Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Sustained Corticosteroid-Free Clinical Remission During Vedolizumab Maintenance Therapy in Patients with Ulcerative Colitis on Stable Concomitant Corticosteroids During Induction Therapy: A Post Hoc Analysis of GEMINI 1
Authors Loftus EV Jr , Sands BE , Colombel JF, Dotan I, Khalid JM , Tudor D, Geransar P
Received 11 February 2020
Accepted for publication 7 May 2020
Published 11 June 2020 Volume 2020:13 Pages 211—220
DOI https://doi.org/10.2147/CEG.S248597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Edward V Loftus Jr,1 Bruce E Sands,2 Jean-Frédéric Colombel,2 Iris Dotan,3 Javaria Mona Khalid,4 David Tudor,5 Parnia Geransar5
1Division of Gastroenterology and Hepatology, Mayo Clinic College of Medicine, Rochester, MN, USA; 2The Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai Hospital, New York, NY, USA; 3Division of Gastroenterology, Rabin Medical Center, Petah Tikva, Israel, and the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; 4Takeda International – UK Branch, London, UK; 5Takeda Pharmaceuticals International AG, Zurich, Switzerland
Correspondence: Edward V Loftus Jr
Division of Gastroenterology and Hepatology, Mayo Clinic College of Medicine, 200 First Street SW, Rochester, MN 55905, USA
Tel +1-507-284-2511
Email [email protected]
Background: Corticosteroid-free clinical remission is important in ulcerative colitis.
Objective: This GEMINI 1 post hoc analysis evaluated vedolizumab efficacy in achieving sustained corticosteroid-free clinical remission in moderately to severely active ulcerative colitis.
Materials and Methods: GEMINI 1 included a 6-week induction period followed by a 46-week maintenance period. Patients received stable corticosteroid dosing at baseline/during induction and tapered dosing during maintenance. Analysis groups included vedolizumab (induction and maintenance); vedolizumab/placebo (vedolizumab induction, placebo maintenance); and placebo (induction and maintenance). The primary endpoint was sustained corticosteroid-free clinical remission (partial Mayo score ≤ 2, no individual subscore > 1, for ≥ 32 weeks). Multivariate analyses identified covariates associated with the primary endpoint. Safety endpoints included adverse events.
Results: Baseline demographics and concomitant corticosteroid use were similar across groups (n=454). A greater proportion (95% confidence interval) of the vedolizumab group achieved sustained corticosteroid-free clinical remission (10.2% [6.9 to 13.6]) vs the placebo group (1.4% [0.0 to 7.3]; difference 8.9% [– 3.8 to 21.4]). Proportions were similar between the vedolizumab/placebo and placebo groups. Covariates associated with sustained corticosteroid-free clinical remission (odds ratio [95% confidence interval]) were treatment (vedolizumab vs placebo: 9.35 [1.25 to 71.43]; p=0.0605), anti-tumor necrosis factor alpha exposure (yes vs no: 0.26 [0.12 to 0.57]; p=0.0008), and disease duration (≤ 2 vs > 2 years: 2.66 [0.99– 7.19]; p=0.0531). Adverse events were similar across groups.
Conclusion: A numerically greater proportion of vedolizumab-treated patients with ulcerative colitis achieved sustained corticosteroid-free clinical remission. Vedolizumab treatment, no previous anti-tumor necrosis factor alpha exposure, and shorter disease duration were associated with sustained corticosteroid-free clinical remission.
Clinicaltrials.gov: NCT00783718.
