Back to Journals » Infection and Drug Resistance » Volume 15
Survival Patterns and Predictors of Mortality among COVID-19 Patients Admitted to Treatment Centers in Oromia Region, Ethiopia
Authors Habtewold EM , Dassie GA , Abaya SG, Debela EA , Bayissa BL, Girsha WD , Abebe AD , Sori HL, Komicha MA, Sori BK, Bajiga GS, Heyi ML, Iticha DG, Jiru TK, Hurissa MB , Bayisa DA, Amante LT, Sima YA , Dhaba DG
Received 26 February 2022
Accepted for publication 1 August 2022
Published 5 September 2022 Volume 2022:15 Pages 5233—5247
DOI https://doi.org/10.2147/IDR.S355060
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Ephrem Mannekulih Habtewold,1 Godana Arero Dassie,1 Shileshi Garoma Abaya,1 Endashaw Abebe Debela,2 Bekana Lemessa Bayissa,3 Worku Dugassa Girsha,1 Alem Deksisa Abebe,1 Hunde Lemi Sori,1 Meyrema Abdo Komicha,1 Birhanu Kenate Sori,4 Gemechu Shumi Bajiga,4 Melese Lemi Heyi,4 Dabesa Gobena Iticha,4 Tesfaye Kebebew Jiru,4 Mengistu Bekele Hurissa,4 Dereje Abdena Bayisa,4 Lemesa Tadese Amante,4 Yadeta Ayana Sima,4 Dejene Gemachu Dhaba4
1Department of Public Health, Adama Comprehensive Specialized Hospital Medical College, Adama, Ethiopia; 2Department of Internal Medicine, Adama Comprehensive Specialized Hospital Medical College, Adama, Ethiopia; 3Department of Surgery, Adama Comprehensive Specialized Hospital Medical College, Adama, Ethiopia; 4Oromia Regional Health Bureau, Addis Ababa, Ethiopia
Correspondence: Ephrem Mannekulih Habtewold, Correspondence: Tel +251-91-336-5954, Email [email protected]
Purpose: To assess survival patterns and predictors of mortality among patients admitted with COVID-19 to treatment centers in the Oromia region of Ethiopia from April 1 to August 31, 2021.
Methods: A prospective cohort study design was employed, taking a sample of 854 patients selected from eight treatment centers in the region. The follow-up duration was the time interval from admission to the treatment center until the final disposition of patients at discharge (death, recovery, or failed to recover). Data were collected by computer tablet with an interviewer-administered questionnaire and checklist designed using CSPro 7.5 and exported to Stata 13 for analysis. Descriptive analysis was used to explore the characteristics of patients. The mortality rate was estimated by number of deaths per 1,000 person-days of observation. The survival duration was estimated by medians with IQR. The Kaplan–Meier method was used to compare the survival experiences of patients. To identify the predictors of time to death after hospitalization, a Cox proportional-hazard model was used. The magnitude of association was estimated using HRs with 95% CIs, and statistical significance was set at P< 0.05.
Results: The mortality rate among hospitalized patients was 9.9 per 1,000 person-days of observation and the median survival time after admission was 9 (IQR 9– 10) days. Higher hazard of death was observed among patients who drank alcohol (AHR 2.0, 95% CI 1.2– 3.3), required anticoagulants (AHR 10, 95% CI 1.2– 91.5), glucocorticoids (AHR 1.7, 95% CI 1.1– 2.8), and oxygen (AHR 4.7, 95% CI 1.1– 22.0), those with acute respiratory distress syndrome (AHR 2.9, 95% CI 1.7– 5.1), and critical patients admitted to intensive care units (AHR 3.4, 95% CI 2.0– 5.9).
Conclusion: The hazard of death is significantly predicted by alcohol use, requiring anticoagulants, glucocorticoids, or oxygen medication, acute respiratory distress syndrome complication, and being critical when admitted to intensive care units.
