Back to Journals » Drug, Healthcare and Patient Safety » Volume 7
Survey on appropriateness of use of nimesulide in nine European countries
Authors Franchi S, Heiman F, Visentin E, Sacerdote P
Received 24 October 2014
Accepted for publication 29 January 2015
Published 16 March 2015 Volume 2015:7 Pages 51—55
DOI https://doi.org/10.2147/DHPS.S76320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shu-Feng Zhou
Silvia Franchi,1 Franca Heiman,2 Elena Visentin,2 Paola Sacerdote,1
1Dipartimento di Scienze Farmacologiche e Biomolecolari, Università degli Studi di Milano, Milan, Italy; 2Cegedim Strategic Data Medical Research Srl, Milan, Italy
Background: Appropriateness of use is a key factor in safeguarding patient’s health as well as a product’s therapeutic properties. This paper presents the results of a survey conducted in nine European countries to verify the appropriateness of use of nimesulide in patients with inflammatory pain.
Methods: Computer-aided telephone interviews were administered to 1,277 nimesulide-prescribing general practitioners in Bulgaria, Czech Republic, Greece, Hungary, Italy, Poland, Portugal, Romania, and Slovakia, covering an estimated 31,719 patients. The interview questionnaire collected information on nimesulide prescriptions with respect to daily dose, treatment duration, and indication.
Results: In the majority of cases, prescriptions of nimesulide did not exceed the recommended daily dose of 200 mg (given as 100 mg twice a day), with a range from 161 mg (Greece) to 190 mg (Slovakia). An adherence to the 15-day treatment limit was observed in over 90% of cases. The average number of treatment days was always less than 15, with a range from 5.4 (Italy) to 13.6 (Czech Republic). Nimesulide was primarily used for the treatment of acute pain and short-term painful episodes in chronic conditions. The presence of gastrointestinal diseases/ulcers was the most frequent reason for not prescribing nimesulide.
Conclusion: The results of this survey demonstrate that nimesulide is generally prescribed in compliance with the information reported in the summary of product characteristics (SmPC) with regard to daily dose and treatment duration, and suggest that it is mainly used for the management of episodes of acute pain in patients with a chronic disorder. These findings indicate the appropriateness of use of nimesulide in the European countries considered in this survey.
Keywords: non-steroidal anti-inflammatory drug, NSAID, appropriateness of use, European Medicine Agency, computer-aided telephone interviews
Introduction
Nimesulide is a non-steroidal anti-inflammatory drug (NSAID), effective in the treatment of a range of inflammatory and painful conditions, including acute pain and primary dysmenorrhea.1
Medicines containing nimesulide are available upon prescription in a number of countries of the European Union, including Bulgaria, Cyprus, Czech Republic, Greece, Hungary, Italy, Latvia, Lithuania, Malta, Poland, Portugal, Romania, Slovakia, and Slovenia. This drug is off-patent and is also marketed by a variety of pharmaceutical companies as a generic drug.
Following the latest review on the safety and efficacy of systemic medicines containing nimesulide,2 the Committee of Human Medicinal Products concluded that the benefits of systemic formulations of nimesulide outweigh the risks, provided that this NSAID is used appropriately. The Committee of Human Medicinal Products recommends nimesulide for the treatment of acute pain and primary dysmenorrhea, with a maximum daily dose of 100 mg twice daily and for less than 15 consecutive days. The European Commission confirmed the positive benefit/risk profile of nimesulide in the above-mentioned indications on January 20, 2012.3
Appropriateness of use is a key factor in safeguarding the health of patients. The marketing authorization holder of the original nimesulide (Helsinn Healthcare, Lugano, Switzerland) and the European Medicine Agency (EMA) agreed on a “Dear Healthcare Professional Communication” (DHPC) to explain the positive benefit/risk profile of nimesulide when used for its approved indications.
After distribution of this DHPC, Helsinn Healthcare sponsored a survey among general practitioners (GPs) of nine European countries in order to verify that nimesulide is used according to the latest recommendations issued by the EMA. This paper presents the results of this survey.
Materials and methods
The objective of this study was to verify the appropriateness of use of nimesulide among GPs who gave their consent to be interviewed and use of the related information. The analysis was based on a survey of anonymized data and did not involve any new studies of human or animal subjects performed by any of the authors.
