Back to Journals » Cancer Management and Research » Volume 11
Survival benefit of higher fraction dose delivered by three-dimensional conformal radiotherapy in hepatocellular carcinoma smaller than 10 cm in size
Authors Su F, Chen K, Liang Z, Qu S, Li L, Chen L , Yang Y, Wu C, Liang X, Zhu X
Received 9 July 2018
Accepted for publication 10 April 2019
Published 30 April 2019 Volume 2019:11 Pages 3791—3799
DOI https://doi.org/10.2147/CMAR.S179540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Fang Su*, Kaihua Chen*, Zhongguo Liang, Song Qu, Ling Li, Long Chen, Yunli Yang, Chunhua Wu, Xia Liang, Xiaodong Zhu
Department of Radiation Oncology, Affiliated Tumor Hospital of Guangxi Medical University, Cancer Institute of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Objective: Even in early-stage hepatocellular carcinoma (HCC), patients are often ineligible for surgical resection, transplantation, or local ablation due to advanced cirrhosis, donor shortage, or difficult tumor location. To compare the safety and efficacy of different fractions on the survival of patients with tumors smaller than 10 cm in size, hepatocellular carcinoma (HCC) patients ineligible for curative therapies were treated with three-dimensional conformal radiotherapy (3DCRT).
Methods: A total of 198 HCC patients who had tumors smaller than 10 cm and were not eligible for surgical resection or local ablation therapy received 3DCRT. Participants were separated into two groups. The treatment for Group A (n=111) was a median total dose of 53 Gy with a fraction of 2.5–4.9 Gy given three times a week, while treatment for Group B (n=87) was a median total dose of 52 Gy with a fraction of 5.0–7.0 Gy given three times a week. Propensity score matching (PSM) was conducted, and after the PSM, 81 pairs of patients arose. The Kaplan–Meier method was adopted to analyze overall survival; multivariate analysis was applied to identify the prognostic factors of survival.
Results: The median follow-up time was 19.7 months (ranging from 1 to 186 months). The median survival for Group A patients versus Group B patients was 14.4 versus 24.8 months (P=0.003), respectively. The overall survival rates at 1, 3, and 5 years were 57.7%, 30.6%, and 18.9% for Group A patients and 73.6%, 43.7%, and 33.3% for Group B patients, respectively (P=0.009). In addition, the results in the PSM model appeared to be similar between the two groups. After PSM, the association between four independent prognostic factors and worse overall survival was discovered as follows: tumor size (>5 cm), Child–Pugh class B, portal vein tumor thrombosis, and fraction of 2.5−4.9 Gy/fx. The two groups also shared similar toxicities.
Conclusions: Higher fraction dose radiotherapy delivered by 3DCRT was effective, as it offered a survival benefit without aggravating the toxicities in patients with small- to medium-sized HCC tumors who were ineligible for curative therapies.
Keywords: three-dimensional conformal radiotherapy, higher fraction dose, hepatocellular carcinoma, curative therapy, survival
Introduction
Hepatocellular carcinoma (HCC) is prevailing in Southeast and East Asia as one of the most common cancers.1 Usually, for small HCC tumors, liver transplantation, surgical resection and local ablation are the prioritized curative therapies.2–4 However, in clinical practice, small HCC tumors cannot always be properly dealt with by these recommended treatments. Even in cases of early-stage HCC, patients may still have difficulty receiving transplantation, surgical resection or local ablation because of donor shortage, advanced liver cirrhosis or difficult tumor location. Therefore, three-dimensional conformal radiotherapy (3DCRT) is often considered as a possible option.
The decision of whether to treat HCC patients with radiotherapy depends on the proliferation of the tumor, in which the tolerance of normal liver tissues and α/β values remain significant factors. Nevertheless, it is not always to obtain a satisfactory predictive value from a mathematical model for complications in normal tissues. In our hospital, 3DCRT with hypofractionation has been used to treat HCC patients with the purpose of conveying a high radiation doses to the tumor. Under this circumstance, a retrospective study was performed to evaluate whether outcomes vary between different fractions of 3DCRT regimens (2.5–4.9 Gy/fx versus 5.0–7.0 Gy/fx) used as a first-line treatment for tumors smaller than 10 cm in size in HCC patients ineligible for curative therapies. The safety and efficacy of the methods were thereby evaluated, and the group of patients who appeared to obtain more benefit from 3DCRT was also explored.