Keywords: COVID-19, Ethiopia, mortality, Oromia, survival
Introduction
COVID-19 is caused by SARS-CoV-2.1 The virus was first identified and reported in Wuhan, China in December 2019.2 SARS-CoV-2 is highly contagious, spreads quickly, and was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020.3 As of December 2021, there had been 265,194,191 confirmed cases of COVID-19 globally, including 5,254,116 deaths.4 COVID-19 is severely affecting many countries worldwide, and Africa was the last continent to be hit by the pandemic. However, Africa is expected to be the most vulnerable continent, with COVID-19 having a major impact. In December 2021, the WHO reported 6,367,815 confirmed cases and 153,296 deaths across African countries.4 Ethiopia confirmed the first COVID-19 case on March 12, 2020.5–7 As of December 6, 2021, there had been 372,215 confirmed cases of COVID-19 with 6,800 deaths reported to the WHO.4 Given the country’s weak institutional capacity, it should be noted that Ethiopia is highly vulnerable to the pandemic.5–7
A large proportion of COVID-19 patients (80%) show few or no symptoms and have a relatively mild course of the disease. However, the disease is known to cause severe pneumonia and multiple complications, especially in certain high-risk groups. The remaining 20% of COVID-19 patients require hospitalization, mainly due to severe respiratory manifestations, and 5% require intensive care and ventilator support.8,9 The major morbidity and mortality of hospitalized COVID-19 patients are attributed to acute viral pneumonitis leading to acute respiratory distress syndrome (ARDS).10
COVID-19 can take a rapidly progressive and fulminant course, giving rise to various complications that can result in a fatal illness. Studies have reported that an estimated 10% of patients die after they are hospitalized for treatment.11 The survival duration of these patients is heterogeneous, estimated to be 5–13 days in different countries.12,13 Case–fatality rates are disproportionate across patients, and hence a higher fatality has been reported among older patients, as opposed to young adults or the pediatric population. Patients with comorbid conditions like hypertension, diabetes mellitus, heart diseases, acute kidney disease, and respiratory diseases are highly likely to die, contrary to those who have no comorbidities.14 Studies have also revealed that smokers in general are more likely to have severe symptoms, be admitted to intensive care units (ICUs), need mechanical ventilation, or die compared to nonsmokers.15,16 Moreover, alcohol consumption has been characterized as one of the causes of acquired immunity disorders that might put an individual at risk for ARDS, and has been identified as a leading cause of deaths among hospitalized patients.17,18
There have been several reports regarding mortality rate and its predictors among hospitalized patients with COVID-19. However, being from countries with different standards of health care, these reports are contradictory with regard to survival duration and predictors of mortality in several aspects. Therefore, it is very important to undertake this investigation to learn from the current contexts. This study can contribute significantly to the existing body of knowledge in terms of serving as baseline evidence for further studies, meta-analyses, or systematic reviews undertaken on similar public health problems.
Methods
Aim, Design, and Setting
This study aimed to assess the survival patterns and predictors of mortality among patients admitted with COVID-19 to treatment centers in the Oromia region of Ethiopia from April 1 to August 31, 2021. A prospective cohort study design was employed, taking a sample of patients selected from a random sample of eight treatment centers across Oromia. The region surrounds Addis Ababa, the capital of Ethiopia and headquarters of the African Union. Oromia is the largest and most populous region in Ethiopia. Administratively, the region is divided into 310 districts. There were four specialized referral hospitals, 19 zonal hospitals, 45 district hospitals, and 1,383 health centers providing services during the study period.19 After the COVID-19 outbreak was identified in Ethiopia, the region established 20 treatment centers. In total, 4,761 cases were admitted to these treatment centers. According to a regional health bureau report, the most cases (563) were admitted to the Bishoftu treatment center, while the least (178) were admitted to Negelle Arsi Hospital.
Participants
All patients with a diagnosis of COVID-19 and admitted to the treatment centers established in Oromia were considered the source population. Patients treated in randomly selected treatment centers during follow-up were considered the study population.
Exposed and Unexposed Groups
Previous studies have revealed that comorbidities, especially preadmission hypertension, are key predictors of COVID-19–related mortality. Accordingly, patients who had a history of hypertension represented the exposed group, whereas patients with no history of hypertension were the unexposed group.