Keywords: vedolizumab, ulcerative colitis, corticosteroid, anti-tumor necrosis factor alpha, clinical remission
Introduction
Ulcerative colitis (UC) is a chronic, progressive, relapsing, and remitting disease characterized by chronic inflammation of the colon leading to abdominal pain, bloody diarrhea, rectal urgency, tenesmus, and extraintestinal manifestations.1–5 While the overall incidence of UC in the United States and Europe has stabilized or decreased in recent years,6 UC incidence is increasing globally and in particular across Asia, Latin America, and Eastern Europe.4,7,8 In addition to the severe negative impact of uncontrolled UC on patient quality of life,9 the progressive disease course increases the risk for structural bowel damage, functional impairment, disability, and the potential for hospitalization and colectomy.1,5,10
The main treatment goals for UC are to improve symptoms, achieve sustained clinical remission (and in particular, corticosteroid-free clinical remission), induce and maintain mucosal healing, and ultimately to improve the course of the disease.10–13 Conventional treatment for UC when first-line aminosalicylates have failed includes corticosteroids, which are used for their anti-inflammatory effects. However, systemic corticosteroid therapy can produce side effects that make longer-term use undesirable, including skin bruising, sleep and mood disturbances, infections, weight gain, hyperglycemia, cataracts, glaucoma, and fractures.13–17 After conventional therapy, second-line treatment options for UC include anti-tumor necrosis factor alpha (anti-TNFα) agents (eg, infliximab, adalimumab, golimumab), which act systemically to suppress the immune response and inflammation, the anti-integrin agent vedolizumab, a humanized monoclonal antibody that selectively targets the α4β7 integrin involved in regulating lymphocyte trafficking to the gut, and the janus kinase inhibitor tofacitinib, which targets multiple cytokine signaling pathways to suppress the immune response and inflammation. First-line corticosteroids are intended for short-term use to induce disease control, whereas second-line treatments should maintain control while allowing tapering of corticosteroid therapy.
Biological agents are efficacious treatments for inducing clinical remission in moderately to severely active UC.18 Various meta-analyses have established that anti-TNFα therapy is more effective than placebo in inducing and maintaining clinical remission in patients with ulcerative colitis.19–21 However, up to one-third of patients may be primary nonresponders to anti-TNFα, and many more responsive patients lose response over time.22–25 Furthermore, it has not been clearly established how well anti-TNFα treatment sustains corticosteroid-free clinical remission.
The efficacy of vedolizumab for inducing and sustaining clinical remission has been established in the pivotal GEMINI 1 study.26 Vedolizumab was effective in achieving corticosteroid-free clinical remission at Week 52, but it was not determined how this endpoint was sustained from earlier time points up to and including Week 52.
This post hoc analysis evaluated the efficacy of vedolizumab in achieving sustained corticosteroid-free clinical remission for at least 32 weeks in the GEMINI 1 study. The effect of anti-TNFα treatment history and other disease characteristics on corticosteroid-free clinical remission was also examined.
Materials and Methods
Study Design
This was a post hoc exploratory analysis of data from the GEMINI 1 study (NCT00783718),26 a Phase 3, randomized, placebo-controlled trial with distinct induction and maintenance phases (details published previously; Supplementary Figure 1). There were 2 induction cohorts. Cohort 1 received double-blind placebo or vedolizumab at Weeks 0 and 2, and Cohort 2 (required to ensure an adequate sample size in maintenance phase) received open-label vedolizumab at Weeks 0 and 2. Patients were assessed for clinical response at Week 6. Patients who discontinued treatment during the maintenance phase or completed 52 weeks of treatment had the option of enrolling in a long-term, open-label safety study (GEMINI LTS [ClinicalTrials.gov NCT00790933]) of vedolizumab administered every 4 weeks.
Patients included in this analysis received stable doses of corticosteroids (prednisone ≤30 mg/day or equivalent) at baseline; the corticosteroid dose was maintained during induction and individually tapered during maintenance for patients achieving clinical response at Week 6 or at a subsequent visit.
GEMINI 1 was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All study procedures were approved by local institutional review boards/ethics committees (see Supplementary list), and all patients provided informed, written consent as previously reported.26
Treatment Groups
Three treatment groups were analyzed (Supplementary Figure 1). The vedolizumab group (vedolizumab during induction and maintenance phases) included patients who responded to vedolizumab during the induction phase and were subsequently randomized to double-blind vedolizumab (every 4 weeks [Q4W], every 8 weeks [Q8W]) and patients who did not respond to vedolizumab during the induction phase and received open-label vedolizumab Q4W during maintenance. The vedolizumab/placebo group included patients who responded at Week 6 following 2 induction doses of vedolizumab administered at Weeks 0 and 2 and were then randomized to placebo for the maintenance phase through Week 52. Finally, the placebo group consisted of patients who received placebo during the induction and maintenance phases.
Assessments and Endpoints
Anti-TNFα-naïve status was assessed at screening using an interactive voice recording system and anti-TNFα-failure status was assessed at enrollment by the investigator using the case report form.
