Back to Journals » Cancer Management and Research » Volume 12
Surgical Outcomes of Transvaginal Neobladder-Vaginal Fistula Repair After Radical Cystectomy with Ileal Orthotopic Neobladder: A Case–Control Study
Received 14 August 2020
Accepted for publication 24 September 2020
Published 19 October 2020 Volume 2020:12 Pages 10279—10286
DOI https://doi.org/10.2147/CMAR.S277001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Dong Hyeon Lee, 1 Wan Song 2
1Department of Urology, Ewha Womans University Medical Center, Ewha Womans University School of Medicine, Seoul, Korea; 2Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
Correspondence: Wan Song
Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-Ro, Gangnam-Gu, Seoul 135-710, Korea
Tel +82-2-3410-3558
Fax +82-2-3410-3027
Email [email protected]
Purpose: To present surgical methods and outcomes in women with bladder cancer (BCa) requiring correction of neobladder-vaginal fistula (NVF) after radical cystectomy (RC) with ileal orthotopic neobladder (IONB).
Materials and Methods: The medical records of 163 women who underwent RC with IONB for BCa between January 2010 and December 2018 were retrospectively reviewed. The presence of NVF was confirmed by cystoscopy and/or voiding cystography. NVF repair was performed using a transvaginal approach, which included circumferential incision of the fistula tract, creation of a plane between the neobladder serosa and the vaginal epithelium, and multi-layered transvaginal closure.
Results: During a median follow-up of 47.9 months, NVF was identified in 12 (8.8%) of the 163 included women. Eight (66.7%) fistulas were located in the proximal anterior vaginal wall and four (33.3%) in the vaginal apex. Median time from RC to NVF repair was 3.4 months (range, 2.1– 5.6 months), median NVF size was 6.0 mm (range, 4.0– 22.0 mm), and median duration of urethral Foley catheter indwelling was 24.0 days (range, 15.0– 43.0 days). Initial repair of NVF was successful in ten (83.3%) patients. Two (16.7%) patients who relapsed retained IONB through the subsequent operation. Two (16.7%) patients developed severe urinary incontinence after NVF repair, requiring anti-incontinence surgery with a synthetic transobturator mid-urethral sling.
Conclusion: The transvaginal approach for NVF repair is feasible, yielding successful surgical outcomes. However, women should be counseled about the risks of relapse and urinary incontinence.
Keywords: neobladder-vaginal fistula, orthotopic urinary diversion, radical cystectomy, surgical outcomes, vaginal approach
Erratum for this paper has been published
Introduction
Radical cystectomy (RC) is the standard surgical treatment for muscle infiltrating or recurrent, high risk, non-muscle-invasive bladder cancer (BCa).1,2 Ileal orthotopic neobladder (IONB) results in a better quality of life3 than other methods of urinary diversion, with 60–70% of women being candidates for IONB at the time of RC.4 Some of these patients, however, may experience surgical complications, such as uretero-intestinal anastomotic stricture, urine leakage, calculus formation and neobladder-vaginal fistula (NVF).5
NVF is a relatively unusual complication, occurring in 2.7–6.0% of women following IONB formation.6–9 Risk factors for NVF include damage to and poor tissue vascularity within the anterior vaginal wall (AVW), an adjacent suture line between the urethral-neobladder anastomosis and the AVW, and local tumor recurrence.5,9 Several surgical modifications have been introduced to reduce the risks of NVF, such as AVW preservation,10 avoidance of overlapping suture lines, and covering the vaginal stump with the omentum.11 These modifications, however, did not protect all patients from these surgical complications.
The treatment of NVF is a surgical challenge, with no consensus regarding the optimal approach and surgical technique for NVF repair. The present study, therefore, describes the experience of our center in surgical technique and outcomes in patients requiring the correction of NVF after RC with IONB.
Materials and Methods
Study Population
This retrospective, descriptive study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (approval number: 2019-10-032), which waived the requirement for informed consent because of the retrospective design of this study. All study protocols were carried out in accordance with the Declaration of Helsinki. A prospectively collected cystectomy-oncology database of 558 patients who underwent RC for BCa between January 2010 and December 2018 by a single urologic oncology surgeon was retrospectively reviewed. Of the 163 women included in the study cohort, 27 were excluded, 24 who underwent ileal conduit urinary diversion and three who underwent ureterocutaneostomy. Ultimately, this study analyzed 136 women who underwent Studer IONB following RC. None of these patients had a history of radiation therapy preoperatively.