Eligibility Criteria
All adult patients aged 18 years and older were included. Since this was a cohort study, patients who had died upon admission were excluded due to no follow-up.
Sample-Size Determination and Sampling
The sample size was calculated using the formula designed for cohort studies to test for differences in the risk of COVID-19–related morality between exposed and unexposed groups. Studies have revealed that being older and having comorbidities, especially preadmission hypertension, are key predictors of COVID-19–related mortality.14 As such, preadmission hypertension was considered a significant risk of death, and the sample was calculated taking the risk difference between patients who did and did not have a history of hypertension. In determining the sample size for the two groups, the required statistical assumptions (95% level of confidence, 80% power, proportion of mortality in exposed group [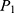 = 0.971], hypothesized proportion of mortality in unexposed group [
= 0.971], hypothesized proportion of mortality in unexposed group [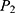 = 0.903], and a 1:1 ratio of the unexposed to exposed groups) were considered. Taking all these assumptions into consideration, the sample size was calculated using Epi Info 7. Accordingly, 832 patients were sampled, and 10% of the estimated sample was added to compensate for a possible nonresponse rate.
= 0.903], and a 1:1 ratio of the unexposed to exposed groups) were considered. Taking all these assumptions into consideration, the sample size was calculated using Epi Info 7. Accordingly, 832 patients were sampled, and 10% of the estimated sample was added to compensate for a possible nonresponse rate.
Considering possible variability in terms of overall settings for management of patients, among 20 established centers, a sample of eight treatment centers (Kurkura, Waliso, Mukaturi, Arsi Negellee, Bokoji, Holota, Guder, and Adama Hospital Medical College) in Oromia was selected by simple random sampling applied using a lottery method. Taking treatment centers as strata, an equal sample of patients was selected from each center. Based on the assumption of random attendance and admission of patients to the treatment center, participants were consequently incorporated in the current study until the required sample size was achieved within the specified study duration.
Data-Collection Tools and Procedure
Data were collected from multiple sources using a structured questionnaire and checklist adapted from a review of previous literature. The tools were designed in accordance with existing COVID-19 treatment practices and prepared using CSPro 7.5. For closed-ended questions, values were inserted via clickable options, and parts of the participants’ responses were written on the open fields provided along with the prompts. The questionnaires were administered through face-to-face interviews, while the checklist was used to review and extract data from patients’ medical records. Data were collected by health professionals with training on COVID-19 and working in the treatment centers during data-collection time. To improve the validity of data-collection tools, pretesting was conducted on 5% of the total sample before embarking on the main study. Training was also given to data collectors and supervisors on the tools and procedures, as well as the study’s purposes. Data collection was supervised to check completeness and consistency. Then, necessary corrections were made and feedback given.
Study Variables
Dependent Variables
Survival duration of COVID-19 patients, magnitude of COVID-19–related mortality, and time to death for COVID-19 patients after admission to treatment center.
Independent Variables
Sociodemographic factors: age, sex, marital status, education, income, and household occupants (with family or alone).
Clinical factors: condition on admission, comorbidities, medication/treatment, complication, admission unit.
Behavioral factors: smoking, alcohol consumption, chewing khat.
Operational Definition of Variables
Khat: A leafy green plant containing stimulant chewed by people in East African countries as a recreational drug to elevate the mood.
Education: This was classified into groups of no education and primary, secondary, and higher education. Patients who had not had any formal education were classified as “no education”, those who had had formal education from grade one to eight “primary”, from grade nine to twelve “secondary”, and above grade twelve “higher education.”
Income: Average monthly household income in Ethiopian birr (ETB). Based on the data, this ranged from 0 to ETB35,000 Birr per month. Considering the contextual variability of income across different settings and time, we used a measure of relative standing (quartile and percentile) for classification. Based on the data, 25% of households earned <ETB1,200 on average per month and were classified as “low income”, the 25% of households earning >ETB5,000 on average per month “high income”, and the remaining 50% of households earning between ETB1,200 and ETB5,000 per month “middle income.”
