Back to Journals » Cancer Management and Research » Volume 9
Survivorship of patients with head and neck cancer receiving care in a tertiary health facility in Nigeria
Authors Okwor VC, Fagbamigbe AF, Fawole OI
Received 24 January 2017
Accepted for publication 24 April 2017
Published 21 July 2017 Volume 2017:9 Pages 331—338
DOI https://doi.org/10.2147/CMAR.S133108
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Vitalis C Okwor,1,2 Adeniyi F Fagbamigbe,1 Olufunmilayo I Fawole1
1Department of Epidemiology and Medical Statistics, Faculty of Public Health, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria; 2Department of Radiation Medicine, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Nigeria
Background: Head and neck cancer (HNC) is a major clinical and public health concern worldwide which impairs the vital functions of life. The treatment of metastatic HNCs is mainly palliative. This study examined the survival patterns and treatment outcomes in patients with HNCs in a tertiary hospital in Nigeria.
Materials and methods: A review of the case files and treatment cards of patients with histological diagnosis of HNCs seen between January 2002 and December 2011 at the Radiotherapy Department, University College Hospital, Ibadan, was conducted. A total of 494 cases were identified, of which 481 had valid records. Analyses were done using Kaplan–Meier survival function and Cox proportional hazard regression techniques at 5% significance level.
Results: The median age of patients was 42 years with a male-to-female ratio of 2:1. Most patients presented at stages 3 (50.7%) and 4 (36.8%). Nasopharyngeal carcinoma was the most common (42.6%) HNC, followed by paranasal sinus (17.7%) and laryngeal cancer (11.6%). The lung was the most common site of metastasis (25.5%). Patients who presented at stages 1 and 4 disease had a median survival of 7.8 years and 1.9 years, respectively. Patients treated with a combination of chemotherapy and radiotherapy had a median survival of 8.0 years compared with those who had a single modality of treatment (~6.3 years).
Conclusion: Patient survival was inversely proportional to the stage of the disease. To encourage the early presentation of HNC cases, health education of the population on routine medical check-ups and on the symptoms suggestive of HNC is recommended. Health care providers should be trained to refer suspected cases promptly to tertiary health facilities for management.
Keywords: head and neck cancer, treatment outcome, survivorship patterns
Introduction
Head and neck cancers (HNCs) are primary malignant neoplasms that occur in several anatomical sites in the head and neck region of humans.1,2 The incidence of HNC is growing worldwide, while survival rates have been reported to improve.3 There is increasing prevalence of HNC in Nigeria.4 The incidence of HNC is threefold higher among men than women and more common in the African Americans than in the White population.5 The incidence of mortality from squamous cell carcinoma of the head and neck (SCCHN) was higher in Blacks than in Whites.6 The 5-year survival rate of HNC was better among Whites than African Americans (61%–64% vs. 40%–52%).7 A Nigerian study found a male predominance in HNC, with reported male-to-female ratios ranging from 2 to 1.8
Studies suggest that HNC affects Nigerians at a younger age when compared with the Caucasians.4,8 Shorter life expectancy in Africans compared with Caucasians and earlier exposure to risk factors of HNC have also been documented.4 The peak occurrence of HNC in Nigeria ranged from the third to sixth decades of life.8,9 There is no consensus on the causes of these differences, but it is thought to be influenced by access to care, stage at diagnosis, insurance status and attitudes of health care providers. HNC is also strongly associated with alcohol consumption, ultraviolet light, some chemicals used in workplaces and certain strains of viruses, such as the human papillomavirus.9
The preference of many patients for non-orthodox medical care contributes to late or complete lack of presentation at health facilities, thereby distorting the true epidemiological picture of the disease.10 Also, survival after development of metastatis is poor. Unfortunately, most patients present at the advanced stage with metastasis to various organs resulting in poor prognosis. The median overall survival for metastatic HNC is reported to be <1 year.11 Management and inherent survivorship of HNC vary greatly and depend on patient’s stage at presentation and location of HNC, treatment history and psychosocial factors such as age and sex.12
HNCs are associated with enormous challenges including social, psychological and devastating effects of treatment. HNC often disfigures patients and causes stigmatization. These challenges are made worse because of the complexity of the anatomical structures, functions affected and the variety of professional disciplines involved in the management of patients. A critical aspect of the care of HNC patients is a good knowledge of patterns of disease progression and treatment failure. These are crucial in guiding future treatment plans and in improving the outcomes of treatment.