Data Collection
The medical records of all patients, recorded at the time of surgery, were reviewed, and their clinical and pathological characteristics were determined, including age at surgery, body mass index (BMI), comorbidities such as diabetes mellitus (DM), pathologic tumor stage, neoadjuvant and/or adjuvant chemotherapy, postoperative complications, and management. The details of the modified surgical technique used at our institution have been described, with all ileal reservoirs constructed as Studer pouches.12 All RCs were performed as open procedures and included anterior exenteration with excision of the upper third of the vagina, as well as standard bilateral pelvic lymphadenectomy.
Follow-Up
In general, patients were followed-up 1 month after surgery, every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. All follow-up visits included physical examination with laboratory tests, urine analysis with cytology, chest radiography, and radiologic evaluation including computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis. Cystoscopy was performed when urine cytology showed abnormal findings and/or patients experienced urinary symptoms, such as hematuria or irritative voiding. A bone scintigraphy scan was performed when clinically indicated.
Suspicion of NVF was based on history taking and physical examination, with the diagnosis confirmed by cystoscopy and/or voiding radiography. Cystoscopy included determination of the number, locations, and sizes of the NVFs.
Surgical Technique and Postoperative Management
The surgical technique for NVF repair was similar to that used in the repair of vesicovaginal fistula (VVF) with an intact bladder13 and included a multi-layer transvaginal closure. NVF repair was performed using a transvaginal approach with the patient under general anesthesia. The patient was placed in an extended lithotomy position, and the suprapubic and perineal areas and vagina were sterilized with iodine-containing wash.
After insertion of the cystoscope through the urethra, the neobladder was filled with normal saline to identify the features of the fistula. The vaginal canal was exposed using a weighted speculum and a US army retractor (Figure 1A). A solution of lidocaine with epinephrine was allowed to infiltrate the AVW for hydrodissection. A sharp, circumferential incision was made around the fistula opening, and the fistula was dissected to create a plane between the serosa of the neobladder and the epithelium of the vagina (Figure 1B). The neobladder was closed with continuous sutures using poliglecaprone 3–0 (Monocryl®; J and J Medical, Somerville, NJ, USA; Figure 1C), and the AVW was repaired with continuous sutures of Polyglactin 2–0 (Vicryl®, J and J Medical; Figure 1D).
A 24Fr urethral Foley catheter was inserted into the neobladder, and 200 mL saline was infused to test for possible leakage. The vagina was packed for 24 hours postoperatively with gauze soaked in povidone iodine. The urethral Foley catheter was maintained for at least 3 weeks, depending on the size of the NVF and the quality of the vaginal tissue. Cystography was performed prior to the removal of the urethral Foley catheter for final confirmation.
Statistical Analysis
The clinical and pathological characteristics of the patients were compared by descriptive statistical analyses. Quantitative variables were reported as median (range) and compared by independent t-tests; and qualitative variables were reported as number (percentage) and compared by Pearson’s chi-square test or Fisher`s exact test. All statistical analyses were performed using IBM SPSS statistics for Windows, version 23.0 (IBM Corp. Armonk, NY, USA). Two-sided P-values <0.05 were considered statistically significant.
Results
The baseline clinicopathologic characteristics of the 136 women who underwent RC with IONB for BCa are summarized in Table 1. Their median age at surgery was 65.0 years (range, 31.0–81.0 years), and their median BMI was 23.8 kg/m2 (range, 15.0–32.8 kg/m2). Sixteen (11.8%) women had DM, and 46 (33.8%) showed locally advanced tumor stage (≥ pT3) at the time of RC. Neoadjuvant chemotherapy was administered to 27 (19.9%) patients and adjuvant chemotherapy to 55 (40.4%). During a median follow-up of 47.9 months, 12 (8.8%) women were diagnosed with NVF. The clinicopathologic characteristics of these 12 women did not differ significantly from those of the 124 women without NVF (all P > 0.05, respectively). None of these patients had evidence of local recurrence.