Admission criteria:
- Every RT-PcR–confirmed severe and critical COVID-19 patient, irrespective of any medical condition.
- Every RT-PcR–confirmed case with established comorbidities, irrespective of severity.
- Every RT-PcR–confirmed mild or moderate cases unable to isolate themselves if all family members were living in a single room.
Mild disease: Confirmed symptoms (has fever, cough, sore throat) of COVID-19 without evidence of viral pneumonia or hypoxia.
Moderate disease: Clinical signs of pneumonia (fever, cough, dyspnea, fast breathing), but no signs of severe pneumonia, including oxygen saturation (SpO2) ≥90% on room air.
Severe disease: Clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) plus one of the following: respiratory rate >30 breaths per minute, severe respiratory distress, or SpO2 <90% on room air.
Critical disease: ARDS or sepsis or septic shock or acute thromboembolism.
Diabetes mellitus: Fasting blood sugar >126 mg/dL or glycated hemoglobin A1c >6.5%.
Data Processing and Analysis
Data were processed and analyzed using Stata13. Before analysis, data processing (cleaning, counting, categorizing, and computing) was performed. Descriptive analysis was used to explore the characteristics of patients. The overall mortality among COVID-19 patients was estimated by proportion within 95% CI and number of deaths per 1,000 person-days of observation. The rate of mortality was also compared across patient characteristics. Survival duration was estimated by medians and IQR. The Kaplan–Meier method was used to compare the survival duration of various groups of patients. Observed survival differences were assessed using log-rank tests at a 5% significance level.
To identify the predictors of time to death among COVID-19 patients, Cox proportional-hazard regression was used. The event of interest under this particular objective was death after admission to the treatment center. Length of follow-up was the duration from admission to the center until the patient died, recovered, or failed to recover. Cox proportional-hazard regression model was developed using a standard model-building approach. First predictors that had crude associations with treatment outcome were identified using simple regression analysis. At this stage of analysis, predictors with P<0.25 were selected as candidates for multiple regression analysis. Then, all predictors were subjected to multiple regression in order to identify those independently associated with the outcomes of interest after adjustment for the effects of potential confounding variables. For the multiple regression model, significance was set at P<0.05. The magnitude of association was estimated using HRs with 95% CIs. The survival analysis proportionality assumption was assessed using log–log plots. Among predictors, variable antibiotic administration as a prognostic factor failed to meet required data assumptions for the relevant statistical tests. To identify time-dependent variable, time-varying covariate testing was performed, and none of the variables was identified as time-dependent.
Results
Sociodemographic Characteristics
In total, 854 COVID-19 patients were included in this study and observed from April 1 to August 31, 2021, for a response rate of 93.23%. The median age of the patients was 48 (IQR 35–62) years. Of these, 318 (37.24%) were aged 18–40 years, 553 (64.75%) were male, 568 (66.51%) were urban residents, 663 (77.63%) were married, and 230 (26.93%) had studied beyond secondary school. Moreover, 54 (6.73%) lived alone and 440 (51.52%) were classified in the middle-income group (Table 1).
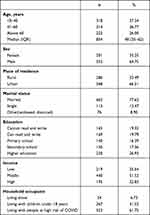 |
Table 1 Sociodemographic characteristics of patients admitted to treatment centers in the Oromia region of Ethiopia, 2021(n=854) |
Substance Use
In sum, 46 (5.39%) patients were active smokers and 133 (15.57%) and 88 (10.30%) active alcohol users and khat chewers, respectively (Table 2).
 |
Table 2 Substance use among patients admitted to treatment centers in the Oromia region of Ethiopia, 2021 |
Condition on Admission
The highest proportion of patients (40.52%) were severe cases, while the lowest proportion (8.55%) were critical (Figure 1).