Studies on survivorship of patients with HNC have been conducted in the more developed countries, but only limited work has been done in Nigeria.1,2,12–14 Hence, assessment of survivorship patterns of patients with HNC in Nigeria could provide literature on the disease for low-income countries. Therefore, this study assessed the survival pattern of patients receiving care for HNC in a tertiary and teaching hospital in Ibadan, Nigeria. The outcome of this study will improve health care providers’ understanding on the outcome of treatment of HNCs. It will also help clinicians with the treatment of patients, enhance efficient counseling of patients and their care givers and assist policy makers in developing guidelines to improve management of cancer patients.
Materials and methods
Study area
The data were collected from records of HNC patients receiving care at the Radiotherapy Department of the University College Hospital, Ibadan, Nigeria. The hospital is a referral center for secondary health facilities in the southern region of the country. It is at the forefront in cancer treatment and management in the country. The radiotherapy department has 18 beds for in-patients and manages >40 patients with HNC each year. The department has seven consultants, 18 resident doctors, 20 nurses, nine radiographers, two physicists and six health attendants. Three clinics are held every week with an average of eight HNC cases per week.
Study population
All patients with HNC managed at the hospital between 1 January 2002 and 31 December 2011 were included in the study. The patients were cases with primary malignant neoplasms at any anatomical site in the head and neck region. These included malignant neoplasms of the oral cavity, nasal cavities, paranasal sinuses, nasopharynx, hypopharynx, oropharynx and salivary gland.
Study design
A retrospective study design was employed by review of hospital records (case series). Case files of all patients with histologically diagnosed SCCHN were included in the study.
Data collection
All radiotherapy treatment records of patients diagnosed with HNC between January 2002 and December 2011 were retrieved by six trained medical record clerks. To ensure data reliability, the retrieved records were cross checked by a radiotherapist. The proforma was used to extract information on sociodemographics, duration of the illness, time of onset of illness, time of presentation and time at diagnosis of cancer and time of metastasis to other sites. Pathological features such as site of the disease, the stage at presentation, the lymph node status, the histological cell type, the histological grade of the disease, the treatment regimen received, the number of cycles received and the site of treatment were also extracted. Grade of the disease was classified as well differentiated (grade 1), moderately differentiated (grade 2), poorly differentiated (grade 3) and undifferentiated (grade 4), while the site(s) of metastasis at presentation was determined from reports of both clinical examination and radiological tests during pretreatment evaluation. The outcome of treatment was determined in two ways, namely, metastasis- and disease-free interval. The former was based on absence or presence of metastasis to other organs and the latter on locoregional recurrence-free interval or distant metastasis-free interval after oncology treatment. The end point of observation or follow-up was distant metastasis and survival pattern.
Study variables
The independent variables were stage of the disease at presentation (1–4), site of the disease (lung, bone, liver, eye, ear, nose, brain and axillary lymph node), the grade of the disease (grades 1–4), treatment received (incomplete chemotherapy, complete chemotherapy, incomplete radiotherapy, complete radiotherapy and both chemotherapy or radiotherapy) and site of metastasis (lung, bone, liver, eye, ear, nose, brain and axillary lymph node). Other included patients’ background characteristics are age and education. The dependent variables were disease-free interval (recurrence or being metastasis free) and outcome of treatment (grouped into disease free, locoregional recurrence, distant metastasis, residual disease and “lost to follow-up”). Lost to follow-up referred to cases whose outcomes were only known as of the time they were last seen. The patients lost to follow-up were censored.
