Back to Journals » Clinical Ophthalmology » Volume 15
Surgical Outcomes of Primary Congenital Glaucoma in Children Under One Year from the Nile Delta
Authors Farid MF, Anany M, Awwad MA
Received 30 December 2020
Accepted for publication 23 February 2021
Published 16 March 2021 Volume 2021:15 Pages 1145—1151
DOI https://doi.org/10.2147/OPTH.S299716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mohamed F Farid, Mohamed Anany, Mohamed A Awwad
Ophthalmology Department, Benha University, Benha, Egypt
Correspondence: Mohamed A Awwad
Ophthalmology Department, Benha University, PO Box: 13511, 1 Fareed Nada Street, Benha, Egypt
Email [email protected]
Purpose: To present outcomes of surgical management of primary congenital glaucoma (PCG) in children less than one year of age in a population based at the Nile Delta.
Methods: A retrospective review of medical records of patients with PCG less than one year of age at presentation who underwent surgical intervention in a tertiary care facility based at the Nile Delta. All patients underwent measurement of intraocular pressure (IOP), horizontal corneal diameter (HCD), cup-to-disc ratio (CDR) before and after surgery and a minimum of 6 months follow up was required. Surgical success was defined as IOP less than 22mmHg without medications and without progression of main disease parameters.
Results: The review revealed 44 eyes of 26 consecutive patients who underwent surgical treatment for PCG. Average age at surgery was 5.2 months and mean follow up was 18.5 months. Preoperative IOP was 28.5± 4 mmHg, HCD was 13.7± 0.7mm, and CDR (when visible) was 0.65± 0.18. A total of 69 surgical procedures were performed with an average of 1.56 procedures per eye. Postoperative IOP was 13.3± 4.8 mmHg, HCD was 12.8± 0.9mm, and CDR was 0.3± 0.2 (P< 0.0001). Surgical success was achieved in 32 eyes (72.7%) while sight-threatening postoperative complications were reported in 3 eyes.
Conclusion: Surgical management of PCG younger than one year of age achieved good success rate in the region of the Nile Delta with low rate of visually significant postoperative complications. However, larger studies with longer follow up are needed to fully reveal the overall characteristics of PCG in the region.
Keywords: primary congenital glaucoma, intraocular pressure, trabeculotomy, trabeculectomy, Ahmed glaucoma valve, the Nile Delta
Introduction
Primary congenital glaucoma (PCG) is defined as idiopathic, isolated developmental anomaly of the angle of anterior chamber which leads to sustained increase in intraocular pressure (IOP). Presenting features, which could be observed in infancy or early childhood, include lacrimation, photophobia and blepharospasm. In addition to increased IOP, common ocular features include corneal cloudiness, Haab’s striae and enlarged corneal diameter.1 Although PCG is the most common glaucoma in infancy, its incidence and management outcomes are quite variable among different populations and different studies.2
PCG has an estimated incidence of 1 in 10,000 to 18,000 live births. However, and owing to its autosomal-recessive transmission pattern, the disease has been reported to occur up to 10 times more frequently in certain ethnic and religious groups where consanguineous relationships, especially cousin–cousin inbreeding, is common.3 Prevalence of PCG in the region of the Middle East has been considered the second highest in the world with an estimated incidence of 1;2500 live births.4 Although Egypt is the most populous country in the region of the Middle East, with its population mainly condensed in the region of the Nile Delta, nation-wide studies of the disease are lacking at present.5 The aim of the current study is to highlight disease characteristics and outcomes of surgical management of PCG patients under one year in a population from the region of the Nile Delta.