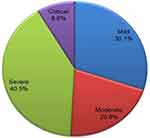 |
Figure 1 Summary of patients’ condition on admission at treatment centers in the Oromia region of Ethiopia, 2021. |
Summary of Signs and Symptoms on Admission
A total of 727 (85.13%) cases had cough, 577 (67.56%) fatigue, and 467 (54.68%) dyspnea on admission. Only a small proportion of patients presented with severe headache (0.7%), rigor (5.74%), and pharyngalgia(8.9%) on admission (Figure 2).
 |
Figure 2 Summary of clinical characteristics (signs and symptoms) of patients admitted to treatment centers in the Oromia region of Ethiopia, 2021, (n=854). |
Summary of Common Comorbidities
In sum, 577 (67.56%) patients did not have any known COVID-19 comorbidities, while 220 (25.76%) had one, 54 (6.32%) had two, and three (0.35%) had three. Although the common diagnosis of patients during admission was a respiratory condition, some had other comorbidities, such as hypertension (134, 15.7%), diabetes mellitus (104, 12.2%), chronic liver disease (six, 0.7%) and cancer (6, 0.7%; Figure 3).
 |
Figure 3 Summary of clinical characteristics (comorbidities) of patients admitted to treatment centers in the Oromia region of Ethiopia, 2021 (n=854). |
Treatment and Management
Once patients had been admitted, resources were utilized as per national guidelines. More than half (459, 53.75%) required oxygen, 467 (54.68%) receiving it intranasally and via face mask, while only 19 (2.22%) required intubation. In addition to oxygen, antibiotics (583, 68.27%), anticoagulants (492, 57.61%), and steroids (211, 24.11%) were utilized (Table 3).
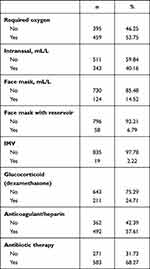 |
Table 3 Summary of medical treatments of patients admitted to COVID-19 treatment centers in the Oromia region of Ethiopia, 2021 |
Summary of Complications
Some patients had at least one complication: ARDS (66, 7.73%), sepsis (43, 5.04%), acute kidney injury (20, 2.34%), and shock (16, 1.87%). An estimated 11.3% (95% CI 9.2%–13.5%) of deaths were observed among all patients (Figure 4).
 |
Figure 4 Summary of complications and clinical outcomes of patients admitted to treatment centers in the Oromia region of Ethiopia, 2021(n=854). |
Mortality Rate
The rate of mortality was measured across various patient characteristics per 1,000 person-days of observation. The highest rate was observed among patients above 60 years of age (16.4) in comparison to younger age-groups and among substance users: cigarette smokers (24.2), alcohol drinkers (18.4), and khat chewers (21.9) compared to nonusers. The mortality rate was also high among patients who required anticoagulants (18.3), glucocorticoids (31.3), antibiotics (11.7), and oxygen therapy (19.3) compared to those who did not require these treatments. The highest mortality rate was observed among patients with ARDS (59.5) complications compared to those who do not have complications and among those admitted to the ICU (53) compared to those who were admitted to the ward (Table 4).
 |
Table 4 Mortality rate among patients admitted to treatment centers in the Oromia region of Ethiopia, 2021 |
Survival Patterns
The median survival time of patients after admission was 9 (IQR 9–10) days. The Kaplan–Meier method was used to compare the survival of various groups. Observed differences were assessed using log-rank tests at a 5% significance level. Significantly longer survival was observed among younger patients compared to older ones and among those in higher income brackets compared to lower. Similarly, significantly longer survival were observed among patients who did not use cigarettes, alcohol, or khat compared to users. Longer survival was also observed among patients who did not require medications like anticoagulants, glucocorticoids, antibiotics, or oxygen therapy compared to their counterparts. Ssignificantly longer survival was observed among patients who did not have ARD complications compared to those who did and among patients who were admitted to the ICU compared to a ward (Figure 5).