Data analysis
Data were analyzed using the Statistical Package for Social Sciences (SSPS) version 16.0. Descriptive statistics was used to describe patients’ characteristics and stages of the cancer. Survival analysis of the cancer progression from the time of presentation to the time of recurrence or being metastasis free was carried out. The rate of survival was determined using Kaplan–Meier survival estimation techniques. Cox proportional hazard regression techniques were used to determine factors influencing the treatment outcome. Chi-square statistics was used to determine the association between independent variables and study outcomes at 5% significance level.
Ethical considerations
Ethical clearance was obtained from the Joint Ethical Review Committee of the University of Ibadan/University College Hospital, Ibadan. The need for consent from patients to review their medical records was waived. To maintain confidentiality, the names of patients were not retrieved. The records of patients were kept confidential, and persons outside the research team had no access to it. The data in the extraction forms were entered into a password-protected computer that was accessible to the researchers only.
Results
Sociodemographic characteristics
A total of 481 cases with information on key variables for the study were retrieved. These transformed to incidence of four HNC patients per month. The cases were aged 11–80 years, with a mean age of 41.8±7.5 years. In all, the males were significantly older than the females with an average age of 42.7 years compared to 39.8 years in the females, respectively (p=0.03). Most (66%) of the patients were males (Table 1).
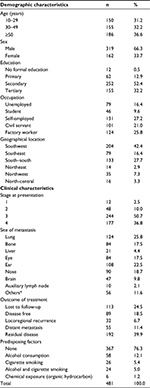  | Table 1 Sociodemographic and clinical characteristics of the subjects Note: *Others include different levels of neck nodes. |
Clinical characteristics
The clinical parameters of the patients are summarized in Table 1. At presentation, about half (50.7%) reported with stage 3 disease, while 36.8% reported with stage 4. About one-fourth of the patients (24.5%) were censored due to “loss to follow-up” or death. A small proportion of the patients (18.5%) were disease free, while 40% of the patients had residual disease. Also, <7% had locoregional recurrence, while 11.4% had distant metastasis to other organs, with the lung being most affected (25.8%), followed by the ear (22.5%) and nose (18.7%).
The distribution of cases presented by anatomical sites showed that the nasopharynx (30.4%) was the most affected anatomical site followed by larynx (7.9%), while hypopharynx had the least (2.9%). The yearly distribution of cancer presentation also showed the highest presentation of HNC cases (60%) in the hospital in 2004 and 2009. The presentations in years 2010 and 2011 decreased compared to the figures in 2008 and 2009 (not shown in the tables).
The test of association between treatment outcomes and the risk factors and the proportion surviving cancer treatments by selected characteristics are shown in Table 2. Results showed that patients’ age, sex, stage at presentation, type of treatment received and anatomical site affected were significantly associated with a disease-free outcome (p<0.05). The proportion of disease-free patients was highest among the younger (10–29) year age group (34.0%) compared to those aged between 30–49 years (8.3%) and 50 years and above (14.0%). Additionally, women reported significantly higher proportion (34.0%) of disease-free outcomes compared to their male counterparts (10.7%), while patients who received complete radiotherapy had significantly higher (38.8%) disease outcome compared to other treatment regimens such as combined therapy (13.2%), incomplete chemotherapy (34.4%) and incomplete radiotherapy (8.4%). Conversely, patient’s education had no significant association with outcome of treatment. The overall survival, that is, the proportion that was disease free after treatment, was 18.5%.
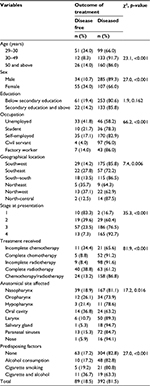  | Table 2 Association between patients’ characteristics and cancer treatment outcomes |
Survival analysis by clinical characteristics
The Kaplan–Meier survival estimate for survivorship of HNC from the time of completion of treatment is shown in Figure 1. Among the three types of treatments given to the patients, the combined therapy consistently had higher survivorship. The median survival time among the patients by the stages of presentation is shown in Table 3. About 50% of patients who presented at stage 1 survived for >7.8 years before experiencing disease recurrence, while those presented at stage 4 had the shortest median survival time (1.9 years); these differences were statistically significant. The combined therapy (radiotherapy and chemotherapy) was the most effective approach with median survival of 8.0 years followed by radiotherapy (7.0 years), while the least (1.3 years) was observed for incomplete chemotherapy recipient group. About 91.6% of the patients survived locoregional recurrence beyond 2.5 years and 65.5% could survive up to 8.5 years. Also, 6 months survival to distant metastases was 97.6%. The overall median survival time was 6.1 years.