Materials and methods
Ethical statement
This study was approved by the Ethics Committee of the Affiliated Tumor Hospital (No. LW2018020), compliance with the Declaration of Helsinki. A written informed patient consent was also obtained. All patients’ information was anonymous.
Patients
A total of 198 HCC patients (a single HCC <10 cm or up to 3 HCCs <5 cm) who received 3DCRT in our hospital during April 1999 and August 2013 were retrospectively analyzed. The eligibility criteria for the this study are as follows: (1) the HCC tumor was ineligible for Surgical resection due to liver cirrhosis and poor liver function (Child–Pugh B), insufficient residual liver volume for resection, and/or patients’ refusal of surgery. (2) The HCC tumor was located on the liver surface, near the large vessels or bile duct, or at the top of the dome where percutaneous ablation is not safe due to tumor location or refusal to accept the surgery. (3) Performance status (PS) score of 0–1 and Child–Pugh class A or B were required for the enrolled patients who received 3DCRT. (4) An incomplete response after transcatheter arterial chemoembolization (TACE) or financial difficulties. Patients with extrahepatic metastases or with Child–Pugh class C tumors were excluded from the study; patients who received reirradiation in the same area were also excluded from the study.
The patients` clinical characteristics were collected from a medical records review (Table 1).
 | Table 1 Clinicopathological data of patients with HCC who underwent different fraction doses of hypofractionated 3DCRT |
Treatment protocols
Patients’ condition and the machine’s capacity of delivering the radiotherapy were fully evaluated before the execution. A preferable suggestion from doctors was a fraction dose of 2.5–4.9 Gy/fx regimen for complicated patients, such as the tumor close to the gastrointestinal tract, the number of intrahepatic tumors >3, or an ALT >80 U/L.
The stereotactic body frame was used to position patients who were trained to breathe shallowly with the help of a tight abdominal belt to restrain breathing. A planning computed axial tomography (CAT) scan was arranged for every patient to facilitate 3D treatment planning through the Topslane planning system. On the CAT scan, delineation of the gross tumor volume (GTV) was performed. The planning tumor volume (PTV) was determined by adding 0.5 cm–1.5 cm to the GTV. The liver motion was also taken into consideration as additional margins were included to indicate motion. Four to eight coplanar/noncoplanar fields were designed with the aid of the eye view from the beam.
In total, 111 patients received the Group A regimen (2.5–4.9 Gy/fx), and 87 patients received the Group B regimen (5.0–7.0 Gy/fx). The Group A regimen was a median total dose of 53 Gy with a fraction of 2.5–4.9 Gy given three times a week, while the Group B regimen was a median total dose of 52 Gy with a fraction of 5.0–7.0 Gy given three fractions every week. The prescribed dose had an isocenter as 100% without inhomogeneity tissue correlation, and the PTV was accounted for to satisfy the coverage of 90% of the isodose curve. The prescription dose of radiotherapy was determined mainly according to the mean dose to the liver, which was limited to 23 Gy and was also limited by the tolerance dose of the gastrointestinal tract. Organs at risk (OARs), including the liver, kidneys, stomach, small intestine, and spinal cord, were under the tolerance dose.
Out of 198 patients, 72 patients (36.4%) had TACE with a median of two cycles (range, 1–4 cycles). The execution of TACE involved the infusion of a mixture of 5 mL of lipiodol, 30–40 mg/m2 of cisplatinum, 50–60 mg/m2 of epiadriamycin or 5 mL of lipiodol, 10–15 mg/m2 of 10-hydroxycamptothecin and 6–7 mg/m2 of mitomycin C, followed by Gelfoam embolization. The 3DCRT treatment was scheduled to start within 2–4 weeks after TACE.
The treatment applied after tumor progression was variable. Twenty-two patients underwent TACE; other treatment options included liver transplantation, radiofrequency ablation, systemic chemotherapy, sorafenib, radiotherapy and liver protection.
Toxicity evaluation and follow-up
The evaluation of toxic reaction followed the Common Terminology Criteria for Adverse Events (CTCAE) Version 2.0. Blood chemistry analyses, routine blood tests, and physical examinations were performed every week during the 3DCRT alone or the combined TACE and 3DCRT periods.