 |
Table 1 Baseline Demographic and Clinical Characteristics of All Patients Who Underwent Radical Cystectomy with Ileal Orthotopic Neobladder |
Median time from RC to NVF repair was 3.4 months (range, 2.1–5.6 months). The characteristics of the 12 patients with NVF and the details of NVF repair are summarized in Table 2. Median operation time was 47.5 min (range, 25.0–100.0 min), and median estimated blood loss was 27.5 mL (range, 10.0–75.0 mL). Eight (66.7%) fistulas were located in the proximal AVW and four (33.3%) in the vaginal apex. Median NVF size was 6.0 mm (range, 4.0–22.0 mm), and median duration of urethral Foley catheter indwelling was 24.0 days (range, 15.0–43.0 days).
 |
Table 2 Surgical Outcomes of Neobladder-Vaginal Fistula Repair |
Following NVF repair, the 12 patients were followed-up for a median 43.1 months (range, 12.9–69.7 months). Initial transvaginal repair of NVF was successful in ten (83.3%) patients. However, two patients who were not diagnosed with DM, #7 and #11, developed recurrent NVF 3.2 and 14.8 months, respectively, after initial NVF repair. Both underwent secondary NVF repair using the same procedure. The operation was successful in Patient #11, whereas Patient #7 developed a recurrent NVF 6.5 months after secondary repair. The third operation was successful. The overall surgical outcomes of NVF repair are depicted in Figure 2.
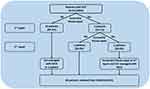 |
Figure 2 Outcome of patients following neobladder-vaginal fistula repair. |
Of the 12 patients, two, #2 and #4, required intermittent self-catheterization. Two others, #1 and #7, experienced severe urinary incontinence, requiring anti-incontinence surgery with a synthetic transobturator mid-urethral sling. Although surgical results were satisfactory, both of the latter patients required the use of 1–2 pads per day. Ultimately, all patients retained their IONBs.
Discussion
The present study showed that, of the 136 women who underwent RC with IONB for BCa, 12 (8.8%) developed NVF. Initial transvaginal repair of NVF was successful in ten (83.3%) of these 12 women, with the other two patients experiencing NVF recurrence, although both retained their IONB through subsequent operations. Even after successful NVF repair, it is necessary to counsel patients about the risk of urinary incontinence. To our knowledge, this is one of the largest case series to evaluate the surgical outcomes of transvaginal NVF repair.
Treatment of NVF after RC in women is challenging. These fistulas do not heal spontaneously. Although the surgical technique used for NVF repair is similar to that used for VVF repair in patients with an intact bladder, several factors mitigate against successful NVF repair. First, the neobladder wall is much thinner and less vascular than the walls of the intact bladder.5 Second, atrophic vaginitis is more frequent in patients undergoing NVF repair, as their vaginas were hypovascular and scarred after anterior exenteration. Furthermore, it is difficult to assess the extent of incontinence in patients with NVF, and repair of NVF could aggravate the incontinence itself.14
Table 3 compares our results with those of recently published studies of NVF repair.6–8,15 These case series included 6–14 patients with NVF, with an incidence of NVF ranging from 2.7% to 6.0%. Recurrent fistulas were identified in 0–50% of patients who underwent NVF repair, with IONB retained by 38.5–100% of patients. Compared with these earlier studies, we observed a higher overall incidence (8.8%) of NVF and a higher success rate of initial NVF repair (83.3%) with IONB retained by 100% of patients.
 |
Table 3 Comparison of the Present Results with Results from Studies Evaluating the Outcomes of Neobladder-Vaginal Fistula Repair |
The main surgical difference between our study and these previous studies was in our ability to preserve the distal two-thirds of the AVW during anterior exenteration. The distal AVW is the most common site of injury during urethral dissection. In addition, we found that the most common site of NVF was the proximal ends of the AVW, followed by the vaginal apex. These results emphasize the need for a tight closure of the vaginal cuff after hysterectomy.11
In addition, in a study by Rosenberg et al,8 a total of 13 patients with NVF repair were retrospectively reviewed. Rates of successful repair were higher for fistulas located in the AVW than in the urethra-neobladder anastomosis (UNA) (4/4, 100% vs 4/9, 44%). Furthermore, post NVF repair continence of patients at the AVW was better than those at the UNA, suggesting that outcomes appear to be better in patients with NVF more proximal to the UNA.