 |
Figure 5 Survival patterns of patients admitted to treatment centers in the Oromia region of Ethiopia, 2021. |
Predictors of Time to Death
On simple regression analysis, among covariates in different age categories and economic class, using cigarettes, alcohol and khat, requiring such medications as anticoagulants, glucocorticoids, antibiotics, or oxygen, ARDS complications, and admission to the ICU or ward were predictors that had crude associations with time to death (P<0.25). After adjustment for potential confounders and assessed for Cox regression assumptions, the final Cox proportional-hazard regression model was fitted. Accordingly, alcohol use, requiring anticoagulants, glucocorticoids, or oxygen, ARDS complication, and admission to the ICU or a ward were found to have significant associations with time to death (P<0.05). Accordingly, after adjustment for the effects of possible confounders, the hazard of death among patients who drank alcohol was double that of nonusers (AHR 2.0, 95% CI 1.2–3.3), ARDS complications almost triple that of no complications (AHR 2.9, 95% CI 1.7–5.1), requiring anticoagulant medication ten times (AHR 10, 95% CI 1.2–91.5), requiring glucocorticoid medication 70% higher (AHR 1.7, 95% CI 1.1–2.8), and requiring oxygen around five times (AHR 4.7, 95% CI 1.1–22.0) that of their counterparts. The hazard of death among patients admitted to the ICU was around three times that of those admitted to a ward (AHR 3.4, 95% CI 2.0–5.9; Table 5).
 |
Table 5 Factors associated with COVID-19–related mortality among of patients at treatment centers in the Oromia region of Ethiopia, 2021 |
Discussion
This study sought to assess survival patterns and predictors of mortality among COVID-19 patients admitted to treatment centers in Oromia, Ethiopia. Almost half the patients were mild and moderate cases, while the lowest proportion were critical. The proportion of severe and critical patients observed in this study was higher than studies done in Jiangsu20 and Saudi Arabia.21 The dissimilarity may be attributable to differences in admission criteria and variability in sociodemographic characteristics.
The median survival time of patients after admission was 9 (IQR 9–10) days. This is longer than the 5 days in a study conducted among hospitalized patients at the Mount Sinai Health System in New York13 and shorter than the 13 days of a pooled meta-analysis of eleven retrospective studies.12 This dissimilarity may be attributable to differences in the quality of care or proportion of critical cases admitted to the treatment centers. The overall mortality rate was 9.9 per 1,000 person-days of observation and the proportion estimated to be 11.3% (95% CI 9.2%–13.5%). This is in agreement with a systematic review and meta-analysis of 44 peer-reviewed articles in which an estimated 10% of patients died after they had been hospitalized.11 Regarding age-specific mortality, the highest rate was observed among patients above 60 years of age (16.4) compared to younger age-groups which was 2.2 per 1,000 person-days of observation, similar to studies conducted in Hubei and other parts of China.22 The highest observed mortality rate among older patients is probably attributable to a high prevalence of comorbidities and decrement in immunounction.
Mortality was higher among substance users — cigarette smokers (24.2), alcohol drinkers (18.4), and khat chewers (21.9) — than nonusers. These findings are supported by studies from the US.23–25 It was also identified that the hazard of death among patients who drank alcohol was double that of nonusers. The association between alcohol use and COVID-related mortality has been elaborated on in several reports. This is probably due to the fact that heavy drinking increases the risk of severe lung infections (including both viral and bacterial pneumonia) and ensuing respiratory problems, which may lead to mortality.26,27 Furthermore, alcohol consumption has been characterized as one of the leading causes of acquired immunity disorders.17 It has also been reported to affect immunorelated health responses and might put an individual at risk of ARDS.18
Mortality differed depending on whether or not the patient received medications or required oxygen therapy. Mortality rates among patients who did not received anticoagulant (18.3), glucocorticoid (31.3), or antibiotic medications (11.7) and among those who required oxygen therapy (19.3) were higher than their counterparts. Requiring anticoagulant and glucocorticoid medications was found to be significant predictors of time to death. Similarly, the risk of death was higher among patients who required oxygen therapy than their counterparts. Some clinical studies on conventional antibiotic therapies have been conducted around the world. Even though significant reduction in COVID-19–related morbidity and mortality was observed among patients who received antibiotics in some of these studies, the results remain controversial. The effectiveness and efficacy of these treatments in reducing COVID-19–related morbidity and mortality depend on different factors. Therefore, personalized medications provided based on clinical findings, as well as the best treatment choice along with an effective dose and minimal side effects, would be a rational consideration for the treatment of patients and reduction in mortality related to COVID-19.28
A higher mortality rate was observed among patients who had ARDS (59.5) complication than those who did not. This is in line with the systematic review and meta-analysis of 44 articles.11 The hazard of death among patients who had ARDS complications was almost three times that of those who did not. This is in line with the findings of a systematic review and meta-analysis of 13 articles. This observed association is probably due to ARDS being related to cytokine storm, a common phenomenon with COVID-19 infections leading to elevated inflammatory markers that may result in higher risk of mortality. A higher mortality rate was observed among patients requiring admission to the ICU (53) than those admitted to a ward, with the hazard of death higher also.
