products have been manufactured by media milling. During the formulation preparation process, many variables show a marked influence on the physicochemical properties of nanosuspensions. Central composite design (CCD) is a multivariate five-level experimental design that can be used to systematically evaluate the influence of different variables on the properties of the formulations (eg, liposomes, microspheres and nanoparticles) and the cross-interaction among the variables.14–17 Hence, a CCD-based surface response methodology was employed in this study to establish quantitative relationships among the various critical influencing factors and the responses and facilitate the optimization of the nanosuspension.
Lung cancer is the leading cause of cancer-related deaths.18 The current treatment options in lung cancer are surgery, radiation, chemotherapy and targeted therapy. Chemotherapy regimens are platinum-based (cisplatin and carboplatin) and in combination with agents such as paclitaxel, docetaxel, topotecan, CPT-11, vinorelbine and gemcitabine.19 Based on the National Cancer Institute record, vinorelbine, paclitaxel, docetaxel and gemcitabine have shown minimal increased survival outcomes, wherever the CPT derivatives (topotecan and CPT-11) are considered as second-line chemotherapy drugs. In this way, CZ48 may offer a promising alternative.
Therefore, we aimed to develop a sustained-release nanosuspension for CZ48 using CCD approach. The optimized CZ48 nanosuspension could overcome the administration challenge and provide a prolonged exposure of CPT at the site of action. In addition, in vitro release and in vivo studies (including pharmacokinetics and organ distribution behaviors) were performed to characterize and evaluate the CZ48 nanosuspensions. Efficacy study was also carried out in non-small-cell lung cancer (NSCLC) xenograft tumor-bearing mouse model.
Methods and materials
Materials
CZ44 (Figure 1C), CPT-20-O-acetate, was used as the internal standard for HPLC assay that simultaneously quantifies the prodrug CZ48 and its active metabolite, CPT.20 CZ48 (purity of 98%) and CZ44 were provided by CHRISTUS Stehlin Foundation for Cancer Research (Houston, TX, USA) as gifts; CPT was purchased from Sigma-Aldrich (St. Louis, MO, USA). Pluronic® F 68 (F-68) and Pluronic® F108 (F-108) were kindly provided by BASF (BASF corporation, NJ, USA); KH2PO4, K2HPO4, NaCl, povidone 40 (PVP 40), polyvinyl acetate (PVA), polyethylene glycol 400, and polysorbate 80 (Tween-80) were purchased from Sigma-Aldrich. Double-distilled water was produced by a Millipore Milli-Q system (Billerica, MA, USA). HPLC-grade acetic acid, dimethyl sulfoxide, acetonitrile, dichloromethane, ethanol and diethyl ether were purchased from Sigma-Aldrich. CZ48 cosolvent was prepared as dimethyl sulfoxide:polyethylene glycol 400:ethanol (2:2:1 by volume).
Methods
CZ48 nanosuspension preparation
Initial screening trials were performed to evaluate the formulation and processing variables of nanosuspensions (ie, concentration of CZ48, type of stabilizers, concentrations of stabilizers, amount of milling media, milling speed and milling time).21 CZ48 nanosuspensions were obtained by media milling method, as described earlier.22 In brief, 0.5 g mixture of CZ48, stabilizers and water were placed in a 7 mL scintillation vial. Glass beads (1 g in weight) were added in the mixture as milling agents. Then, the mixture was milled at 1,600 rpm for various time periods. Each formulation was prepared in triplicate.
Characterization of physical properties
The particle size, polydispersity index (PI), and zeta potential of each formulation were measured by Zeta Pals (Brookhaven Instruments, Holtsville, NY, USA). Samples were diluted to an appropriate concentration by double-distilled, filtered water. Triplicate samples of each preparation were measured, and the mean ±SD values were determined.
CCD experimental design
A CCD was implemented for the optimization of the formulation properties. Based on the results of initial studies (Table S1), a three-factor, five-level CCD was undertaken to evaluate the main effects and the interactions of these three critical influencing factors on the three responses (particle size, PI and zeta potential) of the nanosuspensoin. The test range of each variable and the experimental codes of the optimization are summarized in Table 1. In the present design, 20 experiments (Table 2) were carried out to determine the model coefficients. Three optimal experimental responses were studied: Y1, particle size, Y2, PI and Y3, zeta potential. The responses were modeled by the following quadratic equation 1:
(1) 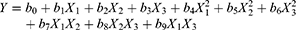
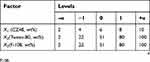 | Table 1 Levels of critical influencing factors and coded correspondent values |
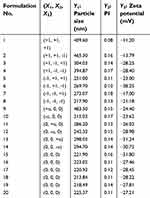 | Table 2 Experimental responses and the results of central composite design (CCD |
where X1, X2 and X3 correspond to the studied factors; Y is the measured response; b0 is an intercept; b1–b9 are the regression coefficients.
Data were analyzed by nonlinear estimation using STATISTICA software. The results of these experiments were compared by ANOVA to determine if the factors and the interactions among the factors were significant. Student’s t-test was used to determine the statistical significance of parameters in the regression model at α=0.05 level. An F-test was performed to determine whether the overall regression relationship between the response Y and the entire set of variables X was significant at a 95% level.
Response surface delineation was performed according to the fitting model. The surface response plots for particle size and zeta potential as functions of influencing factors were conducted by fixing the insignificant factor at its optimized value. The minimum response values and its corresponding experimental settings were solved from the individual regression equations for responses by performing a Visual Basic-language-based computer script calculation with a step width of 0.1.
A validation test was conducted to demonstrate the accuracy and usefulness of this statistic model by performing 6 independent batches of the formulation under the determined optimal formulation conditions. The particle sizes, PI and zeta potential of the prepared nanosuspension formulations were analyzed.