The survey was conducted in October 2012 (about 6 months after the distribution of the DHPC) in Bulgaria, Czech Republic, Greece, Hungary, Italy, Poland, Portugal, Slovakia, and Romania. Computer-aided telephone interviews (CATI) methodology was used. This method is suitable for the scope of the survey as it allows surveyors to reach a large number of doctors across a wide range of sampling points, thus guaranteeing thorough representation. Previously this method has been successfully applied to medical research involving cardiovascular disease and asthma.4,5 CATI is based on telephone interviews read by the interviewer and selecting responses from multiple-choice options on a computerized questionnaire with built-in branch logic, hence skipping inapplicable questions and probing for more detail when required. The CATI approach enhances data accuracy and intercountry standardization.6,7
GP statistics and lists were obtained from official data derived from the Ministry of Health of the countries in scope along with the “One-Key database”, the world reference for information on health care professionals.8 Only prescribers of nimesulide were recruited for the interview. The prescription of nimesulide was ascertained by means of a preliminary screening question: “On average, in a month, how many patients do you prescribe a product containing nimesulide for any disease?” (prescribers if >0).
The interview questionnaire included the following items: daily dose, patient characteristics, treatment duration, reason for use, and an estimate of the average number of patients prescribed nimesulide per month. Representativeness of the GP sample is supported by random selection of contacts and the distribution of samples by geographic area.
The caseload of patients was collected by means of the last patient technique (each physician is asked to provide information on the last patient to whom nimesulide is prescribed); this indicates that one patient case per physician is collected for each response and carried forward to the base of physicians selected from each country. Owing to its random selection process, the last patient technique can be considered truly representative of prescribing behavior. Descriptive statistics are used to report data.
Results
Prescribers and monthly caseload of patients
Of the 1,387 GPs contacted, 1,277 were nimesulide prescribers and were included in the analysis. Prescribers of nimesulide represented 92% of the contacted physicians in all the countries in the survey, with the exception of Hungary (85%), Portugal (81%), and Greece (76%). The number of nimesulide-prescribing GPs ranged from 90 in Slovakia and Greece to 200 for Poland and 201 for Italy. The sample size of interviewed GPs is reliable, given the 95% confidence level with a confidence interval ranging from 6.89 (Poland) to 10.28 (Greece). Cumulatively, 119,300 estimated patients were referred to the 1,277 nimesulide-prescribing GPs (a range of 2,300 patients in Slovakia and 46,800 patients in Italy). Of this figure, 31,719 patients in total were estimated to be prescribed nimesulide per month, ranging from 1,242 patients in Greece to 7,457 patients in Italy.
Prescriptions by therapeutic indication
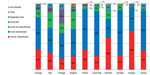
Analyzing the reported cases by therapeutic indication, it is noted that the majority of the prescriptions involved patients with acute inflammatory pain of various origins. On average, 72% of nimesulide prescriptions were for symptomatic treatment of osteoarticular diseases (Figure 1).
Prescribed daily dose
Approximately 30% of the respondents prescribed a dose of 100 mg once a day. Sixty percent reported a dose of 100 mg twice a day, with a minority of patients being prescribed 100 mg three times a day, from 1% in Bulgaria, Czech Republic, Romania, and Hungary, to 10% in Portugal. The average daily dose was 168 mg, ranging from 161 mg in Greece to 190 mg in Slovakia (Figure 2).
Prescribed treatment duration
Cases with a treatment duration up to 15 days were 94% on average, with 98%–100% cases in Italy, Portugal, Bulgaria, Romania, and Greece, and 91%–92% of patients in Slovakia and Poland. Only 1% of the treated patients were exposed for more than 15 days in Italy, Portugal, and Poland; 4% in Romania; and 9%, 12%, and 16% in Slovakia, Hungary, and the Czech Republic, respectively (Figure 3). The average number of treatment days was 9.4, ranging from 5.4 in Italy to 13.6 in the Czech Republic. These data demonstrate an average treatment duration of less than 15 days in the large majority of the cases, and constitute further confirmation of the appropriateness of use of nimesulide.
Reasons for not prescribing nimesulide
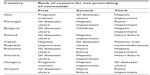
In general it appears that respondents were aware of all clinical situations that preclude or limit the use of nimesulide. The presence of gastrointestinal diseases (including ulcers) was the first reason for not prescribing nimesulide in five countries (Portugal, Bulgaria, Romania, Slovakia, and Greece). Hepatic impairment was the first reason for non-prescription of nimesulide in the Czech Republic and the second/third reason in Portugal, Poland, Slovakia, Hungary, Italy, Bulgaria, and Romania (Table 1). On average, 59% of nimesulide prescriptions were as second-line treatment.