Our experience suggests that the transvaginal approach is feasible and should be considered as an initial approach in patients with NVF. Both sides of the proximal AVW and the vaginal apex are easily accessible through the transvaginal approach. Moreover, this approach avoids the potential risk of neobladder or bowel injury that could occur using an abdominal approach. In addition, outcomes using the transvaginal approach were successful in one patient with a 22-mm-sized NVF and two patients with recurrent NVF. Thus, NVF size and history of previous treatment failure should not be a limiting factor for the transvaginal approach.15
In our study, two patients experienced de novo stress urinary incontinence. A short, non-compliant urethra is frequently identified in these patients because dissection of AVW causes damage to the sphincter mechanism and/or urethral blood supply.8 In general, a synthetic mid-urethral sling is not recommended for the treatment of incontinence after NVF repair due to the increased risks of NVF erosion in atrophic vaginas, as well as injuries to the bowel and neobladder. However, two patients in our study (#2 and #12) lacked vaginal atrophy and had good tissue quality, enabling a urologist specializing in female reconstruction surgery to perform anti-incontinence surgery with a transobturator mid-urethral sling without complications. Injection of bulking agents is the preferred surgical option8,15 as it can compensate for the loss of urethral coaptation due to intrinsic sphincteric deficiency.8 However, de novo NVF has been reported following injection of bulking agent.7,16 In addition, patients should be fully counseled that restoration of continence may not persist and that they may require repeated injections.
Tissue interposition has been reported to be effective for NVF repair.14 An omental flap is preferred, as it is robust and does not require additional incision.8 If an omental flap is unavailable, a Martius labial fat flap can be used because it offers extra-vascularization and a better epithelization surface. If both flaps do not reach the NVF site, gracilis muscle flaps can be considered. However, none of the patients in the current study required tissue interposition, as most of the NVFs were <2 cm in size, and the tissue used for repair was well vascularized and of sufficient strength. Despite the lack of tissue interposition, the success rate of NVF repair was comparable to success rates in studies that used tissue interposition.6,8,15
The usefulness of tissue sealant at anastomosis sites is unclear.17–20 Polyglycolic acid (PGA) sheets (Neoveil®; Gunze Co., Tokyo, Japan) are a new type of hemostasis reinforcement material, which promotes tissue sealing and prevents leakage. PGA sheets have been reported to reduce the rates of pancreatic fistula18 and cervical anastomotic fistula in patients with esophageal cancer.20 Few studies in urologic surgery have tested the efficacy of PGA sheets for hemostasis after partial nephrectomy.19 Further studies are needed to determine the role of bio-absorbable material in reducing fistula formation in patients undergoing urologic surgery.
This study has several strengths. First, it is one of the largest case series to date of patients undergoing transvaginal NVF repair with a detailed description of NVF characteristics. In addition, the median follow-up was relatively long, 43.1 months, enabling assessment of the clinical course and outcomes following subsequent surgical intervention.
However, this study also had several limitations. First, it was retrospective in design and included patients treated at a single tertiary referral center by a single surgeon, raising concerns about selection bias. Second, despite this being the largest case series to date, the number of patients was quite low because this condition is uncommon. Third, it was difficult to determine the impact of perioperative chemotherapy on NVF. Finally, there was no data related to the quality of life after VNF repair that we could not report it. Large, prospective, multicenter studies are therefore warranted.
In conclusion, NVF is a distressing complication for women following IONB, and NVF repair is a surgical challenge. This study suggests that a transvaginal approach is feasible and can result in successful surgical outcomes. However, women should be counseled about the risks of relapse and urinary incontinence.
Funding
This research was supported by the Basic Science Research Program through a National Research Foundation of Korea grant funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6007678).
Disclosure
All the authors have approved the manuscript and agree with submission to your esteemed journal. The authors declare that they have no conflicts of interest.