The primary endpoint for this analysis in patients using oral corticosteroids at baseline was sustained corticosteroid-free clinical remission, defined as a partial Mayo score ≤2, with no individual subscore >1 for a duration of ≥32 weeks through Week 52 (ie, corticosteroid-free clinical remission had to be achieved at the latest at Week 20).10 The secondary endpoint was time to sustained corticosteroid-free clinical remission. Safety and tolerability were assessed as the incidence and type of adverse events (AEs) occurring during maintenance therapy.
Patients were assessed at weeks 2, 4, and 6 during induction therapy, and subsequently every 4 weeks until Week 52. At each visit, a partial Mayo score (comprising the Mayo score minus the sigmoidoscopy subscore; the score ranges from 0 to 9 and higher scores indicate more active disease) was calculated.27
Data Collection and Substitution
For patients in the vedolizumab group who discontinued GEMINI 1 early and then entered the GEMINI LTS study, missing GEMINI 1 maintenance data were substituted using data from the GEMINI LTS study. This was not possible for the placebo and vedolizumab/placebo groups owing to the lack of a placebo arm in the GEMINI LTS study. Any patient with missing data in the placebo or vedolizumab/placebo arms was considered not in remission. Patients with missing induction data were not substituted.
Statistical Methods
Analyses were performed for the overall population and for the anti-TNFα-naïve and anti-TNFα-failure subgroups. Proportions of patients and percentage-point differences (along with their 95% confidence intervals [CIs]) across vedolizumab, vedolizumab/placebo, and placebo groups were described for the primary endpoint of sustained corticosteroid-free clinical remission. Time to sustained corticosteroid-free clinical remission was estimated using Kaplan-Meier survival analysis. Logistic regression and chi-square analyses were performed to identify covariates significantly associated (p < 0.05) with the primary endpoint. Covariates included severity of UC (moderate vs severe), duration of UC (≤2 years vs >2 years), anti-TNFα treatment (yes vs no), and treatment (vedolizumab vs vedolizumab/placebo or placebo). Descriptive statistics were used to summarize baseline patient demographics, disease characteristics, and safety.
Results
Baseline Characteristics
The overall population of patients receiving corticosteroid at baseline (N=454) included 313, 67, and 74 patients in the vedolizumab, vedolizumab/placebo, and placebo groups, respectively; the number of anti-TNFα-naïve and anti-TNFα-failure patients in each treatment group is presented in Table 1. Patient demographics, baseline disease characteristics, and corticosteroid use at baseline were similar among treatment groups in each population (Table 1).
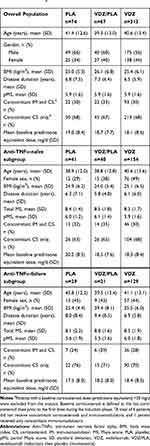 |
Table 1 Baseline Demographics for Patients with Baseline Corticosteroid Usea |
Sustained Corticosteroid-Free Clinical Remission
Among all patients who received corticosteroids at baseline, the proportion of patients who achieved sustained corticosteroid-free clinical remission (≥32 weeks) in the vedolizumab group (10.2%) was higher than in the placebo group (1.4%) (difference 8.9% [95% CI –3.8 to 21.4]); proportions were similar between the vedolizumab/placebo and placebo groups (Figure 1). In anti-TNFα-naïve patients, 16.2% of patients in the vedolizumab group versus 0% of patients in the placebo group (difference 16.2% [95% CI –1.0 to 33.0]) achieved sustained corticosteroid-free clinical remission. In the anti-TNFα-failure subgroup, the corresponding rates were 5.4% versus 3.4% (difference 2.0% [95% CI –18.2 to 22.0]) (Figure 1). There were no differences between vedolizumab/placebo and placebo in the anti-TNFα treatment subgroups.
Kaplan-Meier analyses of time to sustained corticosteroid-free clinical remission in the overall population demonstrated that the vedolizumab group had the highest rate (13.0%) of corticosteroid-free clinical remission for ≥32 weeks until Week 52 compared with the vedolizumab/placebo (8.5%) or placebo (1.8%) groups (Figure 2A). In the anti-TNFα-naïve subgroup, corresponding Kaplan-Meier estimates were 19.0% and 10.8% in the vedolizumab and vedolizumab/placebo groups, respectively; estimates in the placebo group could not be determined owing to a lack of events (Figure 2B). In the anti-TNFα-failure subgroup, corresponding Kaplan-Meier estimates were 7.8% and 5.6% for the vedolizumab and placebo groups, respectively; estimates were not determined in the vedolizumab/placebo group owing to the small number of events (Figure 2C).