The patient follow-up was scheduled for every three months within the 1st year and then every six months after the treatment was completed. Each follow-up included physical examination, routine blood and blood chemistry tests, serum AFP, abdominal B ultrasound, and CT scan. Survival calculations were recorded from the date of starting the treatment until the final follow-up or death. Recurrence diagnosis was based on two concurrent imaging technologies or the combination of increased serum AFP and consistent ultrasound examinations or CT findings.
Statistical analysis
All statistical analyses were carried out using SPSS 20.0. The Mann–Whitney U test was used to analyze the statistical significance of differences in continuous data, and the chi-squared test was applied to assess the significance of differences between categorical data. The Kaplan–Meier method was adopted to estimate overall survival; the log-rank test was employed for comparison between groups. Cox proportional hazards model was used to perform multivariate analysis. For all tests, a P-value<0.05 was considered statistically significant.
Propensity score matching (PSM)
PSM was performed to reduce selection and confounding bias. In the analysis, the propensity scores were created by using logistic regression for HCC patients in Group A and in Group B. Clinical variables were incorporated into a logistic regression model that was applied to produce propensity scores (PS) for every patient along a continuous range from 0 to 1. The nearest neighbor matching was used to obtain a one-to-one match between the two groups.
Results
Patients’ characteristics
Table 1 shows the clinical and laboratory baseline characteristics of Group A (n=111) and Group B (n=87) patients. Group A patients showed worse liver function, including longer prothrombin times and higher levels of ALT and bilirubin (P<0.05). More advanced patients (T3 and T4) or patients with PVTT received the Group A regimen (P<0.05).
Dose distribution
Because of the performance status of patients, the location of the tumor and the limits of the OARs, the prescription dose and the fraction of radiotherapy were different. To ensure the comparability of the radiation dose, the total dose was converted to a biologically equivalent dose (BED) through a linear-quadratic (L-Q) model with an α/β ratio of 10 Gy. Group B showed a significantly higher BED of the tumor than Group A (P<0.001), and the radiotherapy fractions in Group B were significantly less than those of Group A. The percentage of the whole liver covered by at least 5 Gy (V5) was significantly higher in Group B; however, there were no significant differences in the dose for V10, V20, and V30 of the whole liver and the mean dose to normal liver, as shown in Table 2.
 | Table 2 Mean values of dosimetric parameters in patients |
Survival analysis in all patients
The median follow-up time was 19.7 months (range, 1–186 months), and 68 patients died in Group B (78.2%), while 94 patients died in Group A (84.7%). The median survival for Group A versus Group B was 14.4 versus 24.8 months (P=0.003), respectively. The 1-, 3-, and 5-year overall survival (OS) was 57.7%, 30.6%, and 18.9% for Group A patients and 73.6%, 43.7%, and 33.3% for Group B, respectively (P = 0.009; Figure 1).
 | Figure 1 Overall survival curves of the entire patient population who underwent different fraction doses of hypofractionated three-dimensional conformal radiotherapy (P=0.009). |
However, the cumulative recurrence rate was similar between groups A and B (P=0.446). Moreover, the site of tumor recurrence was similar between the two groups(P=0.699). Among the extrahepatic metastatic sites, the lung was the most prevalent site, occurring in 18 of 29 patients (62.1%). Sites involving bone (4 patients) and other organs (7 patients), such as the peritoneal seeding or the adrenal gland, were infrequently seen (Table 3).
 | Table 3 Characteristics for hepatocellular carcinoma recurrence |
Univariate analysis identified the following prognostic factors in all patients: serum AFP ≥400 ng/mL, serum alanine aminotransferase>80 U/L, tumor size>5 cm, Child–Pugh class B, portal vein thrombosis, and fractions of 2.5−4.9 Gy/fx. All these prognostic factors except serum AFP were also identified by multivariate analysis as predictors of poor prognosis (Table 4).