Patients and Methods
A retrospective study was performed for all consecutive pediatric patients diagnosed with PCG at pediatric ophthalmology unit, Benha University Hospital, which is a referral tertiary care hospital to the population of Qalyubiyya Governorate, the Nile Delta region, Egypt from the unit’s inauguration year (2015) through 2019. Patients who resided outside the Nile Delta region at the time of diagnosis were excluded together with those who had less than 6 months follow up. Data collection was approved by the regional institutional ethics review board of Benha University Hospital. The requirement to obtain patient and parental consent to review participating children’s medical records was waived by the board due to the retrospective nature of the study. However, patient data remained confidential in compliance with HIPAA regulations and the Declaration of Helsinki. Medical records were checked for the following data, age at surgery, gender, laterality, family history of congenital glaucoma and presenting symptoms. All patients were diagnosed with PCG based on standard clinical assessment and criteria with absence of other ocular or systemic conditions.3 Patients were included if they presented with corneal enlargement and haze with optic nerve cupping and elevated IOP in the absence of any associated anomalies either ocular or systemic known to be associated with or to cause glaucoma. At presentation and at each postoperative visit, IOP was measured using a Perkins handheld applanation tonometer (Haag-Streit, Koniz, Switzerland) following orally administered chloral hydrate sedation (50 mg/kg), or using general inhalational anesthesia (1% halothane) immediately following induction of anesthesia and before intubation. In late follow up visits of older and cooperative children, IOP was measured in the outpatient clinic using Goldmann applanation tonometry. Anterior segment evaluation was performed by hand-held slit lamp, horizontal corneal diameter (HCD) was measured with calipers and cup-to-disc ratio (CDR) was assessed using indirect ophthalmoscopy or slit lamp biomicroscopy for older and cooperative children.
When the diagnosis was confirmed, surgical interventions were scheduled as early as possible, usually within two days from diagnosis. All patients were fully examined 1 and 3 days after surgery, and weekly till the end of the first month, then every two weeks till the end of the third month, then every 3 months thereafter till the end of follow up. Operative notes included type of surgery, number of interventions, intraoperative and postoperative complications. Success was defined as IOP less than 22 mmHg (excluding hypotony) without IOP lowering medications with no progression of signs of the disease such as HCD and CDR.6 Cases with IOP less than 22 mmHg aided with antiglaucoma medications and without progression of CHD and CDR were termed qualified success.7 Statistical analysis was performed using SPSS (version 13, SPSS Inc., Chicago, IL). All results are presented as mean values ± standard error of mean.
Results
Chart review revealed a total of 49 patients with PCG aged less than one year presented to pediatric unit from its inauguration in 2015 and onward. On further analysis, it was found that 10 patients were residents outside the region of the Nile Delta at time of presentation and therefore were excluded. In addition, postoperative follow up was less than 6 months in 8 patients and therefore were excluded together with another 5 patients whose medical files were found to have been missing relevant data valuable for interpretation. As a result, this leaves 26 patients whose files met the inclusion criteria and underwent final data analysis. Detailed information of all patients before surgery and at the last postoperative visit is illustrated in a Supplementary Table.
Patient cohort included 44 eyes of 26 PCG patients (12 males and 14 females) who underwent surgical treatment from 2015 to 2019. Positive family history was found in 9 patients (34.6%) while history of parental consanguinity was present in 12 cases (46.1%). Out of the 26 patients, there was 18 patients with bilateral disease and 8 patients with unilateral disease. In unilateral cases, the right eye was affected in 5 patients and the left eye in 3 patients. Average age of patients at time of surgery was 5.2±2.9 months (range 1 to 11) and mean follow up was 18.5±11.8 months (range 6 to 48). Corneal haze or cloudiness was the most common presentation (20 patients, 76.9%) while corneal scarring in the form of Haab’s stria was the least common presentation (4 patients, 15.3%) (Table 1). Preoperative IOP was 28.5±4 mmHg (range 17 to 37). Because of the referral status of our hospital, 6 patients (23%) were prescribed topical anti-glaucoma medications at presentation (Table 1). Mean value of HCD before operation was 13.7±0.7mm (range 12 to 15.5mm) and in cases with visible posterior segment (26 eyes), mean value of CDR was 0.65±0.18 (range 0.2 to 0.9).