  | Figure 1 Kaplan–Meier curve for disease survivorship by therapies taken. Abbreviation: Cum survival, cumulative survival. |
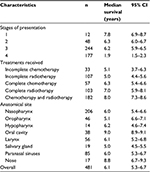  | Table 3 Median survival by clinical variables at presentation and treatments received Abbreviation: CI, confidence interval. |
Survival analysis of HNC patients by baseline variables
Disease progression (the event) was observed in a total of 279 patients. The shortest survival time was 6 months, and the maximum was 11 years. The median survival time was 6.8 years. After controlling for other variables, a unit increase in age of the patient raised the risk of disease progression by 1.11 times. That is, the younger patients were more likely to survive than the elderly. Also, the progressions were 0.35 and 0.26 times less likely among the unemployed and the students compared to factory workers, respectively. Patients who presented at stages 1 and 2 were 0.47 and 0.58 times less likely to experience disease progression compared with those who presented at stage 4, respectively. The risk of disease progression was 1.43 times higher among recipients of chemotherapy than those who received the combination of therapies, while it was 1.10 times higher among the recipients of radiotherapy (Table 4). This relationship is shown in Figure 1; the combination of therapies improved survivorship.
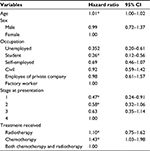  | Table 4 Hazard ratio of progression of diseases according to the baseline variables Note: *Significant at p<0.05. Abbreviation: CI, confidence interval. |
Discussion
In this study, we assessed the differentials in survivorship of HNC with regard to stage at presentation, therapies received and background of the patients involved in the study. We found that monthly average incidence of HNC obtained in our study was higher than values reported in another teaching hospital settings in Nigeria.15,16 This may be due to high volume of referrals to the health facility used in our study. Also, most tertiary institutions in the country do not have treatment facilities such as Radiotherapy at the University College Hospital, Ibadan. The 40–49 years age group was the most affected, which corresponded with the peak age group reported in Lagos, Nigeria.17 The higher prevalence of HNC in males compared to females is also consistent with a previous study conducted in Enugu, Nigeria.18 This may be attributed to higher tendency of men to smoke cigarette and drink alcohol compared to women.19 This may also be due to men’s greater ability to afford and therefore utilize health facilities, hence they present earlier than women. The reduction in number of cases in 2010 and 2011 compared with 2008 and 2009 figures may indicate a reduction in cases or may be due to industrial action by hospital trade unions. This makes the trend in disease presentation difficult to decipher.
Nasopharyngeal cancer was the most common HNC observed. This was also the finding in Zaria, Nigeria,8 and Texas, USA,20 but contrary to findings reported in Maiduguri, Nigeria,15 and Ilorin, Nigeria,21 which all reported oral cavity as the commonest site of HNC. These studies were carried out in the Northern part of Nigeria compared to our study where most patients reside in the Southern region. Perhaps, these differences are attributable to regional differentials in the types of cancer in Nigeria. An earlier study suggested that the primary site of involvement may be related to the geographical location and the sociocultural practice of the people of that region.22 Overall, the lung was the commonest site of distant metastasis, which is in contrast to a London study that reported that bone was the most common site of distant metastasis, followed by the lungs and liver.23
As reported by other authors in Nigeria, there was late presentation by HNC patients in our study. This is consistent with other studies in Nigeria.8,18 Onotai and Nwogbo18 noted that most patients presented to the hospital late and often with advanced-stage disease because of their inability to afford treatment, ignorance, incorrect diagnosis and reliance on native medications. Unfortunately, late presentation reduces the prognosis of the disease and militates against achieving good quality of life.3,11,12 Hence, there is the need for further studies to describe health-seeking behavior of patients at settings such as in our study area and to identify reasons for late presentation to guide the development of appropriate interventions.