In vitro drug release study
The in vitro release studies in PBS solution (pH 7.4) and human plasma, respectively, were performed using the dialysis bag diffusion technique with 0.2 wt% Tween-80 in the release medium to maintain the sink condition.23 The PBS solution was made from 0.4 mM KH2PO4, 2 mM K2HPO4 and 140 mM NaCl. Approximately, 1 mg of the formulation was transferred to the dialysis bag (molecular weight cutoff 6,000–8,000 Da) with PBS or human plasma in a shaker with the speed of 100 rpm at 37.0±0.5°C. Samples (200 μL) were withdrawn at the predetermined time points of 0.25, 0.5, 0.75, 1, 2, 3, 4, and at 6 hrs for release from PBS and 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 10, 24, 32, and 48 hrs for release from human plasma. Samples were assayed for CZ48 by a validated HPLC method.24 The profiles of cumulative amount of CZ48 released versus time were constructed. The extent of CZ48 release was calculated as the total percentage (%) released at 6 hrs for release from PBS and 48 hrs for that from plasma. The first-order release kinetic model was used to derive the release kinetic parameter and release rate constant (k), for all of the three formulations. Data were presented as mean ± SD (n=3).
In vivo pharmacokinetic evaluation and organ distribution study of CZ48 formulations
All experiments were conducted in accordance with NIH Guidelines for the care and use of animals and with approved animal protocol from the Institutional Animal Care and Use Committee in University of Houston. Male Swiss athymic nude mice (20~25 g) were a gift from Stehlin Foundation for Cancer Research (Houston, TX, USA). Mice were maintained in individual ventilated cages under standard laboratory conditions (12 hr light/dark cycle) with free access to food and water. Then, the mice were randomly divided into experimental groups (6 mice per group) for treatment with CZ48 formulations.
Mice were dosed with CZ48 cosolvent at 5 mg/kg, CZ48 NS-S at 25 mg/kg or CZ48 NS-L at 25 mg/kg through the tail vein. There were six groups of mice for each formulation which were for sampling at 15 mins, 30 mins, 2 hrs, 4 hrs, 8 hrs, and 12 hrs, respectively. The animals of 15-min and 30-min groups were sacrificed after 15 mins and 30 mins, respectively, post-dose under anesthesia using Avertin (tribromoethanol and amyl alcohol) based on the Christus Stehlin Foundation Standard Operating Procedure for mouse anesthesia. A terminal blood collection was withdrawn from the heart, and the whole body was flushed by normal saline before heart, liver, spleen, lung, kidney, and brain were harvested. For the animals of 2-hr group, one additional blood sample was taken at 1-hr time point from the facial vein, then follow the same procedure as the 15-min and 30-min groups. For the animals of 4-hr, 8-hr, and 12-hr groups, one additional blood sample for each mouse was collected from facial vein before sacrifice, which was at 3-hr for 4-hr groups, 6-hr for 8-hr groups, and 10-hr for 12-hr groups. In this way, only one blood sample was taken from each mouse in 15-min and 30-min groups , and two blood samples from each mouse in other groups.
The blood samples were immediately centrifuged at 8,000×g for 20 mins to separate the plasma fraction from the blood cells, and the samples were stored at –80 °C until HPLC analysis. The HPLC assay was based on a well-established gradient HPLC method for the simultaneous quantifications of CZ48 and CPT concentrations in plasma samples.24 This HPLC method also has been validated in supplementary data in different mice organs.
The pharmacokinetic parameters of CZ48 and CPT (ie, area under the plasma concentration–time curve [AUC], the elimination half-life [t1/2], the volume of distribution at steady state [Vss] and total plasma clearance [CL]) were derived by a compartmental model using WinNonlin Professional Version 3.0. The absolute bioavailability (Fab) was calculated by Equation 2. The organ/plasma partition coefficient (Kp) of CZ48 and CPT for the heart, liver, spleen, lung, kidney and brain was obtained experimentally from the AUCorgan/AUCplasma ratios toward the end of the study by Equation 3.25
(2) 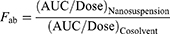
(3) 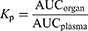
Efficacy studies
Athymic Swiss nude mice (25 ~ 30 g) were used for efficacy studies. NSCLC H460 cell lines were purchased from American Type Culture Collection (Mansaas, VA, USA), which was widely used in the NSCLC efficacy evaluation in subcutaneous tumor model because of its fast growth rate and high implant successful rate.
Passage of tumor into mice
For the efficacy studies, the cells were suspended in RPMI 1640 medium at a concentration of 107 cells/mL. One-quarter millilters of the suspension was injected subcutaneously in the mid-dorsal portion of four to six mice with a 25-gauge needle for tumor growth. After 2 weeks, the mice were sacrificed and the tumors were removed, pooled, minced up, and centrifuged. The supernatant was removed and one part of RPMI 1640 media was added to two parts of the tumor cells for passage into all study mice. About 100 μL of the suspension was injected in the mid-back of the study mice to induce tumor growth.
Randomization of mice into dosing groups
When the estimated tumor volumes were about 100 mm3, the mice were weighed and tumor volumes were measured. Calipers were used to measure tumor volume which is defined as the product of the tumor length (L), width (W) and height (H). The mouse weight and tumor volume were added to Microsoft FoxPro 7.0 with the mouse ID number that was earlier generated using FoxPro 7.0 and the program was used to randomize the mice into groups.