Discussion
Nimesulide is an NSAID with a multifactorial mode of action, characterized by a fast onset of analgesic activity, and is particularly suitable for the treatment of acute inflammatory pain.9 It has been available on the market and widely prescribed since 1985. As with all NSAIDs, nimesulide prescriptions must be based on careful evaluation of the patient’s characteristics and clinical condition. Nimesulide must be used under medical supervision in accordance with the approved indications and in compliance with the information included in the product’s SmPC.1,3 The use of this product is currently recommended in acute inflammatory and painful conditions at a dose of 100 mg twice daily for a maximum of 15 days, and must not be used in patients at risk of hepatotoxic reactions or gastrointestinal bleeding. As with all NSAIDs, severe heart failure an renal impairment represent additional contraindications.1 The results of this survey show that nimesulide is generally prescribed according to the recommended indications. In fact, it is prescribed for acute painful conditions in the majority of cases. Most of the prescriptions do not exceed the recommended dose of 100 mg twice a day. The average treatment duration is less than 15 days in 98%–100% of the prescriptions in Italy, Portugal, Bulgaria, Romania, and Greece, and in 91%–92% of the prescriptions in Slovakia and Poland, with a range of treatment days from 5.4 in Italy to 13.6 in the Czech Republic. The overall results of this survey indicate that when nimesulide is used in patients with acute pain associated with chronic disease, the treatment is generally within the recommended 15-day limit and within the recommended dose, thus supporting the symptomatic use of this drug. The recommendations of the SmPC with respect to the contraindications are also adhered to. Interestingly, gastrointestinal disorders/ulcers are indicated as the first reason for not prescribing nimesulide in six of nine countries.
Thanks to the appropriate use of nimesulide in clinical practice, no critical safety issues were identified in recent years based on spontaneous reports, reviews, or published studies. Consistently, Periodic Safety Update Reports should be submitted to the EMA according to a 3-year periodicity,10 instead of every 6 months, as in the past. On the whole, this evidence confirms the positive benefit/risk profile of nimesulide when used appropriately.
From this point of view, it is important to note that the marketing authorizations for systemic formulations of nimesulide in the European Member States were renewed via the Mutual Recognition Procedure with unlimited validity in April 2014.
Conclusion
The results of this survey indicate that nimesulide is generally prescribed in compliance with the information reported in the SmPC with regard to daily dose and treatment duration. With reference to indications, a large number of patients with acute or chronic osteoarticular disease received nimesulide. The evidence of predominant use in symptomatic treatment, combined with the results showing a short duration of treatment, suggests that nimesulide was mainly used for the management of episodes of acute pain in patients with a chronic disorder. These findings demonstrate the appropriateness of use of nimesulide in the European countries considered in this survey.
Disclosure
This study was financially supported by Helsinn Healthcare, Switzerland. CSD Italy Srl was responsible for the survey, data entry, and data analysis. CSD Medical Research Srl was responsible for medical writing and editorial assistance. The authors report no other conflicts of interest in this work. All authors were responsible for data interpretation and critically revised and approved the final manuscript.
References
European Medicines Agency. Summary of Product Characteristics. Annex III to the Commision Decision on Article 31 referral for nimesulide-containing medicinal products. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Nimesulide/human_referral_000275.jsp&mid=WC0b01ac0580024e9a. Updated January 20, 2012. Accessed July 28, 2014. | |
European Medicines Agency Nimesulide – referrals. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Nimesulide/human_referral_000275.jsp&mid=WC0b01ac0580024e9a. Updated April 19, 2012. Accessed July 28, 2014. | |
European Medicines Agency. Assessment report for Nimesulide containing medicinal products-Procedure number: EMEA/H/A-31/1261- 20 January 2012 – Report Nr.EMA/73856/2012 -Patient Health Protection. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Nimesulide_31/WC500125574.pdf. Accessed July 28, 2014. | |
Ketola E, Klockars M. Computer-assisted telephone interview (CATI) in primary care. Fam Pract. 1999;16:179–183. | |
Anie KA, Jones PW, Hilton SR, Anderson HR. A computer-assisted telephone interview technique for assessment of asthma morbidity and drug use in adult asthma. J Clin Epidemiol. 1996;49:653–656. | |
Kelly J. Computer-assisted Telephone Interviewing (CATI). In: PJ Lavrakas. Encyclopedia of Survey Research Methods. Thousand Oaks, CA, UA: Sage Publications; 2008. | |
Bailey KD. Methods of Social Research. 4th ed. New York, NY, USA: Simon & Schuster; 1994. | |
Cegedim. Healthcare Professional Databases. Available from: http://www.cegedim.com/solutions/life-sciences/cci/Pages/default.aspx. Accessed July 28, 2014. | |
Rainsford KD; Members of the Consensus Report Group on Nimesulide. Nimesulide – a multifactorial approach to inflammation and pain: scientific and clinical consensus. Curr Med Res Opin. 2006;22:1161–1170. | |
European Medicines Agency. List of European Union Reference Dates and frequency of submission of PSURs (EURD List). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000361.jsp&mid=WC0b01ac058066f910. Accessed July 28, 2014. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.