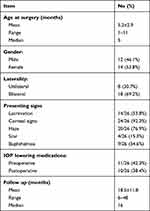 |
Table 1 Patients’ Demographics and Clinical Characteristics |
All procedures were performed by the same author (MFF). Average follow up was 18.5±11.8 months (range 6–48 months). Single glaucoma surgery was performed in 29 eyes (65.9%), two surgeries were performed in 11 eyes (25%), and 4 eyes underwent more than 2 surgeries (9%). Regarding the first surgical procedure, 11 eyes (25%) underwent trabeculotomy, 8 eyes (18%) underwent combined trabeculotomy-trabeculectomy with mitomycin-C, 25 eyes (56.8%) underwent trabeculectomy with mitomycin-C. Secondary procedures were carried out in eyes because of persistently elevated IOP after the primary surgery and they include trabeculectomy with mitomycin-C (10 eyes), Ahmed glaucoma valve (5 eyes), trabeculotomy (one eye), combined trabeculotomy/trabeculectomy with mitomycin-C (3 eyes) and diode laser cyclophotocoagulation (6 eyes). In total, 69 procedures were performed to the 44 eyes with an average of 1.56 procedures per eye. Postoperatively, IOP, HCD and CDR were significantly improved to 12.4±4.1 mmHg (range 0 to 24), 12.9±0.9mm (range10.5 to 15.5mm), 0.3±0.2 (range 0.7 to 0) (p<0.00001) (Table 2), (Figure 1). Postoperatively, success (IOP less than 22 mmHg without medications and excluding hypotony) was achieved in 32 eyes (72.7%) while qualified success (IOP less than 22 mmHg but with medications) was achieved in 8 eyes (18.1%) which makes the total success (true success plus qualified success) achieved in 40 eyes (90.9%). At the last postoperative visit, anti-glaucoma medications were used to control IOP in 10 eyes. Data of cycloplegic refraction after surgery was available for 14 eyes only with average spherical equivalent - 4.7 diopter.
 |
Table 2 Main Disease Parameters Before and After Surgery and Degree of Improvement |
Among all study variables, variables which were found to correlate with success were age at time of surgery and preoperative IOP. Binary logistic regression analysis for predictors of success was performed. Age at time of surgery was positively correlated with success; the older the age, the better the outcomes (p=0.022), whereas preoperative IOP was negatively correlated; the lower the preoperative IOP, the better the outcomes (p=0.032) (Table 3). The Kaplan–Meier survival analysis curve for the whole study is provided in Figure 2.
 |
Table 3 Binary Logistic Regression Analysis for Predictors of Success |
 |
Figure 2 Kaplan–Meier survival analysis curve for the whole study eyes. |
Sight-threatening postoperative complications were recorded in 3 eyes (6.8%); valve extrusion (one eye), consensual orbital cellulitis and endophthalmitis following Ahmed glaucoma valve implantation (one eye),8 and severe persistent hypotony secondary to cyclitic membrane formation (one eye). Other non-visual threatening complications included intraoperative localized iridodialysis (one eye), pupillary irregularities (7 eyes), cataract (7 eyes), posterior synechia (2 eyes), transient retinal hemorrhages (one eye), shallow anterior chamber (one eye), and tube endothelial touch (one eye). Management of sight-threatening complications were performed accordingly by the same author (MFF). The case of consensual orbital cellulitis and endophthalmitis was managed by valve removal, injection of periocular and intravitreal fortified antibiotics with final favorable visual and anatomical outcomes.8 The case of persistent hypotony secondary to cyclitic membrane was initially managed by repeated reformation of anterior chamber with periocular steroid injection. Unfortunately, surgical debulking of the cyclitic membrane was eventually needed with poor functional and anatomical outcomes (Figure 3).