Efficacy study design
The concentrations of CZ48 (50 mg/kg) in the nanosuspensions were diluted to the required concentrations, and the tail vein injection dose was limited up to 0.2 mL or less. The control groups received equal volumes of placebo formulations. The mice were dosed with CZ48 formulations and assessed twice weekly for a total of 8 doses (4 weeks). The assessment included body weight for toxicity, as well as tumor size and survival for efficacy. If the body weight loss was >15% or the tumors were >7,000 mm3, the mice were sacrificed. The evaluation parameters of the study were tumor growth rate (defined as V/V0, V is the tumor volume on the day of sacrifice and V0 is the tumor volume on the first day of dosing), toxicity (body weight loss >15%) and survival.
Statistical analysis
The statistical significance of the difference in release,pharmacokinetic parameters and tumor growth rate among the formulation groups was evaluated by one-way ANOVA, followed by post-hoc Tukey’s test at P<0.05, using MINITAB student 14 software. SASS was used for Kaplan–Meier survival analysis among groups in the efficacy studies.
Results
CCD approach for formulation optimization
Based on the preliminary results, the stabilizers of combined F-108 and Tween-80 were selected for further CCD optimization. Particle size, PI and zeta potential were considered as the critical responses of our nanosuspensions. CZ48, Tween-80 and F-108 concentrations were chosen as the critical factors, as they affect the particle size and zeta potential.
The characteristics of the nanosuspensions prepared with the three factors at five levels of CCD were tabulated for individual experimental runs. The experiments at the center points (n=6) were performed to estimate the coefficient of variation (or reproducibility of the experiment), which was <5%. Factor levels of each experimental run and values of each nanosuspension properties are shown in Table 2. The particle sizes ranged from 215 to 484 nm (~2-fold), indicating that a fine control of the selected factors enabled the preparation of nanosuspension with the desired particle size. The measured values were fitted to Equation 1 to describe the relationship between the critical influencing factors and responses and obtain the following second-order polynomial equations of particle size (Y1, Equation 4) and zeta potential (Y3, Equation 5):
(4) 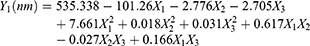
(5) 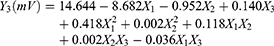
The quadratic model was significant with F values of 148 and 79.9 (p<0.0001) for particle size and zeta potential, respectively, indicating that response variables Y and the set of X variables were significantly related. Moreover, the high regression coefficients (R2) of these equations were 0.959 and 0.895, demonstrating a good correlation between the selected factors and responses. PI had no correlation with the selected factors.
Among the three factors, CZ48 and F-108 concentrations had considerable impacts on the mean particle size, but Tween-80 concentration did not. The dependence of particle size on the drug and F-108 concentrations was plotted (Figure 2A), based on the regression equation (Equation 4) at 10 wt% of Tween-80 (X2=10). The minimum particle size of 190 nm could be achieved by operating the experiment under the formulation conditions of 5.9 wt% of CZ48 (X1), 10 wt% of Tween-80 (X2) and 28 wt% of F-108 (X3).
The dependence of zeta potential on the concentrations of CZ48 and F-108 was also plotted (Figure 2B), based on the regression Equation 5. The zeta potential value of the nanosuspension, prepared by the optimal conditions for particle size model, was in the stable range. Therefore, the experimental conditions optimized by particle size model have been utilized empirically for the preparation of NS-S with a particle size of 197.22±7.12 nm, zeta potential of -26.52±0.93 mV and PI of 0.11±0.03. The model was demonstrated to be valid since a fine agreement existed between the predicted and observed values with a bias of <3% (Table 3). Another nanosuspension with a larger particle size (NS-L) of the same composition was prepared by reducing the milling time from 24 hrs to 2 hrs. NS-L had a particle size of 589.35±23.27 nm, zeta potential of -27.91±0.76 mV and PI value of 0.12±0.03.
 | Table 3 Predicted values and experimental results of CZ48 nanosuspension prepared under the optimal conditions |
In vitro drug release study
In in vitro drug release study, when PBS was used as the medium, the release rate constants (k) of CZ48 from nanosuspensions were significantly smaller (21.36±1.92%/hr for NS-S and 30.72±0.11%/hr for NS-L) compared to that from cosolvent (84.62±2.76%/hr), though a complete release (>98%) was achieved for all three formulations after 4 hrs (Figure 3A). Between CZ48 nanosuspensions of different particle sizes, the release rate of CZ48 from NS-L was significantly slower than that from NS-S at p<0.05. By contrast, when plasma was used as the medium (Figure 3B), the release of CZ48 from nanosuspensions was 10 times slower than that from cosolvent (0.60%/hr vs 6.48±0.90%/hr). After 48 hrs, a complete release of CZ48 was observed from cosolvent, but the release extents of CZ48 from nanosuspensions were rather limited (about 40%). After the last sample collection, the drug in the dialysis bag was quantified for mass balance; the recovery of the total amount of CZ48 was about 98%.
In vivo pharmacokinetic evaluation and organ distribution study of CZ48 formulations
The in vivo performances of three CZ48 formulations were evaluated in nude mice at a single dose of 5 mg/kg, 25 mg/kg and 25 mg/kg for cosolvent, NS-S and NS-L, respectively, by i.v. injection.
Pharmacokinetics of CZ48 and CPT from three CZ48 formulations
The plasma concentration–time profiles were constructed using sparse sampling approach. The mean concentration normalized by dose–time profiles of CZ48 and CPT was constructed (Figure 4). Compartmental modeling was used to derive the pharmacokinetic parameters of CZ48 and CPT (Table 4).
 | Table 4 Pharmacokinetic parameters of CZ48 and active metabolite CPT from cosolvent, NS-S and NS-L after i.v. administration in mice (n=6) |
As expected, the plasma concentration–time profiles of CZ48 were markedly different between the nanosuspensions and cosolvent (Figure 4A). Cosolvent showed a significantly higher initial concentration, C0. On the other hand, the plasma concentrations of CZ48 from dosing of nanosuspensions were always significantly higher than those from cosolvent at 3 hrs post-dose, indicating that a sustained level of CZ48 can be achieved using the developed nanosuspensions. As a result, the half-lives (t1/2) of CZ48 from NS-S and NS-L were 8.00 hrs and 5.58 hrs, respectively, about 11-fold and 8-fold longer than that from cosolvent (0.70 h). The AUC0-∞/Dose of CZ48 was comparable among the three groups.