Influence of Disease Characteristics
Analysis of predictive modeling factors that may influence sustained corticosteroid-free clinical remission revealed that anti-TNFα treatment (yes vs no, odds ratio [OR] = 0.259; p = 0.0008) was significantly and independently associated with achieving the primary endpoint (Figure 3). There were also strong trends for association of vedolizumab treatment (vedolizumab vs placebo, OR = 9.346; p = 0.0605) and disease duration (“short” [≤2 years] vs “long” [>2 years], OR = 2.660; p = 0.0531) with achieving the primary endpoint that approached but did not reach statistical significance (Figure 3). Similar trends were observed in the anti-TNFα-naïve subgroup, but not in the anti-TNFα-failure subgroup (in which too few events in the placebo group precluded hazard ratio estimation).
Safety
Vedolizumab exhibited a favorable safety/tolerability profile in patients with moderately to severely active UC. Among patients in each treatment group who were receiving corticosteroid therapy at baseline, overall incidences were similar for any AE, any drug-related AE, any AE resulting in treatment discontinuation, any serious AE, any serious infection, and any death (Table 2). The most common AEs during the study (occurring in >3% of vedolizumab patients) in each group are presented in Supplementary Table 1.
 |
Table 2 Adverse Eventsa During Maintenance Therapy in Patients Using a Corticosteroid at Baselineb |
Discussion
Important treatment goals in UC are to achieve and then sustain corticosteroid-free clinical remission, allowing patients to benefit from short-term corticosteroid use while avoiding the safety issues associated with longer-term corticosteroid use.12,13,28-30 This study demonstrated that a greater proportion of UC patients receiving ongoing corticosteroid therapy at baseline achieved sustained corticosteroid-free clinical remission with vedolizumab than placebo, both in the overall population and in the anti-TNFα treatment subgroups. However, differences between treatment groups did not achieve statistical significance. After adjusting for prior anti-TNFα status, disease duration, and disease severity, the likelihood of achieving sustained corticosteroid-free clinical remission was greater among patients receiving vedolizumab compared with placebo.
A previous GEMINI 1 post hoc analysis demonstrated sustained clinical remission with vedolizumab maintenance therapy, but achievement of concomitant corticosteroid-free status plus sustained clinical remission was not evaluated.31 Although additional studies have evaluated clinical outcomes that encompass corticosteroid-sparing, these analyses used less stringent criteria to assess corticosteroid-free clinical remission associated with vedolizumab use.26 The original analysis of GEMINI 1 demonstrated that more UC patients receiving vedolizumab (Q4W: 45.2%; Q8W: 31.4%) than placebo (13.9%, both p ≤0.01) achieved corticosteroid-free clinical remission at Week 52.26 A subsequent analysis found that more UC patients receiving vedolizumab rather than placebo achieved the composite endpoints of clinical remission at Week 52 while also remaining corticosteroid-free for at least the previous 90 days (Q4W 45.2% and Q8W 30.0% vs placebo 13.9%) or 180 days (Q4W 42.5% and Q8W 28.6% vs placebo 11.1%); this benefit was also demonstrated for clinical remission at Weeks 6 and 52 while also corticosteroid-free at Week 52 (Q4W 58.6% and Q8W 39.1% vs placebo 17.4%).32 Another analysis reported higher rates of corticosteroid dose reductions (74% vs 57%) and proportions of patients who were corticosteroid-free for ≥90 days (51% vs 24%) and ≥180 days (48% vs 21%) at Week 52 for vedolizumab compared with placebo.33 In each of these analyses, endpoints were evaluated only among patients who responded after 6 weeks of vedolizumab induction therapy.26,32,33 The current analysis evaluated sustained corticosteroid-free clinical remission for ≥32 weeks up to and including Week 52 among all patients regardless of a response to vedolizumab induction therapy at Week 6: this greater stringency likely accounts for the consistent albeit lower rates of sustained corticosteroid-free clinical remission observed with vedolizumab (10.2%) versus placebo (1.4%) compared with the previous analyses.