References
1. Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU international consultation on bladder cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45–57. doi:10.1016/j.eururo.2012.08.009
2. Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475. doi:10.1016/j.eururo.2016.06.020
3. Gakis G, Stenzl A. Considerations for orthotopic diversions in women. Curr Opin Urol. 2015;25(6):550–554. doi:10.1097/MOU.0000000000000224
4. Stein JP, Esrig D, Freeman JA, et al. Prospective pathologic analysis of female cystectomy specimens: risk factors for orthotopic diversion in women. Urology. 1998;51(6):951–955. doi:10.1016/S0090-4295(98)00099-5
5. Tunuguntla HS, Manoharan M, Gousse AE. Management of neobladder-vaginal fistula and stress incontinence following radical cystectomy in women: a review. World J Urol. 2005;23(4):231–235. doi:10.1007/s00345-005-0013-7
6. Ali-El-Dein B, Ashamallah A. Vaginal repair of pouch-vaginal fistula after orthotopic bladder substitution in women. Urology. 2013;81(1):198–202. doi:10.1016/j.urology.2012.08.103
7. Bailey GC, Blackburne A, Ziegelmann MJ, Lightner DJ. Outcomes of surgical management in patients with stress urinary incontinence and/or neovesicovaginal fistula after orthotopic neobladder diversion. J Urol. 2016;196(5):1478–1483. doi:10.1016/j.juro.2016.06.009
8. Rosenberg S, Miranda G, Ginsberg DA. Neobladder-vaginal fistula: the university of southern california experience. Neurourol Urodyn. 2018;37(4):1380–1385. doi:10.1002/nau.23454
9. Rapp DE, O’Connor RC, Katz EE, Steinberg GD. Neobladder-vaginal fistula after cystectomy and orthotopic neobladder construction. BJU Int. 2004;94(7):1092–1095. doi:10.1111/j.1464-410X.2004.0339.x
10. Chang SS, Cole E, Cookson MS, Peterson M, Smith JA
11. Ali-el-Dein B, Shaaban AA, Abu-Eideh RH, el-Azab M, Ashamallah A, Ghoneim MA. Surgical complications following radical cystectomy and orthotopic neobladders in women. J Urol. 2008;180(1):206–210. doi:10.1016/j.juro.2008.03.080
12. Song W, Yoon HS, Kim KH, et al. Role of bowel suspension technique to prevent early intestinal obstruction after radical cystectomy with ileal orthotopic neobladder: a retrospective cohort study. Int J Surg. 2018;55:9–14. doi:10.1016/j.ijsu.2018.04.044
13. Eilber KS, Kavaler E, Rodriguez LV, Rosenblum N, Raz S. Ten-year experience with transvaginal vesicovaginal fistula repair using tissue interposition. J Urol. 2003;169(3):1033–1036. doi:10.1097/01.ju.0000049723.57485.e7
14. Kaufman MR. Neobladder-vaginal fistula: surgical management techniques. Curr Urol Rep. 2019;20(11):67. doi:10.1007/s11934-019-0934-0
15. Carmel ME, Goldman HB, Moore CK, Rackley RR, Vasavada SP. Transvaginal neobladder vaginal fistula repair after radical cystectomy with orthotopic urinary diversion in women. Neurourol Urodyn. 2016;35(1):90–94. doi:10.1002/nau.22687
16. Pruthi RS, Petrus CD, Bundrick WJ. New onset vesicovaginal fistula after transurethral collagen injection in women who underwent cystectomy and orthotopic neobladder creation: presentation and definitive treatment. J Urol. 2000;164(5):1638–1639. doi:10.1016/S0022-5347(05)67047-4
17. Gonde H, Le Gac C, Gillibert A, et al. Feedback on the use of three surgical sealants for preventing prolonged air leak after robot-assisted anatomical lung resection. J Thorac Dis. 2019;11(7):2705–2714. doi:10.21037/jtd.2019.06.43
18. Kwon HE, Seo HI, Yun SP. Use of neoveil or tachosil to prevent pancreatic fistula following pancreaticoduodenectomy: a retrospective study. Medicine (Baltimore). 2019;98(17):e15293. doi:10.1097/MD.0000000000015293
19. Hongo F, Kawauchi A, Ueda T, et al. Laparoscopic off-clamp partial nephrectomy using soft coagulation. Int J Urol. 2015;22(8):731–734. doi:10.1111/iju.12808
20. Song YN, Qi Y, Zhang CY, et al. A new technology for reducing anastomotic fistula in the neck after esophageal cancer surgery. J Thorac Dis. 2019;11(7):3084–3092. doi:10.21037/jtd.2019.07.28
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