Similar to CZ48, the pharmacokinetic behaviors of CPT (the active metabolite of CZ48) were also altered to a great extent by the use of nanosuspensions (Figure 4B and Table 4). Most notably, the apparent elimination of CPT was significantly reduced, which was evidenced by a 31-fold and 15-fold increase in estimated t1/2 values by dosing NS-S and NS-L, 12.51 and 5.81 hrs, respectively, compared to 0.40 hrs from cosolvent. This is probably due to a depot effect from nanosuspensions that gradually released CZ48. In addition, CPT exposure normalized by the dose was 2-fold higher with NS-S dosing than that with cosolvent dosing.
Organ distributions of CZ48 and CPT from three CZ48 formulations
The biodistribution study in mice was also comparatively evaluated for CZ48 and CPT from cosolvent, NS-S and NS-L. Different organ distribution patterns of CZ48 and CPT were observed among cosolvent, NS-S and NS-L (Figure 5). The drug concentrations in different organs showed similar trends as that in plasma. The concentration–time profiles of nanosuspensions were distinct from those of cosolvent for both CZ48 and CPT, with a slower elimination phase. The mean organ parameters were derived from the mean concentration–time profiles for each formulation by WinNonlin using compartmental models (Table 5).
 | Table 5 CZ48 and CPT organ distribution parameters from cosolvent, NS-S and NS-L in mice after i.v. administration (n=6) |
 | Figure 5 Organ distribution profiles of CZ48 and CPT from cosolvent (A and B), NS-L (C and D) and NS-S (E and F) in mice (n=6). |
The exposures of CZ48 from both nanosuspensions were higher in the reticuloendothelial system (RES), such as liver, spleen and lung, but lower in kidney and brain than those from cosolvent. In addition, CZ48 from NS-S was mainly distributed in liver and lung, while cumulated in liver from NS-L. The half-lives of CZ48 from nanosuspensions were longer than those from cosolvent in all the tested organs.
By comparing the biodistribution patterns of CPT among the three formulations, nanosuspensions yielded longer half-lives in all organs compared to cosolvent. NS-S displayed the highest exposure and longest half-life in lungs among the three formulations.
The Kp (AUCorgan/AUCplasma) values versus time are plotted in Figure 6. Through nanosuspension administration, the Kp values of CZ48 in liver, spleen and lung were much higher than those from cosolvent, which may be due to the significant uptake of nanoparticles by RES. However, no significant difference was observed among the Kp values of CPT from the three formulations, which may be due to the fact that only free CZ48 can be biotranformed to CPT by carboxylesterases (CEs).
 | Figure 6 Profiles of partition coefficient (Kp, AUCorgan/AUCplasma) of CZ48 and CPT from cosolvent (A and B), NS-L (C and D) and NS-S (E and F) in mice (n=6). |
Efficacy study
Based on the pharmacokinetic and organ distribution study in mice, NS-S was selected as the lead formulation to perform the efficacy study. Seven groups of tumor-xenograft mice were used: receiving no treatment (NT), cosolvent placebo (CP), nanosuspension placebo (NP), CZ48 cosolvent (Co, 5 mg/kg), NS-S of low dose (NS-S-L, 5 mg/kg), NS-S of medium dose (NS-S-M, 25 mg/kg) and NS-S of high dose (NS-S-H, 50 mg/kg), respectively, using the same formulations as in the pharmacokinetic studies. The groups for NT, CP and NP were used as control groups and cosolvent as a reference for comparison.
Average body weight
Significant body weight loss was considered as a sign of toxicity. The average body weights versus the days post first dose were monitored for each group (Figure 7). No statistical difference was observed in the body weights among the groups except NS-S-H group with apparent weight losses.
Tumor growth rate
The comparative tumor growth, V/V0 ratio, versus time is shown in Figure 8 for different groups. At day 11 of treatment, the mice in each control group (NT, CP and NP) and reference group (Co) were started to be sacrificed due to the tumor size which grew to >7,000 mm3. Therefore, the comparison to day 11 was more precise as compared to that to day 29, due to the decreased observation number resulting from animal death. The growth rate (/day) to day 11 treatment was calculated according to the exponential tumor growth model for each group, and ANOVA followed by Tukey’s post-hoc statistical analysis was conducted (Table 6). Both NS-S-M and NS-S-H groups had statistically slower tumor growth rate as compared to those of the control groups. Moreover, the tumor growth rate of NS-S-H group was statistically slower than that of NS-S-M group.
 | Table 6 Tumor growth rate from the first day of dosing until day 11 |
Survival rate
The survival rates of mice in the three control groups, cosolvent reference group and three nanosuspension treatment groups are shown in Figure 9. The Kaplan–Meier plot was used for the comparison of survival analysis. There was no significant difference among these three control groups without CZ48 treatment (NT, CP and NP) with a p-value of 0.3356. The p-values for the 10-way comparison are summarized in Table 7. NS-S-M group was statistically different from all the control groups, cosolvent group and other treatment groups with a p-value of 0.0002 ~ 0.0085. The high-dose group showed significantly stronger tumor suppression compare to low- and medium-dose groups, but with a lower survival rate, probably due to a higher toxicity reflected in body weight loss.
 | Table 7 Summary of significance testing by Kaplan–Meier survival analysis among different groups |
Discussion
CZ48 nanosuspensions were prepared by media milling method, as described earlier.21 The effect of milling time on particle size was established by fixing all other factors (Figure S1). The particle size decreased as milling time increased, and reached a plateau at approximately 24 hr. Therefore, we used selected milling time up to 24 hrs for nanosuspension preparation in stabilizer screening and CCD experiment.