 | Table 4 Multivariate analysis of clinicopathological factors predictive of poor overall survival |
Characteristics and survival analysis of PSM
PSM identified 81 pairs of patients. When only these pairs were viewed, there was no significant difference in baseline characteristics between groups A and B (Table 1). The median survival for patients in the PSM model who received the Group A regimen versus patients who received the Group B regimen was 14.7 versus 21.6 months, respectively (P=0.032). The 1-, 3-, and 5-year OS was 58.0%, 34.6%, and 19.8% for Group A and 74.1%, 43.2%, and 33.3% for Group B, respectively (P=0.041, Figure 2). Multivariate analysis identified tumor size (>5 cm), Child–Pugh class B, PVTT, and fractions of 2.5−4.9 Gy/fx as mortality risk factors (Table 4).
 | Figure 2 Overall survival curves of the propensity score-matched patient population who underwent different fraction doses of hypofractionated three-dimensional conformal radiotherapy (P=0.041). |
Toxicity
The treatment-related toxicity was similar in both groups. Twenty-six patients (13.1%) experienced toxicity of CTCAE version 2.0 grade ≥3 in both groups (Table 5). Gastrointestinal bleeding was observed in two patients who received the Group A regimen and in two patients who received the Group B regimen. Two patients developed upper gastrointestinal bleeding 3 months and 12 months after the end of the Group A regimen radiotherapy. Of these patients, one case had esophagogastric varices discovered by gastroscopy detection, which was considered to be related to liver cirrhosis. Two patients had upper gastrointestinal bleeding 6 months and 14 months after the completion of the Group B regimen radiotherapy. These patients eventually died from hypovolemic shock.
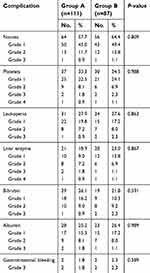 | Table 5 Complications after treatment |
Discussion
Several treatment modalities are available for patients with HCC, including local ablation, TACE, and radiation therapy. The main curative treatment for HCC is hepatic resection. With the persistently updated surgical techniques and improvement of postoperative management, the 5-year OS has reached approximately 70%, particularly for HCC <5 cm. In addition,6 Ablative therapies are effective treatment options. Ablative therapies are most effective for tumors <3 cm (preferably <2 cm) that are in an appropriate location away from other organs and major vessels and/or bile ducts.7 However, complete ablation is difficult to achieve for larger or perivascular tumors.8 Some research reported that local ablation was not feasible in 6.0% −16.2% of HCC patients because of high-risk tumor location or poor detection on ultrasonography.9,10 Therefore, 3DCRT is considered a treatment option for small- to medium-sized HCCs ineligible for curative therapies. Our study evaluated small- to medium-sized tumors (<10 cm) in HCC patients treated with 3DCRT. The results revealed OS in the entire study population at 1, 3, and 5 years was 57.7%, 30.6%, and 18.9% for Group A, and 73.6%, 43.7%, and 33.3% for Group B, respectively. These outcomes were similar to previous studies, which confirmed the importance of 3DCRT in locally HCC.11–19 The 3DCRT technique is technically simple and should be considered in patients with HCC that is not eligible for ablation or resection.
The size of the fraction dose is an important factor associated with OS and local control of disease. Previous studies have reported superior outcomes with an increase in fraction dose for HCC patients.20,21 Wang et al reported that TACE in combination with hypofractionated 3DCRT (6–8 Gy/fx) obtained better OS and lengthened median survival than conventional 3DCRT (2 Gy/fx) without increasing the acute adverse event rates.20 Hou et al found that hypofractionated intensity-modulated radiotherapy (2.5–4.0 Gy/fx) obtained longer median survival than conventional 3DCRT (1.8–2.0 Gy/fx).21 These findings support our results. Our study retrospectively reviewed 198 HCC patients with a tumor size <10 cm who were ineligible for curative therapies and were treated with hypofractionated 3DCRT. The enrolled patients were divided into two groups, Group A and Group B. Group A received a median total dose of 53 Gy with fractions of 2.5–4.9 Gy given three fractions per week; Group B received a median total dose of 52 Gy with a 5.0–7.0 Gy/fx given three fractions per week. To reduce selection and confounding bias, we performed analysis on propensity-score matched patient pairs. It showed that a longer median survival was found in Group B (5.0–7.0 Gy/fx) than Group A (2.5–4.9 Gy/fx) in the PSM population (21.6 versus 14.7 months, respectively, P=0.032). The 1-, 3-, and 5-year OS rates were 58.0%, 34.6%, and 19.8% for patients who received the Group A regimen and 74.1%, 43.2%, and 33.3% for patients who received the Group B regimen after PSM, respectively (P=0.041). Moreover, although acute adverse events were seen in most patients, they were generally not serious and well tolerated. The rate and severity of acute adverse events were similar in both groups. These results indicate that a slightly higher dose fraction may be beneficial for patients with HCC that is not eligible for ablation or resection. by improving the local control and OS with an acceptable acute adverse events rate.