The median survival was longest among patients who presented at stage 1, indicating that the best treatment outcome is achieved when HNC cases present in the early stage when the disease is still localized and the patients more responsive to treatment therapies. Hence, patients can survive longer unlike in advanced-stage disease where other organs of the body may have been affected. In the advanced stage of the cancer, patients may also develop nutritional deficiencies that also reduce response to treatment. Hence, in high-income countries such as the USA, American Cancer Society advocates early reporting and immediate treatment of HNC by general health practitioners.13 Patients with locoregional recurrence had relatively better survivorship than those with distant metastasis. This difference could be due to availability of salvage therapy in patients with locoregional recurrence.24
Males had higher risk of rapid disease progression, probably due to their poorer adherence to treatment.8 The median survival was higher among patients on combined chemotherapy and radiotherapy treatment, which is actually the standard treatment than those on single treatment.25 The implication of this is that clinicians need to encourage patients to opt for combined treatment despite the fact that it may be more expensive, inconvenient for the patients and more demanding for caregivers. However, in the long term, it improves patients’ survival. About 87% of the patients presented at stages 3 and 4, when surgery is less effective. The remaining 13% who presented relatively earlier were predominantly patients with nasopharyngeal carcinoma who were treated with chemoradiation therapy. Therefore, surgery was only/mainly done to establish a diagnosis, hence limited this study to the patients treated with concurrent chemoradiation therapy, radiotherapy alone and chemotherapy alone. The ideal treatment is concurrent chemoradiation therapy for HNC. However, there is only one radiotherapy machine in our center with high number of patients with different pathologies awaiting their turn. And also, some patients face challenges of providing fund for chemotherapy and radiotherapy at the same time in the absence of health insurance. Patients are treated based on what they can afford as well as available facilities. Hence, the need for some of the patients to commence radiotherapy separately from chemotherapy even with the full knowledge that concurrent chemoradiation therapy is the best option.
Patients with malignancy of the salivary gland had lower median survival time compared to other sites probably due to the deep location nature of the medial part of the deep lobe, which is also the part that most often causes recurrence. The oral cavity tumor had highest median survival, and this could be attributed to early disease detection. A greater proportion of the patients had radiotherapy. This indicates that the patients received radical radiotherapy with palliative intent.
The study has some limitations. This included missing values for some variables due to incomplete information in patients’ case record files and also because some patients defaulted treatment. The missing values are due to the retrospective nature of the study. Hence, future studies could employ observational and prospective study design. This study was conducted in a tertiary health facility; hence, the results may not be generalizable to other levels of health care delivery or the general population.
Conclusion
The most commonly affected primary anatomical site of HNC was the nasopharynx, while the lung was the most common site of distant metastasis. Younger patients with HNC survived for a relatively longer period compared to older patients. Patients’ survival was inversely proportional to the stage of the disease. Treatment outcomes and survivorship were enhanced by combining chemotherapy and radiotherapy. However, survivorship was worsened by late presentation for the treatment at advanced stage of the disease. Health education of the general population to improve awareness on signs of HNC and thereby promote early presentation by care providers is recommended. The general practitioners and other health care providers should be encouraged to refer any suspected cancer cases promptly to teaching hospitals.
Acknowledgments
The authors acknowledge the support received from staff from the Department of Radiotherapy, University College Hospital, Ibadan, and the technical support given to the second author by the Consortium for Advanced Research Training in Africa (CARTA).
CARTA has been funded by the Wellcome Trust (UK) (Grant No: 087547/Z/08/Z), the Department for International Development (DfID) under the Development Partnerships in Higher Education (DelPHE), the Carnegie Corporation of New York (Grant No: B 8606), the Ford Foundation (Grant No: 1100-0399), and the Bill and Melinda Gates Foundation (Grant No: 51228).
Disclosure
The authors report no conflicts of interest in this work.