Due to the energy introduced into the system to reduce the particle size, surface-active compounds (surfactants) should be used to stabilize the particles in the thermodynamically unstable suspension system. There are only a limited numbers of nonionic and ionic surfactants that have been approved as excipients for parenteral use. Because of the safety concerns about ionic surfactants, 5 nonionic surfactants were selected as stabilizer candidates, which were polyvinylpyrrolidone (PVP) K40, polyvinyl alcohol (PVA), Pluronic® F68 (F-68), Pluronic® F108 (F-108) and polysorbate 80 (Tween-80).26,27
In order to identify stabilizer candidates for the nanosuspensions of CZ48, the 5 stabilizers in three concentrations at stabilizer/CZ48 ratios of 1:1 (H), 1:4 (M) and 1:10 (L) as well as their combination with Tween-80 (L:L) were screened. The results are shown in Table S1. Among the 5 stabilizers, only F-108 and F-68 could further decrease the particle size significantly when higher concentrations were used (p<0.05). The particle sizes of nanosuspensions using F108 were significantly smaller than those using F-68. This is consistent with an earlier report that F-108 is excellent in stabilizing nanoparticles, due to its strong affinity to the surface of nanoparticles.28 In addition, the surfactant F-108 has also been used in the formulation of i.v. injectable itraconazole, and the clinical trial of its suspension has also been conducted.29
Moreover, nanosuspensions with single stabilizer resulted in a significantly larger particle size than those with a combination of Tween-80. The smallest size (366±13.2 nm) was achieved by using both F-108 and Tween-80 as stabilizers without any formulation optimization. Considering stabilizers in combination perform better long-term stabilization, and Tween-80 uniquely facilitates a longer drug circulation, we preferably used the combination of F-108 and Tween-80 for CZ48 nanosuspension preparation.30 In addition, our nanosuspensions appeared to be a homogeneous and stable system with a PI of 0 ~ 0.2 and a zeta potential value of -25 ~ -32 mV.
It has been reported that too much dispersant could actually promote Ostwald ripening, and an increase of stabilizer concentration led to a concentration-dependent increase in particle size once the stabilizer concentration exceeded a critical concentration.31 This theory is consistent with our CCD results. Of the three factors studied, the concentrations of CZ48 and F-108 had considerable impacts on the mean particle size with a p<0.05, but the concentration of Tween-80 did not. This might be due to the fact that Tween-80 only played a role as a wetting agent in the formulation, and the effect on particle size was minimal. The zeta potential was not significantly affected by the experimental conditions employed in this study. All the CCD model regression parameters are shown in Table S2.
In order to obtain a nanosuspension exhibiting a good stability, a minimum zeta potential of ±20 mV is desirable.7 Most of the nanosuspensions produced by FDA-approved surfactants have a negative charge.32 Moreover, the negatively charged particles have a similar charge of the cellular membrane, and the particles will be strongly adsorbed onto the membrane for cellular uptake. In this way, nanosuspension with a negative zeta potential is preferred. Our optimized CZ48 nanosuspension (NS-S) was obtained with a particle size of 197 nm and zeta potential of –26.5 mV. Previous research in our laboratory has proven that only the nanosuspension of size >500 nm yields a significant difference in pharmacokinetic behavior in vivo from that of 200 nm.33 Therefore, another nanosuspension (NS-L) with particle size of 600 nm was selected for comparison as the second model formulation for further investigation.
The chemical stability of CZ48 under different conditions has already been established by Dr. Yousif Rojeab.34 Physical stability evaluations of NS-S and NS-L revealed that both formulations were stable over 6 months. It also displayed consistency in CZ48 loading of 50 mg/mL, from batch to batch, when prepared under the uniform conditions.
The in vitro release characteristics of CZ48 from the selected nanosuspension formulations of different particle sizes were evaluated in PBS and human plasma. In PBS, the immediate release profile of cosolvent and slower release profiles of CZ48 nanosuspensions, including NS-S and NS-L, could be explained by their different physical nature. In cosolvent formulation, CZ48 molecules are completely dissolved in the cosolvent mixture. The dissolved CZ48 molecules are readily transferred from the dialysis bag to the outside medium. In contrast, nanosuspensions contain solid drug particles of nanometer sizes, and the drug molecules need to be dissolved into the diffusion layer first and then into the bulk medium before being released from the dialysis bag.35 The slow dissolving process contributes to the slower and sustained-release profiles of CZ48 from nanosuspensions. The mean values of initial rate of nanosuspensions of various sizes increased as the particle size decreased, which is consistent with the previous work in our laboratory by Qi.33 According to Noyes–Whitney equation, the dissolution rate will increase due to the increase of surface area when particle size reduces.36 In plasma, the limited-release extent and slow-release rate might be explained by the high protein binding of CZ48 (82.48±2.52%) in plasma.37 The drug molecules also need to be dissociated into the diffusion layer first and then into the bulk medium before being released from the dialysis bag. However, the drug molecules which bound to the protein were hard to permeate through the dialysis bag. Therefore, the particle size of nanosuspensions did not apparently affect the in vitro release rate of CZ48 in plasma. The results indicated that our nanosuspension can suppress the release and achieve a sustained release of CZ48, which was attributed to the solid status of CZ48 in nanosuspension.