Discussion
In this retrospective study, we present the results of 44 consecutive eyes surgically treated for PCG in a tertiary referral ophthalmic facility which receives patients from the region of the Nile Delta. In our cohort, patients were surgically treated at an average age of 5.2 months. Bilateral cases surpass the unilateral ones (18 vs 8), a figure which correlates with the predominance of bilateral disease in PCG in previous reports.2,9,10 All eyes that underwent surgical procedures were observed for up to an average of 18.5 months after the operation. Following surgery, there was a significant reduction in IOP, HCD and CDR measurements with relatively low incidence of visual threatening complications (three eyes, 6.8%), and this is likely to be related to the early presentation and early surgical intervention.
Management of PCG has always been surgical with some adjunctive role to the medical treatments. The main goal of surgery for PCG is to maintain good visual function and to avoid structural ocular changes by reducing IOP. In our patients’ cohort, the average IOP was significantly reduced from 28.5±4 mmHg to 12.4±4.1 mmHg. Success was achieved in 32 eyes (72.7%) while qualified success was achieved in 40 eyes (90.9%), a figure which matches surgical success rate in the few published reports regarding management of PCG in Egypt.5,11 In addition, we have found that success in our cases is positively correlated with age (the younger the age, the better the outcomes) and negatively correlated with the level of IOP at presentation (the lower the IOP, the better the outcomes) (Table 3). Other reports on surgical treatment of PCG from other parts of the world present success rates in reducing IOP <22 mmHg ranging from 43% to 90%.12–14 Early detection, referral and management of patients could be responsible for our quite good success rate. In addition, access to IOP lowering medications both before and after surgery could also be a contributing factor.
Out of 44 eyes which underwent primary surgery, severe complications were reported in three eyes while 30 eyes were free from any complications. Out of those eyes, 24 eyes underwent single glaucoma surgery. This figure emphasises the correlation between multiple glaucoma surgeries and the rate of postoperative complications. Generally, 34% of eyes in the current report required more than one surgical procedure to control the disease, and this figure is in accordance with published reports regarding management of PCG in Egypt.5 Regarding management, all sight-threatening complications were managed accordingly by the same author (MFF). Management of the case of consensual orbital cellulitis and endophthalmitis following Ahmed glaucoma valve implantation was in accordance with management of similar cases in literature,15,16 and it was discussed in detail in a previous article.8 With regard to the case of persistent hypotony secondary to formation of dense inflammatory cyclitic membrane, associated UBM signs included severe corneal edema, lost anterior chamber, dense peripheral anterior synechia, iridocorneal and lenticulo-corneal touch, posterior synechia, dense cataract and ciliary body detachment (Figure 3A). B-scan ultrasonography showed shallow cilio-choroidal detachment, and choroidal thickening with attached retina (Figure 3B). Reformation of anterior chamber using sodium hyaluronate with Periocular injection of Dexamethasone and Atropine were performed twice without success. The patient finally underwent surgical Synechiolysis, cataract extraction, anterior vitrectomy and surgical debulking of the cyclitic membrane using vitrector (Figure 3C). Postoperative, corneal edema and depth of anterior chamber improved. Unfortunately, persistent inflammation of anterior segment continued with development of phthisis bulbi.
In addition to its retrospective nature, limitations of the current study include the relatively small number of patients and short term follow up. There has been almost uniform agreement in literature that the success of pediatric glaucoma operations deteriorates with time, as reflected by the increased need to perform further procedures with increasing age.17–19 Therefore, we believe that lower values of success rate in our patient cohort could be achieved if longer period of follow up were obtained. The current study also lacks surgical functional outcomes in the form of assessment of effect of surgical intervention on visual functions of the patients. However, young patients’ age with difficulty in obtaining reliable visual data could be an excuse for this shortcoming. In conclusion, this article is one of few to address characteristics and outcomes of management PCG in patients under one year of age in Egypt and more specifically, its most populous part, the region of the Nile Delta where there is high prevalence of PCG due to high consanguineous marital relations. In general, the outcomes were promising with high success rate of conventional surgical interventions and low rates of visually significant complications. Larger studies with larger number of patients and longer follow up periods are needed for complete revelation of the disease characteristics in the area.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Papadopoulos M, Khaw PT. Childhood glaucoma. In: Taylor D, Hoyt CS, editors. Pediatric Ophthalmology and Strabismus.