References
Rischin D, Maccallum P, Centre C, Le Q. Overview of advances in head and neck cancer. J Clin Oncol. 2015;33(29):3225–3227. | ||
Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33(29):3314–3321. | ||
Ringash J. Survivorship and quality of life in head and neck cancer. J Clin Oncol. 2015;33(29):3332–3337. | ||
Adeyemi BF, Adekunle LV, Kolude BM, Akang EE, Lawoyin JO. Head and neck cancer: a clinicopathological study in a tertiary care centre. J Natl Med Assoc. 2008;100(6):690–694. | ||
Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. | ||
Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the U.S. 1998–2003. Cancer. 2008;15(113):2901–2909. | ||
Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2007;12(1):20–37. | ||
da Lilly-Tariah OB, Somefun AO, Adeyemo WL. Current evidence on the burden of head and neck cancers in Nigeria. Head Neck Oncol. 2009;9:1–14. | ||
Ridge JA, Glisson BS, Lango MN. Head and neck tumors, cancer management: a multidisciplinary approach. J Oncol. 2008;3:369–408. | ||
Tovey P, Broom A, Chatwin J, Hafeez M, Ahmad S. Patient assessment of effectiveness and satisfaction with traditional medicine, globalized complementary and alternative medicines, and allopathic medicines for cancer in Pakistan. Integr Cancer Ther. 2005;4(3):242–248. | ||
Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13(1):35–46. | ||
Hutcheson KA, Carol ML. Head and neck cancer survivorship management. In: Foxhall LE, Rodriguez MA, editors. Advances in Cancer Survivorship Management. Texas: Springer; 2014:145–166. | ||
Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203–239. | ||
Wan FCV, Panwar A, Lydiatt WM. Cancer survivorship care in the head and neck patient : current practices. Austin J Otolaryngol. 2015;2(3):1034. | ||
Amusa YB, Olabanji JK, Ogundipe OV. Pattern of head & neck malignant tumour in a Nigerian teaching hospital: a ten year review. West Afr J Med. 2004;23(4):280–285. | ||
Otoh EC, Johnson NW, Danfillo IS, Adeleke OA, Olasoji HA. Primary head and neck cancers in North Eastern Nigeria. West Afr J Med. 2004;23(4):305–313. | ||
Nwawolo CC, Ajekigbe AT, Oyeneyin JO, Nwankwo KC, Okeowo PA. Pattern of head and neck cancers among Nigerians in Lagos. West Afr J Med. 2001;20(2):111–116. | ||
Onotai LO, Nwogbo AC. Primary head and neck malignant tumours in Port Harcourt, Nigeria. J Med. 2012;3(2):122–125. | ||
FMoH. National HIV/AIDS and Reproductive Health and Serological Survey, 2012 (NARHS Plus). Abuja, Nigeria: Federal Ministry of Health; 2013. | ||
Ahamad A, Ang KK. Nasal Cavity and Paranasal Sinuses. In: Halperin EC, Perez CA, Brady LW, editors. Perez and Brady’s Principles and Practice of Radiation Oncology. Vol. 39. 5th ed. Washington, DC: Lippincott Williams & Wilkins; 2008. | ||
Ologe FE, Adeniji KA, Segun-Busari S. Clinicopathological study of head and neck cancers in Ilorin, Nigeria. Trop Doct. 2005;35(1):2–4. | ||
Bhurgri Y, Bhurgri A, Usman A, et al. Epidemiological review of head and neck cancers in Karachi. Asian Pac J Cancer Prev. 2006;7(2):195–200. | ||
Lee N, Colevas AD, Karen KF. Cancer of the Nasopharynx, Text Book of Radiation Oncology. 2nd ed. New York: Wiley; 2004. | ||
Ian K. Mouth, Secondary Nodes of Neck, Paranasal Sinuses, Ear, Salivary Glands. In Bomford CK, editor. Walter and Miller’s Textbook of Radiotherapy, Radiation Physics, Therapy and Oncology. Vol. 23. 6th ed. New York: Churchill Livingstone; 2003. | ||
Kuperman D, Arquette MAD. Head and Neck Cancer, the Washington Manual of Oncology. 2nd ed. Washington, DC: Springer; 2010. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