In pharmacokinetic study, CZ48 nanosuspensions of two different particle sizes (NS-S and NS-L) exhibited distinct plasma and biodistribution patterns from that of cosolvent. Significantly lower C0 of CZ48 from both nanosuspensions than that from the cosolvent formulation could be due to the slower dissolution rates of CZ48 from the nanosuspensions than from cosolvent. Longer β half-life for CZ48 nanosuspensions than that from cosolvent was partially attributed to the sustained drug release from nanosuspensions. Another possible reason for the prolonged β half-life could be due to the RES uptake of nanoparticles in nanosuspensions, and then the drug was released from phagocytic cells to blood circulation due to the drug concentration gradient, which resulted in a longer blood circulation compared to that from cosolvent.38 Two-compartmental model was used to characterize the CZ48 plasma profiles from nanosuspensions, while one-compartmental model was used for the CZ48 profile from the cosolvent. It could be due to the drug accumulation in the peripheral compartment from CZ48 nanosuspensions. The decreased C0 and prolonged half-lives of nanosuspensions, as compared with cosolvent, could be of clinical significance and merits. For example, the decreased C0 could be beneficial in reducing side effects of the drug caused by the excessively high C0.39 CZ48 nanosuspension with a prolonged half-life requires a less frequent dosing regimen to provide a sustained therapeutic plasma level of CPT, which is more convenient to patients and favors a better patient compliance.6 More importantly, the CPT from NS-S showed a significantly longer elimination half-life compared to that from NS-L and cosolvent. Our results clearly demonstrated the slow in vitro release of CZ48 from nanosuspensions that may be translational to a sustained release in vivo, which ultimately may contribute to a significantly longer retention of both CZ48 and CPT in blood circulation for an enhanced CPT exposure.
The organ distribution patterns of CZ48 from nanosuspensions were distinct from that of CZ48 cosolvent. CZ48 distributions among organs from cosolvent were relatively even among organs, due to the rapid dissolution and highly hydrophobic properties of CZ48, except a high accumulation in lung that may be due to the drug precipitation with a subsequent retention in lung, which was consistent with the finding of bifendate nanosuspension study in rabbits.32 Further research is needed in the future. The liver and spleen were observed with the highest exposure of CZ48 from nanosuspensions, which could be due to the reticuloendothelial system (RES) uptake. Liver and spleen are two major RES organs of the body defense systems to clear foreign particles.40 The pattern of high drug distributions in liver and spleen from nanosuspensions is very similar to that of liposome and other nanoparticle formulations.35,41–43 Following the uptake, the RES acts as a depot and drug can be released slowly back to the systemic circulation, which may contribute to the sustained plasma drug level achieved from nanosuspensions.17 CZ48 exerts the antitumor activity by CE-mediated hydrolysis to the active metabolite CPT in vivo.44,45 In vitro study of CZ48 metabolism has shown that among blood and CE-containing tissues such as liver, spleen, lung and kidney, the liver has the highest metabolic capacity to convert CZ48 to CPT instead of blood, consistent with our observation that the highest exposure of CPT is in liver.46 Because the nanosuspension particles will be trapped by the RES and released slowly, only the free CZ48 molecules can be biotransformed to CPT. The CPT from nanosuspension yielded a significantly longer elimination half-life in different organs compared to those from cosolvent. All these properties may offer potential merits for CZ48 chemotherapy. The much higher spleen exposure of CZ48 from nanosuspensions may pose a potential concern of off-target accumulation and toxicity and requires further investigation.
The present in vivo studies demonstrated that our nanosuspension formulation had substantially lower toxicity than the cosolvent. Four times tail vein injection of cosolvent made severe tissue damage, but nanosuspension injection did not result in any damage. In addition, when the blood was taken after the animal was sacrificed, much darker and thicker blood was observed by cosolvent administration compared to NT group and nanosuspension groups. Because of the low solubility of CZ48, the highest tolerated dose of CZ48 cosolvent was 5 mg/kg. Any increase of the cosolvent concentration will cause animal death immediately due to the significant drug precipitation in the blood circulation. However, the dose of nanosuspension can be given up to >50 mg/kg. Cosolvent and NS-S-L at the same dose level (5 mg/kg) did not exhibit significant efficacy over the control/reference groups. For most of the chemotherapy agents, there is a therapeutic window. The phenomenon of lack of efficacy was not well understood, but might be due to the fact that dose level could not reach the minimum therapeutic concentration, or it failed to keep the therapeutic concentration for a long enough duration. Whereas, treatment with NS-S-M yielded significant tumor growth suppression and animal survival rate increase, compared to control groups. The significant onset of tumor suppression was observed 3 days after the first dose was given. This dose is the same as the pharmacokinetic study, which resulted in sustained circulation of CZ48 and CPT for >24 hrs in rodent animals. In this way, the significant efficacy may be attributable in part to the favorable pharmacokinetics of systemically delivery drugs. For high-dose group of CZ48 nanosuspension administration, the significant tumor suppression could be investigated, but the toxicity caused animal death (5 in 10 mice) and significant body weight loss.