2. Taylor RH, Ainsworth JR, Evans AR, Levin AV. The epidemiology of pediatric glaucoma: the Toronto experience. J AAPOS. 1999;3:308–315. doi:10.1016/S1091-8531(99)70028-5
3. Papadopoulos M, Cable N, Rahi J, Khaw PT; BIG Eye Study Investigators. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007;48:4100–4106. doi:10.1167/iovs.06-1350
4. Bejjani BA, Lewis RA, Tomey KF, et al. Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet. 1998;62:325–333. doi:10.1086/301725
5. Bayoumi NH. Surgical management of primary congenital glaucoma in Egypt. J Egypt Ophthalmol Soc. 2016;109(2):85. doi:10.4103/2090-0686.193398
6. Ben-Zion I, Tomkins O, Moore DB, Helveston EM. Surgical results in the management of advanced primary congenital glaucoma in a rural pediatric population. Ophthalmology. 2011;118(2):231–235. doi:10.1016/j.ophtha.2010.02.027
7. Denis D, Pommier S, Coste R, Fogliarini C, Benso C, Cornand E. Deep sclerectomy in congenital glaucoma: results of a study lasting more than 3 years. J Fr Ophtalmol. 2008;2:173–179. doi:10.1016/S0181-5512(08)70350-4
8. Farid MF, Awad MA, Belal EA. Consensual orbital cellulitis and endophthalmitis complicating pediatric glaucoma drainage implant. Austin Ophthalmol. 2016;1(1):1004.
9. Alanazi FF, Song JC, Mousa A, et al. Primary and secondary congenital glaucoma: baseline features from a registry at King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia. Am J Ophthalmol. 2013;155:882–889. doi:10.1016/j.ajo.2012.12.006
10. Debnath SC, Teichmann KD, Salamah K. Trabeculectomy versus trabeculotomy in congenital glaucoma. Br J Ophthalmol. 1989;73:608–611. doi:10.1136/bjo.73.8.608
11. Bayoumi NH. Deep sclerectomy in pediatric glaucoma filtering surgery. Eye. 2012;26(12):1548–1553. doi:10.1038/eye.2012.215
12. Tanimoto SA, Brandt JD. Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol. 2006;17:132–137. doi:10.1097/01.icu.0000193091.60185.27
13. Alsheikheh A, Klink J, Klink T, et al. Long-term results of surgery in childhood glaucoma. Graefes Arch Clin Exp Ophthalmol. 2007;245:195–203. doi:10.1007/s00417-006-0415-2
14. Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003;136:994–1000. doi:10.1016/S0002-9394(03)00714-1
15. Goldfarb J, Jivraj I, Yan D, DeAngelis D. A case of pseudomonas orbital cellulitis following glaucoma device implantation. J Glaucoma. 2019;28(1):e14–6. doi:10.1097/IJG.0000000000001095
16. Zheng CX, Uhr JH, Deaner JD, et al. Orbital cellulitis following uncomplicated glaucoma drainage device surgery: case report and review of literature. J Ophthalmic Vis Res. 2020;15(3):412.
17. Morad Y, Donaldson CE, Kim YM, et al. The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol. 2003;135:821–829. doi:10.1016/S0002-9394(02)02274-2
18. Budenz DL, Gedde SJ, Brandt JD, et al. Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology. 2004;1 11:2204–2210. doi:10.1016/j.ophtha.2004.05.017
19. Rolim de Moura C, Fraser-Bell S, Stout A, et al. Experience with the Baerveldt glaucoma implant in the management of pediatric glaucoma. Am J Ophthalmol. 2005;139:847–854. doi:10.1016/j.ajo.2004.12.028
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