The previous analysis of corticosteroid-free clinical remission at Week 52 in the GEMINI 1 study reported that the treatment difference between vedolizumab and placebo was generally independent of prior treatments for UC.26 However, a subsequent analysis of corticosteroid dose reductions at Week 52 demonstrated that vedolizumab treatment was associated with greater reductions than placebo in the anti-TNFα-naïve population compared with the anti-TNFα-failure population.33 In the present analysis, rates of sustained corticosteroid-free clinical remission were higher in both anti-TNFα-naïve and anti-TNFα-failure patients receiving vedolizumab compared with placebo, and while neither group difference reached statistical significance, the difference with treatment was greater in the anti-TNFα-naïve group.
Besides evaluating rates of sustained corticosteroid-free clinical remission in the 3 treatment groups, a multivariate analysis was also undertaken, which adjusted for well-established disease characteristics (ie, disease severity, disease duration, and anti-TNFα treatment history). This analysis demonstrated that vedolizumab was more likely to achieve sustained corticosteroid-free clinical remission compared with placebo in the overall population. In addition to vedolizumab treatment, anti-TNFα-naïve status and shorter disease duration were independently associated with an increased likelihood of sustained corticosteroid-free clinical remission.
There have been few studies of anti-TNFα agents that have evaluated corticosteroid-free clinical remission, and even fewer involving the sustainability of this endpoint. In the ACT1 study, the rates of corticosteroid-free status and corticosteroid-free symptomatic remission were higher with infliximab (63% and 47%, respectively) than placebo (42% and 33%, respectively).34 In the ULTRA2 study, adalimumab was more effective than placebo at achieving sustained corticosteroid-free clinical remission at both Week 32 and Week 52 (10% vs 1.4%; p = 0.002).35 In an open-label follow up to the ULTRA1 study, over half (53–56%) of patients using corticosteroids at baseline were free of corticosteroid use at Week 52; approximately one-quarter of these patients (24.6–26.1%) were in corticosteroid-free clinical remission and 90% had been free of corticosteroids for at least 90 days.36 A valid comparison of these results with the current findings from GEMINI 1 is not feasible because of substantial differences in patient population, study design, and study endpoints.
While anti-TNFα agents have a generally favorable safety/tolerability profile, they are still associated with several potentially serious safety concerns. In ULTRA2, which allowed concomitant corticosteroid (tapering permitted) or immunosuppressant therapy, a significantly greater proportion of adalimumab-treated patients developed injection site-related (12.1% vs 3.8%) or hematologic-related AEs (1.9% vs 0) compared with patients receiving placebo.35 Serious AEs (12% each) and serious infections (1.6% and 1.9%, respectively) occurred in similar proportions of patients in the adalimumab and placebo groups. In contrast, vedolizumab was associated with a favorable safety/tolerability profile in patients with moderately to severely active UC. The incidences of AEs, treatment-related AEs, AEs resulting in treatment discontinuation, serious adverse events, and deaths were similar in each treatment group. The safety/tolerability profile of vedolizumab described in this post hoc analysis is consistent with that previously reported in this patient population in GEMINI 1.26
The clinical benefit of corticosteroid-free clinical remission is substantial and has the potential to reduce or eliminate severe AEs associated with corticosteroid use. In a population-based study of long-term corticosteroid-related AEs (N=2,167), the risk of clinically relevant AEs such as sleep disturbance, weight gain, skin thinning or bruising, and mood problems was dose-related (adjusted ORs; 2.8, 2.2, 3.0, and 2.4, respectively), among patients in the highest quartile of cumulative prednisone-equivalent dosage (>4.7 mg; eg, 10 mg/day prednisone for 18 months).30 Nearly all patients reported 1 or more corticosteroid-related AE and over half reported at least 1 corticosteroid-related AE that was very bothersome (eg, weight gain).30 Serious potentially corticosteroid-related AEs including cataracts (15%) and fractures (12%) were also common.30
This post hoc analysis has several limitations. First, the study was not designed, and therefore not sufficiently powered, to detect significant differences between treatment groups for the primary endpoint. Second, bias may exist against the results obtained in the placebo and vedolizumab/placebo groups because missing maintenance data could only be substituted by long-term data (from the GEMINI LTS study) for patients receiving vedolizumab. The current results must also be viewed in the context that only a small number of patients achieved the primary endpoint in the overall population, with even lower patient numbers in the anti-TNFα subgroups.