We performed multivariate analysis by considering a number of prognostic factors previously shown to be associated with the OS of HCC patients. The multivariate analysis identified tumor size (>5 cm), Child–Pugh class B, portal vein thrombosis, and fractions of 2.5−4.9 Gy/fx as prognostic factors after PSM. Our results identified tumor size >5 cm as an independent prognostic factors, which is also consistent with the previous studies.22,23 Tumor size >5 cm was related with increased invasiveness and reflected higher incidence of portal venous invasion and intrahepatic metastasis.24,25 A large tumor is likewise connected with the presence of vascular invasion and satellite lesions, which are conducive to tumor recurrence and poor prognosis.26,27 Our study also demonstrated that Child–Pugh class was an independent prognostic factor for OS, which led to a similar conclusions as previous studies.28 Child–Pugh class is generally considered to be a significant prognostic factor, due to HCC being a complex cancer inserted on preneoplastic cirrhosis, and thus variables of both diseases that cause death should be considered.29 There were twelve Child–Pugh class B patients in our study. Three of them eventually died from liver function decompensation caused by tumor progression or infection at 1, 3, and 4 months. Therefore, we recommend that Child–Pugh class A patients are the best candidates for 3DCRT. PVTT is a risk factor most likely because it is associated with the recurrence and emergence of de novo tumors.26 Moreover, our study found a fraction dose of 2.5−4.9 Gy was an independent prognostic factor, which might explain the longer median survival found in the group with higher fraction doses (5.0–7.0 Gy/fx).
The synergistic effect of TACE and 3DCRT has been demonstrated.30 TACE prolongs survival by the arterial injection of anticancer drugs and embolizing agents, which subsequently induce ischemic necrosis.31,32 In our study, due to the limited economic conditions of patients, only some patients used TACE in combination, but also achieved good results.
We found that lower percentages of V5 were observed in Group A patients compared with those in Group B. Group B provided a higher dose with no significant increase in V10, V15, V20, V30 and the mean dose of the whole liver. The overall toxicity, including liver toxicity in both treatment groups was similar. In addition, it was interesting to find that the cumulative recurrence rate and the site of recurrence tumors were similar between the two groups. It is generally known that recurrence is a major cause of death in HCC patients. Because 3DCRT is a local treatment, our results indicated that regular monitoring and systemic approaches were needed to prevent intrahepatic and extrahepatic metastasis.
There were some drawbacks in our study. First, this study is based on a retrospective analysis and might be affected by selection bias. Sufficient imaging data are lacking in this research, which makes it impossible to evaluate the efficacy on tumors after radiotherapy. Second, it was a single-center study carried out in the region with a high prevalence of HBV infection. Therefore, other study groups need external validation. Third, this study was not randomized. A large-scale randomized controlled trial would be ideal but might be difficult to carry out because in general practice, factors, such as body constitution and tumor location would affect the choice of treatment.33 Fourth, there was a small study population with patients receiving other surgical and systemic therapies before and after enrollment in the study.
Conclusions
Our PSM findings indicate that higher fraction dose radiotherapy that 3DCRT delivers is effective as it offers survival benefits without raising serious toxicity to patients with small- to medium-sized HCC tumors who are ineligible for curative therapies. It is without a doubt that prospective randomized control trials with large sample sizes should be carried out for further verification.
Acknowledgments
This study was sponsored by the Guangxi Medical and Health Key Research Project (Zhong200532), and the Youth Science Foundation of Guangxi Medical University (GXMUYSF201317).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Parkin DM, Whelan SL, Ferlay J, et al. Cancer incidence in five continents. Volume VII. IARC Sci Publ. 1997;VII(143):
2.
3. Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44(Suppl 19):119–121. doi:10.1007/s00535-008-2244-z
4. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi:10.1055/s-0030-1247133
5.