Conclusion
In this study, we have successfully developed nanosuspensions NS-S with the particle size of about 200 nm via an implementation of CCD-based surface response methodology and NS-L with the particle size of about 600 nm with the same composition by increasing media milling time. The CZ48 released in vitro was much slower from the nanosuspensions (in a sustained manner) than from cosolvent. Moreover, CZ48 nanosuspensions exhibited more favored pharmacokinetic properties in contrast to the cosolvent with a prolonged elimination half-life of both CZ48 and CPT, and an enhanced CPT exposure. As more effective chemotherapy is correlated to a prolonged active CPT exposure, the developed nanosuspension formulations offer potential therapeutic merits for clinical trials. The efficacy study proved that nanosuspension is a viable pharmaceutical carrier that delivered CZ48 in a sustained manner and achieved significant tumor growth suppression at a higher tolerable dose. This study provides a formulation strategy that has great potential to overcome the delivery barriers for many other anticancer drugs. We anticipate that CZ48 nanosuspension will be a lead candidate for the clinical trials on CZ48 in the near future.
Abbreviation list
CZ48, C20-propionate ester of camptothecin; CPT, camptothecin; CCD, central composite design; NSCLC, non-small-cell lung cancer; PVP 40, povidone 40; PVA, polyvinyl acetate; PEG 400, polyethylene glycol 400; F-68, Pluronic® F 68; F-108, Pluronic® F108; Tween-80, polysorbate 80; PI, polydispersity index; wt%, percent of weight; NS-S, the nanosuspension with particle size of 200 nm; NS-L, the nanosuspension with particle size of 600 nm; Fab, absolute bioavailability.
Acknowledgments
This research was supported by Stehlin Foundation for Cancer Research, and the Program for Pearl River New Stars of Science and Technology in Guangzhou (No. 201610010143), Medical Scientific Research Foundation of Guangdong Province (No. A2017138), and the Fundamental Research Funds for the Central Universities (No. 21617459).
We also thank Dr. Yang Teng and Dr. Mo Yang for their assistance in efficacy study and Dr. Yang Wang for her assistance in critical review and revision of the current draft.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cao Z, Kozielski A, Liu X, Wang Y, Vardeman D, Giovanella B. Crystalline camptothecin-20(S)-O-propionate hydrate: a novel anticancer agent with strong activity against 19 human tumor xenografts. Cancer Res. 2009;69(11):4742–4749. doi:10.1158/0008-5472.CAN-08-4452
2. Cao Z, Harris N, Kozielski A, Vardeman D, Stehlin JS, Giovanella B. Alkyl esters of camptothecin and 9-nitrocamptothecin: synthesis, in vitro pharmacokinetics, toxicity, and antitumor activity. J Med Chem. 1998;41(1):31–37. doi:10.1021/jm9607562
3. Botella P, Rivero-Buceta E. Safe approaches for camptothecin delivery: structural analogues and nanomedicines. J Control Release. 2017;247:28–54. doi:10.1016/j.jconrel.2016.12.023
4. Tejpar S, Yan P, Piessevaux H, et al. Clinical and pharmacogenetic determinants of 5-fluorouracyl/leucovorin/irinotecan toxicity: results of the PETACC-3 trial. Eur J Cancer. 2018;99:66–77. doi:10.1016/j.ejca.2018.05.009
5. Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Curr Med Chem. 2003;10(1):41–49.
6. Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 1992;81(7):676–684.
7. Singh SK, Vaidya Y, Gulati M, Bhattacharya S, Garg V, Pandey NK. Nanosuspension: principles, perspectives and practices. Curr Drug Deliv. 2016;13(8):1222–1246.
8. Wang L, Du J, Zhou Y, Wang Y. Safety of nanosuspensions in drug delivery. Nanomedicine. 2017;13(2):455–469. doi:10.1016/j.nano.2016.08.007
9. Maniam G, Mai CW, Zulkefeli M, Dufès C, Tan DM, Fu JY. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front Pharmacol. 2018;9:1358. doi:10.3389/fphar.2018.01358
10. Cooper ER. Nanoparticles: a personal experience for formulating poorly water soluble drugs. J Control Release. 2010;141(3):300–302. doi:10.1016/j.jconrel.2009.10.006
11. Pignatello R, Ricupero N, Bucolo C, Maugeri F, Maltese A, Puglisi G. Preparation and characterization of eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS PharmSciTech. 2006;7(1):E27. doi:10.1208/pt070127
12. Xiong R, Lu W, Yue P, et al. Distribution of an intravenous injectable nimodipine nanosuspension in mice. J Pharm Pharmacol. 2008;60(9):1155–1159. doi:10.1211/jpp.60.9.0006
13. Ganta S, Paxton JW, Baguley BC, Garg S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int J Pharm. 2009;367(1–2):179–186. doi:10.1016/j.ijpharm.2008.09.022
14. Sarkar M, Grossman RG, Toups EG, Chow DS. Rational design and development of a stable liquid formulation of riluzole and its pharmacokinetic evaluation after oral and IV administrations in rats. Eur J Pharm Sci. 2018;125:1–10. doi:10.1016/j.ejps.2018.09.004
15. Deepika D, Dewangan HK, Maurya L, Singh S. Intranasal drug delivery of frovatriptan succinate-loaded polymeric nanoparticles for brain targeting. J Pharm Sci. 2019;108(2):851–859. doi:10.1016/j.xphs.2018.07.013
16. Attivi D, Wehrle P, Ubrich N, Damge C, Hoffman M, Maincent P. Formulation of insulin-loaded polymeric nanoparticles using response surface methodology. Drug Dev Ind Pharm. 2015;31(2):179–189. doi:10.1081/DDC-200047802
17. Gil EC, Colarte AI, Bataille B, Pedraz JL, Rodríguez F, Heinämäki J. Development and optimization of a novel sustained-release dextran tablet formulation for propranolol hydrochloride. Int J Pharm. 2006;317(1):32–39. doi:10.1016/j.ijpharm.2006.02.049
18. Luo W, Rao M, Qu J, Luo D. Applications of liquid biopsy in lung cancer-diagnosis, prognosis prediction, and disease monitoring. Am J Transl Res. 2018;10(12):3911–3923. Review.