Conclusions
In conclusion, this post hoc study of GEMINI 1 demonstrated that vedolizumab was associated with a greater likelihood of achieving sustained corticosteroid-free clinical remission, using strictly defined criteria, compared with placebo in patients with UC. Vedolizumab treatment, anti-TNFα-naïve status, and disease duration ≤2 years were independently associated with achieving sustained corticosteroid-free clinical remission. Larger studies specifically designed to assess sustained corticosteroid-free clinical remission in UC are warranted to confirm and expand the current findings.
Abbreviations
AE, adverse event; anti-TNFα, anti-tumor necrosis factor alpha; BMI, body mass index; CI, confidence interval; CS, corticosteroid; IM, immunomodulator; LTS, long-term safety; PLA, placebo; pMS, partial Mayo score; Q4W, every 4 weeks; Q8W, every 8 weeks; SD, standard deviation; UC, ulcerative colitis; VDZ, vedolizumab.
Data Sharing Statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Acknowledgments
Editorial assistance and medical writing support were provided by Chris Barnes, PhD, of ProEd Communications, Inc.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Takeda. Editorial assistance and medical writing support were funded by Takeda.
Disclosure
EVL has received financial support for research from: AbbVie, Takeda, Janssen, UCB, Amgen, Pfizer, Genentech, Celgene, Receptos, Gilead, MedImmune, Seres Therapeutics, and Robarts Clinical Trials; and has served as a consultant for AbbVie, Takeda, Janssen, UCB, Amgen, Pfizer, Eli Lilly, Celltrion Healthcare, Allergan, Bristol-Myers Squibb, Gilead, Genentech, Celgene, and Boehringer Ingelheim. BES has received financial support for research from: Takeda, Janssen, Theravance Biopharma, Pfizer; and has served as a consultant for: 4D Pharma, AbbVie, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Capella Bioscience, Celgene, Celltrion Healthcare, Eli Lilly and Company, EnGene, F. Hoffmann-La Roche, Ferring, Gilead Sciences, Ironwood Pharmaceuticals, Janssen, Lyndra, MedImmune, Oppilan Pharma, Otsuka America Pharmaceutical, Palatin Technologies, Pfizer, Progenity, Prometheus Laboratories, Protagonist Therapeutics, Rheos Medicines, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Synergy Pharmaceuticals, Takeda, Target PharmaSolutions, Theravance Biopharma, TiGenix, UCB, Valeant Pharmaceuticals North America, Vivelix Pharmaceuticals. J-FC has served as a consultant/advisory board member for: AbbVie, Amgen, Arena Pharmaceuticals, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Geneva Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Immunic, Ipsen, Landos, Medimmune, Merck & Co., Novartis, O Mass, Otsuka, Pfizer, Takeda, Tigenix, and Viela Bio; has served as a speaker for: AbbVie, Allergan, Amgen, Ferring Pharmaceuticals, and Takeda; has received research support from: AbbVie, Janssen Pharmaceuticals, and Takeda; and has stock options in: Intestinal Biotech Development and Genfit. ID has served as a consultant/advisory board member for: Pfizer, AbbVie, Janssen, Takeda, Genentech, Neopharm, Celltrion, Celgene, Arena, Gilead, Medtronic/given imaging, Rafa Laboratories; has served as a speaker for: Takeda, AbbVie, Janssen, Genentech, Pfizer, Ferring, Falk Pharma, Celltrion; has received research support from: Pfizer, AbbVie. JMK was an employee of Takeda International - UK Branch at the time this study was conducted. DT was a biostatistics consultant on temporary contract at Takeda Pharmaceuticals AG at the time this study was conducted. PG is an employee of Takeda Pharmaceuticals International AG and holds Takeda stock or stock options. The authors report no other conflicts of interest in this work.