As to the strengths of this study, a prospective cohort design was used to assess clinical characteristics and predictors of treatment outcomes among patients admitted with COVID-19 to the treatment centers. We used a multidisciplinary research team and employed an advanced statistical model for analysis. Regarding study limitations, in some of the treatment centers, chemistry machines were not functional during the study period and laboratory investigations were not performed for all patients included in the study. Therefore, some important variables to be operationalized from laboratory investigation were left out of the analysis. It was also infeasible to intensively observe all prognostic factors at admission to the treatment centers, due to so many restrictions in the centers during the study period. Immediately upon its accomplishment, the study findings were presented to the funding organization and all stakeholders working on COVID-19 prevention and control programs. A lot has changed in the treatment of COVID-19 patients since the time this study was conducted. With all its limitations, the current study can make a significant contribution to the existing body of knowledge in terms of serving as baseline evidence for further studies, meta-analysis, and systematic reviews that will be performed in developing countries with similar public health problems.
Conclusion
The study sought to assess survival and predictors of mortality among patients admitted to the treatment centers. The median survival time of patients after admission was 9 (IQR 9–10) days. The overall mortality rate was 9.9 per 1,000 person-days of observation, and the proportion of mortality estimated to be 11.3% (95% CI 9.2%–13.5%). It was also identified that the risk of mortality was significantly predicted by alcohol use, requiring anticoagulants, glucocorticoids, or oxygen, ARDS complications, and admission to the ICU.
Abbreviations
ACEI, angiotensin-converting enzyme inhibitor; APACHE, Acute Physiology and Chronic Health EvaluatiOn; ARB, angiotensin-receptor blocker; ARDS, acute respiratory distress syndrome; CT, computed tomography; EUA, emergency-use authorization; HFNC, high-flow nasal cannula oxygen; ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS, length of stay; NIMV, noninvasive mechanical ventilation; PE, pulmonary embolism; SOFA, Sequential Organ Failure Assessment.
Data Sharing
Upon formal request, the data for the current study can be obtained from the principal investigator — Ephrem Mannekulih.
Ethics Approval and Informed Consent
Ethics approval was obtained from the Institutional Ethical Review Board (IERB) of Adama Hospital Medical College, and a letter of cooperation was obtained from Oromia Region Health Bureau and then submitted to the organizations or institutions concerned, such as hospitals and treatment centers. A consent form as per the required ethical principles was prepared, then participants were informed and requested to participate in the study. Accordingly, participants were informed about the objectives of the study and the benefits and risks of participation. The autonomy of participants was exercised by ensuring their right to totally refuse to participate in the study or discontinue the interview process at any time. Participant confidentiality was ensured by avoiding any of their unique identifiers that could potentially disclose their information. The data collected will not be used for any other purpose. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
Acknowledgments We hugely acknowledge Oromia Health Bureau for covering all expenses for this crucial study. We would also like to acknowledge Adama Hospital Medical College for logistics and others for support as needed. All health-care providers working in the selected treatment centers and the participants are thanked for their genuine cooperation during the course of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was entirely funded by the Oromia Regional Health Bureau.
Disclosure
The authors declare that they have no competing interests.