6. Ishii H, Furuse J, Kinoshita T, et al. Hepatectomy for hepatocellular carcinoma patients who meet the Milan criteria. Hepato-gastroenterology. 2008;55(82–83):621–626.
7. Benson AB
8. Sofocleous CT, Boas FE. Radiation segmentectomy for hepatocellular carcinoma: readyfor prime time? Radiology. 2018;287(3):1059–1060. doi:10.1148/radiol.2018180163
9. Kagawa T, Koizumi J, Kojima S, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010;116(15):3638–3644. doi:10.1002/cncr.25142
10. Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(3):1050–1058. doi:10.1148/radiol.2018171768
11. Lim DH, Lee H, Park HC, et al. The efficacy of high-dose 3-dimensional conformal radiation therapy in patients withsmall hepatocellular carcinoma not eligible for other local modalities. Am J Clin Oncol. 2013;36(2):162–166. doi:10.1097/COC.0b013e3182438dae
12. Jung J, Kong M, Hong SE. Conventional fractionated helical tomotherapy for patients with small to medium hepatocellular carcinomas without portal vein tumor thrombosis. Onco Targets Ther. 2014;7:1769–1775. doi:10.2147/OTT.S69618
13. Zhou ZH, Liu LM, Chen WW, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007;80(951):194–201. doi:10.1259/bjr/33521596
14. Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307–316.
15. Wu DH, Zhi FC, Chen LH. Evaluating the efficacy of transcatheter arterial chemoembolization combined with hypofractionated 3- dimensional conformal radiotherapy for hepatocellular carcinoma. Chinese J Digestion. 2004.
16. Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25(6):1189–1196. doi:10.1111/j.1478-3231.2005.01170.x
17. Liu MZ, Wang XS, Cai L, et al. [External radiation and combined transcatheter arterial chemoembolization for unresectable primary liver cancer]. Chin J Cancer. 2005;24(1):82–86.
18. Liao XF, Hui-Juan HE, Zhou ZS. Three-dimensional conformal radiotherapy combined with interventional therapy in treatment of primary hepatocellular carcinoma. J Pract Oncol. 2010;25(6):681–684.
19. Chen WJ, Yuan SF, Zhu LJ, Sun XN, Zheng W. Three-dimensional conformal radiotherapy in combination with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma. J BUON. 2014;19(3):692–697.
20. Wang C, Li S, Sun A, et al. The comparison of outcomes between hypofractionated and conventional 3D-CRT regimens used in combination with TACE as first-line treatment of advanced hepatocellular carcinoma. Tumour Biol. 2015;36(7):4967–4972. doi:10.1007/s13277-015-3144-5
21. Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Jpn J Clin Oncol. 2016;46(4):357–362. doi:10.1093/jjco/hyv205
22. Jiang JH, Guo Z, Lu HF, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol. 2015;21(15):4627–4634. doi:10.3748/wjg.v21.i15.4627
23. Chiche L, Menahem B, Bazille C, et al. Recurrence of hepatocellular carcinoma in noncirrhotic liver after hepatectomy. World J Surg. 2013;37(10):2410–2418. doi:10.1007/s00268-013-2127-1
24. Truant S, Boleslawski E, Duhamel A, et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol. 2012;38(12):1189–1196. doi:10.1016/j.ejso.2012.07.112
25. Zhang Q, Chen H, Li Q, et al. Combination adjuvant chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin after liver transplantation for hepatocellular carcinoma: a preliminary open-label study. Invest New Drugs. 2011;29(6):1360–1369. doi:10.1007/s10637-011-9726-1
26. Li T, Fan J, Qin LX, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–1963. doi:10.1245/s10434-010-1540-z
27. Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–1092. doi:10.1002/lt.20472
28. Bae SH, Kim MS, Cho CK, et al. Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona clinic liver cancer-C stage hepatocellular carcinoma. J Korean Med Sci. 2013;28(2):213–219. doi:10.3346/jkms.2013.28.2.213
29. Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40(3):225–235. doi:10.1007/s00535-005-1566-3
30. Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi:10.1016/j.ijrobp.2011.03.019
31. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi:10.1016/S0140-6736(02)08649-X
32. Maluccio MA, Covey AM, Porat LB, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19(6):862–869. doi:10.1016/j.jvir.2008.02.013
33. Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34(17):2046–2053. doi:10.1200/JCO.2015.64.0821
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