References
1. Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18(7):1356–1363. doi:10.1002/ibd.22839
2. Dart RJ, Samaan MA, Powell N, Irving PM. Vedolizumab: toward a personalized therapy paradigm for people with ulcerative colitis. Clin Exp Gastroenterol. 2017;10:57–66. doi:10.2147/CEG.S110547
3. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. doi:10.1056/NEJMra0804647
4. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. doi:10.1053/j.gastro.2011.10.001
5. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi:10.1016/S0140-6736(16)32126-2
6. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
7. da Silva BC, Lyra AC, Rocha R, Santana GO. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol. 2014;20(28):9458–9467. doi:10.3748/wjg.v20.i28.9458
8. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi:10.1038/nrgastro.2015.150
9. Williet N, Sarter H, Gower-Rousseau C, et al. Patient-reported outcomes in a French nationwide survey of inflammatory bowel disease patients. J Crohns Colitis. 2017;11(2):165–174. doi:10.1093/ecco-jcc/jjw145
10. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6(10):965–990. doi:10.1016/j.crohns.2012.09.003
11. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338. doi:10.1038/ajg.2015.233
12. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784. doi:10.1093/ecco-jcc/jjx009
13. Kornbluth A, Sachar DB. Practice parameters committee of the American College of Gastroenterology. ulcerative colitis practice guidelines in adults: American College of Gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501–523. doi:10.1038/ajg.2009.727
14. Rubin DT, Mody R, Davis KL, Wang CC. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143–1155. doi:10.1111/apt.12727
15. Rubin DT, Patel H, Shi S, Mody R. Assessment of corticosteroid-related quality of care measures for ulcerative colitis and Crohn’s disease in the United States: a claims data analysis. Curr Med Res Opin. 2017;33(3):529–536. doi:10.1080/03007995.2016.1267616
16. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One. 2016;11(6):e0158017. doi:10.1371/journal.pone.0158017
17. Cross RK. Safety considerations with the use of corticosteroids and biologic therapies in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2017;23(10):1689–1701. doi:10.1097/MIB.0000000000001261
18. Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160(10):704–711. doi:10.7326/M13-2403
19. Lopez A, Ford AC, Colombel JF, Reinisch W, Sandborn WJ, Peyrin-Biroulet L. Efficacy of tumour necrosis factor antagonists on remission, colectomy and hospitalisations in ulcerative colitis: meta-analysis of placebo-controlled trials. Dig Liver Dis. 2015;47(5):356–364. doi:10.1016/j.dld.2015.01.148
20. Lv R, Qiao W, Wu Z, et al. Tumor necrosis factor alpha blocking agents as treatment for ulcerative colitis intolerant or refractory to conventional medical therapy: a meta-analysis. PLoS One. 2014;9(1):e86692. doi:10.1371/journal.pone.0086692
21. Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2014;39(7):660–671. doi:10.1111/apt.12644
22. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4(4):355–366. doi:10.1016/j.crohns.2010.04.004
23. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–787. doi:10.1136/gut.2010.221127
24. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. doi:10.1038/ctg.2015.63
25. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi:10.1056/NEJMoa050516
26. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi:10.1056/NEJMoa1215734
27. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–1666. doi:10.1002/ibd.20520
28. Regueiro MD, Greer JB, Hanauer SB. Established management paradigms in IBD: treatment targets and therapeutic tools. Am J Gastroenterol Suppl. 2016;3(3):8–16. doi:10.1038/ajgsup.2016.16
29. Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148(5):1035–1058 e1033. doi:10.1053/j.gastro.2015.03.001
30. Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–426. doi:10.1002/art.21984
31. Feagan BG, Schreiber S, Wolf DC, et al. Sustained clinical remission with vedolizumab in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2019;25(6):1028–1035. doi:10.1093/ibd/izy323
32. Sands BE, Hanauer SB, Columbel J-F, et al. Reductions in corticosteroid use in patients with ulcerative colitis or crohn’s disease treated with vedolizumab. UEG J. 2013;1(suppl 1):A370.
33. Loftus EV, Siegel CA, Panaccione R, et al. Corticosteroid dose reduction in ulcerative colitis patients treated with vedolizumab during the GEMINI 1 trial. Am J Gastroenterol. 2015;110(suppl1):
34. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194–1201. doi:10.1053/j.gastro.2011.06.054
35. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–265. doi:10.1053/j.gastro.2011.10.032
36. Reinisch W, Sandborn WJ, Panaccione R, et al. 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis. 2013;19(8):1700–1709. doi:10.1097/MIB.0b013e318281f2b7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