19. Chen Q, Yang Y, Lin X, et al. Platinum(iv) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem Commun (Camb). 2018;54(42):5369–5372. doi:10.1039/c8cc02791a
20. Han Z, Cao Z, Chatterjee D, Wyche J, Pantazis P. Propionate and butyrate esters of camptothecin and 9-nitrocamptothecin as antileukemia prodrugs in vitro. Eur J Haematol. 1999;62(4):246–255.
21. Kocbek P, Baumgartner S, Kristl J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int J Pharm. 2006;312(1–2):179–186. doi:10.1016/j.ijpharm.2006.01.008
22. Van Eerdenbrugh B, Vermant J, Martens JA. et al. A screening study of surface stabilization during the production of drug nanocrystals. J Pharm Sci. 2009;98(6):2091–2103. doi:10.1002/jps.21563
23. Kostanski JW, DeLuca PP. A novel in vitro release technique for peptide containing biodegradable microspheres. AAPS PharmSciTech. 2000;1(1):E4. doi:10.1208/pt010427
24. Liu X, Wang Y, Vardeman D, Cao Z, Giovanella B. Development and validation of a reverse-phase HPLC with fluorescence detector method for simultaneous determination of CZ48 and its active metabolite camptothecin in mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867(1):84–89. doi:10.1016/j.jchromb.2008.03.013
25. Chow EC, Durk MR, Cummins CL, Pang KS. 1Alpha,25-dihydroxyvitamin D3 upregulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(-/-) and fxr(+/+) mice and increased renal and brain efflux of digoxin in mice in vivo. J Pharmacol Exp Ther. 2011;337(3):846–859. doi:10.1124/jpet.111.179101
26. Bummer P. Interficial phenomena. In: Gennaro A, editor. Remington: The Science and Practice of Pharmacy.
27. Van Eerdenbrugh B, Van Den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364(1):64–75. doi:10.1016/j.ijpharm.2008.07.023
28. Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv. 1995;17(1):31–48. Review. doi:10.1016/0169-409X(95)00039-A
29. Mouton JW, van Peer A, de Beule K, Van Vliet A, Donnelly JP, Soons PA. Pharmacokinetics of itraconazole and hydroxyitraconazole in healthy subjects after single and multiple doses of a novel formulation. Antimicrob Agents Chemother. 2006;50(12):4096–4102. doi:10.1128/AAC.00630-06
30. Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs–a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1–3):151–170. Review. doi:10.1016/j.jbiotec.2004.06.007
31. Li M, Azad M, Davé R, Bilgili E. Nanomilling of drugs for bioavailability enhancement: a holistic formulation-process perspective. Pharmaceutics. 2016;8(2):
32. Möschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–156. doi:10.1016/j.ijpharm.2012.09.034
33. Qi YL Impacts of Size on Pharmacokinetics and Biodistributions of Mebendazole Nanoformulation in Mice and Rats. Dissertation. University of Houston (2008).
34. Yousif R Microemulsion formulations of CZ48, lactone-stabilized camptothecin-C20- propionate, for transdermal delivery. [Dissertation]. University of Houston; 2007.
35. Hsu JP, Lin MJ. Dissolution of solid particles in liquids. J Colloid Interface Sci. 1993;141:60–66. doi:10.1016/0021-9797(91)90302-O
36. Aulton ME. Pharmaceutics: The Science of Dosage Form Design. Edinburgh: Churchill Livingstone; 2002.
37. Pfuma E Pharmacokinetics and pharmacodynamics of combination CZ48 and manumycin A in an athymic mouse model. [Ph.D. Dissertation]. University of Houston; 2009.
38. Liu Y, Zhang D, Duan C, et al. Studies on pharmacokinetics and tissue distribution of bifendate nanosuspensions for intravenous delivery. J Microencapsul. 2012;29(2):194–203. doi:10.3109/02652048.2011.642015
39. Kim WY, Nakata B, Hirakawa K. Alternative pharmacokinetics of S-1 components, 5-fluorouracil, dihydrofluorouracil and alpha-fluoro-beta-alanine after oral administration of S-1 following total gastrectomy. Cancer Sci. 2007;98(10):1604–1608. doi:10.1111/j.1349-7006.2007.00573.x
40. Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–1659. doi:10.1016/j.addr.2004.02.014
41. Allen TM, Everest JM. Effect of liposome size and drug release properties on pharmacokinetics of encapsulated drug in rats. J Pharmacol Exp Ther. 1983;226(2):539–544.
42. Freise J, Müller WH, Magerstedt P. Uptake of liposomes and sheep red blood cells by the liver and spleen of rats with normal or decreased function of the reticuloendothelial system. Res Exp Med (Berl). 1981;178(3):263–269.
43. Peters K, Leitzke S, Diederichs JE, et al. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemother. 2000;45(1):77–83.
44. Burgess DJ. Injectable Dispersed Systems: Formulation, Processing, and Performance. London: Taylor & Francis; 2005.
45. Liehr JG, Harris NJ, Mendoza J, Ahmed AE, Giovanella BC. Pharmacology of camptothecin esters. Ann N Y Acad Sci. 2000;922:216–223. Review.
46. Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol. 1998;38:257–288. Review. doi:10.1146/annurev.pharmtox.38.1.257
Supplementary materials
 | Table S1 Effects of different stabilizers and combination with Tween-80 on particle size, PI and zeta potential of nanosuspensions by media milling preparation method |
 | Figure S1 Dependence of particle size (nm) on the milling time (hrs) (n=3), particle size decreased as milling time increase up to 24 hrs. |
 | Table S2 Summary of central composite design (CCD) fitting parameters |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