References
1. Lai -C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi:10.1016/j.ijantimicag.2020.105924
2. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
3. World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Geneva, Switzerland: World Health Organization; 2020.
4. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi:10.1016/S1473-3099(20)30120-1
5. Debela BK. The COVID-19 pandemic and the Ethiopian public administration: responses and challenges. Good Public Govern Global Pandemic. 2020;113:1.
6. Zikargae MH. COVID-19 in Ethiopia: assessment of how the Ethiopian government has executed administrative actions and managed risk communications and community engagement. Risk Manag Healthc Policy. 2020;13:2803. doi:10.2147/RMHP.S278234
7. Baye K. COVID-19 Prevention Measures in Ethiopia: Current Realities and Prospects. International Food Policy Research Institute; 2020.
8. Bajema KL, Oster AM, McGovern OL, et al. Persons evaluated for 2019 novel coronavirus—United States, January 2020. Morb Mortal Wkly Rep. 2020;69(6):166. doi:10.15585/mmwr.mm6906e1
9. Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14(11):1149–1163. doi:10.1080/17476348.2020.1804365
10. World Health Organization. Novel Coronavirus ( 2019-nCoV): situation report, 11; 2020.
11. Potere N, Valeriani E, Candeloro M, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Critical Care. 2020;24(1):1–12. doi:10.1186/s13054-020-03022-1
12. Martín‐Moro F, Marquet J, Piris M, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190(1). doi:10.1111/bjh.16801
13. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi:10.1016/j.jacc.2020.05.001
14. Varghese GM, John R, Manesh A, Karthik R, Abraham O. Clinical management of COVID-19. Indian J Med Res. 2020;151(5):401. doi:10.4103/ijmr.IJMR_957_20
15. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
16. Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi:10.18332/tid/119324
17. Simou E, Britton J, Leonardi-Bee J. Alcohol and the risk of pneumonia: a systematic review and meta-analysis. BMJ open. 2018;8(8):e022344. doi:10.1136/bmjopen-2018-022344
18. Sarkar D, Jung MK, Wang HJ. Alcohol and the immune system. Alcohol Res. 2015;37(2):153.
19. Central Statistical Agency. Population projection of Ethiopia for all regions at woreda level from 2014–2017. Addis Ababa, Ethiopia Federal democratic republic of Ethiopia: Central Statistics Agency; 2014.
20. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71(15):706–712. doi:10.1093/cid/ciaa199
21. Barry M, AlMohaya A, AlHijji A, et al. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J Epidemiol Glob Health. 2020;10(3):214. doi:10.2991/jegh.k.200806.002
22. Li H, Wang S, Zhong F, et al. Age-dependent risks of incidence and mortality of COVID-19 in Hubei Province and other parts of China. Front Med. 2020;7:190. doi:10.3389/fmed.2020.00190
23. Baillargeon J, Polychronopoulou E, Kuo Y-F, Raji MA. The impact of substance use disorder on COVID-19 outcomes. Psychiatr Serv. 2021;72(5):578–581. doi:10.1176/appi.ps.202000534
24. Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30–39. doi:10.1038/s41380-020-00880-7
25. Wei Y, Shah R. Substance use disorder in the COVID-19 pandemic: a systematic review of vulnerabilities and complications. Pharmaceuticals. 2020;13(7):155. doi:10.3390/ph13070155
26. Stockwell T, Andreasson S, Cherpitel C, et al. The burden of alcohol on health care during COVID‐19. Drug Alcohol Rev. 2021;40(1):3–7. doi:10.1111/dar.13143
27. Medina‐Mora ME, Cordero‐Oropeza M, Rafful C, Real T, Villatoro‐Velazquez JA. COVID‐19 and alcohol in Mexico: a serious health crisis, strong actions on alcohol in response—Commentary on Stockwell et al. Drug Alcohol Rev. 2021;40(1):13–16. doi:10.1111/dar.13177
28. Sahebnasagh A, Avan R, Saghafi F, et al. Pharmacological treatments of COVID-19. Pharmacol Rep. 2020;72:1–33.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
