Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Surgery and Guillain-Barré Syndrome: A Single-Center Retrospective Study Focused on Clinical and Electrophysiological Subtypes
Authors Bao L , Chen X, Li Q, Zhang R, Shi H, Cui G
Received 4 December 2019
Accepted for publication 22 March 2020
Published 15 April 2020 Volume 2020:16 Pages 969—974
DOI https://doi.org/10.2147/NDT.S241128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Lei Bao,* Xueting Chen,* Qingjie Li,* Ruixue Zhang, Hongjuan Shi, Guiyun Cui
Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Bao
Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221004, People’s Republic of China
Tel/Fax +86 516 85802129
Email [email protected]
Background: Surgery-related Guillain-Barré syndrome (GBS) is often underestimated and sometimes difficult to diagnose. This study aimed to elucidate the clinical features and electrophysiological subtypes of post-surgical GBS.
Methods: We retrospectively reviewed 17 patients who developed post-surgical GBS after a recent surgery between 2015 and 2019. Clinical characteristics, electrophysiological examinations, lumbar puncture results and prognosis were assessed. As controls, we selected 66 patients hospitalized with non-surgical GBS.
Results: The median duration from the surgery to the onset of GBS symptoms was 16.0 days. The main types of surgeries preceding GBS were orthopedic, gastrointestinal and neurosurgery. Symmetrical distal limbs weakness was present in all 17 post-surgical GBS patients. The incidence of respiratory failure, autonomic dysfunction and muscle atrophy in post-surgical GBS patients was significantly higher than that in non-surgical GBS patients. Hughes Functional Grading Scale (HFGS) scores were also higher in the post-surgical GBS group both at the time of peak disease and 6 months after discharge. Electrophysiological studies revealed significant motor amplitudes reduction with relative preserved nerve conduction velocities and distal latencies, suggesting axonal subtypes of GBS.
Conclusion: GBS should be considered in patients with rapidly progressive muscle weakness after surgery. Such patients often exhibit axonal subtypes of GBS with severe motor dysfunction, high risk of respiratory failure, and poor prognosis.
Keywords: Guillain-Barre syndrome, post-surgical GBS, surgery, electrophysiology, axonal neuropathy
Introduction
Guillain-Barré syndrome (GBS) is an acute immune-mediated polyradiculoneuropathy, characterized by flaccid paralysis, acute demyelinating changes in the peripheral nervous system and albumino-cytological dissociation in the cerebrospinal fluid (CSF).1 About two-thirds of patients have antecedent infections 6 weeks before the onset of GBS.2 However, GBS has also been reported to be triggered by non-infectious factors such as trauma, vaccination, autoimmune diseases, immunosuppression and administration of ganglioside.3,4
Two publications from Mayo Clinic and Massachusetts General Hospital firstly reported surgical procedures as a trigger for GBS.5,6 Since then, there have been a large number of published case reports on GBS triggered by surgery. Retrospective series noted that between 5% and 19% of patients with GBS had undergone a surgical procedure during the 6 or 8 weeks preceding the onset of symptoms.7,8 In practice, however, surgery-related GBS is often underestimated and sometimes difficult to diagnose. The importance of diagnosing post-surgical GBS is appreciated because it can rapidly progress and become life-threatening by affecting the respiratory musculature. Thus, prompt and accurate diagnosis is essential for a better prognosis.
Up to present, studies describing the role of surgery in GBS have focused on clinical factors and potential triggers. Few studies have reported the clinical and electrophysiological subtypes of post-surgical GBS. In this study, the clinical characteristics of post-surgical GBS were described, with emphasis placed on the electrophysiological findings. Through our present research, we aimed to aid clinical physicians in recognizing and diagnosing this relatively rare cause of GBS.
Methods
Ethical Statements
The Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University approved this study protocol. The protocols were in accordance with the Declaration of Helsinki. Written informed consents were acquired from all participants or their legal guardians.
Participants
This study used a case–control design and has been approved by the institutional review board for conducting research. Seventeen GBS patients with a recent history of surgery were included between January 2015 and October 2019 at the department of Neurology of the Affiliated Hospital of Xuzhou Medical University, Jiangsu, China. The inclusion criteria for the post-surgical GBS patients were (1) first occurrence of GBS for a given patient; (2) GBS symptom onset within 6 weeks of surgery; and (3) received follow-up. Exclusion criteria included a history of antecedent infections and prior use of intravenous gangliosides (single sialic acid ganglioside, cerebroside peptide). For comparison, the control group consisted of 66 patients with other causes of GBS and 30 healthy volunteers in the same study period. All patients in this study met the diagnostic criteria for GBS.9 Briefly, the criteria include the presence of progressive weakness and areflexia, relative symmetry, mild sensory involvement, cranial nerve involvement, autonomic dysfunction. These findings were supported by electrodiagnostic criteria and cerebrospinal fluid (CSF) results (albumin cytological dissociation). Clinical data for each patient were collected, including basic information, surgeries, previous infections, days to onset of symptoms, electrophysiology, cerebrospinal fluid (CSF) analysis, treatment, and prognosis.
Evaluation of Clinical Severity and Prognosis
The Hughes Functional Grading Scale (HFGS) is widely used to evaluate the disability for GBS, ranging from 0 to 6, with higher scores indicating more severe disability.10 HFGS scores at the time of peak disease and 6 months after discharge of all patients were evaluated and collected.
Electrophysiological Study
Nerve conduction studies (NCS) were performed on all patients around 10–14 days after onset of clinical symptoms, using the standard technique. Briefly, limb temperature was maintained at > 32°C during the procedures. During the motor NCS, we stimulated the median, ulnar, peroneal, and tibial nerves and recorded the compound motor action potentials (CMAP) at the abductor pollicis brevis, abductor digiti minimi, extensor digitorum brevis, and abductor hallucis. Data from the forearm and lower leg segment were chosen for the analysis of the ulnar and peroneal nerves. During the sensory NCS, we stimulated the median, ulnar, and sural nerves and recorded the sensory nerve action potential (SNAP) from the index finger, little finger, and the lateral malleolus. CMAP amplitude, distal motor latency, motor nerve conductive velocities (MCVs), conduction block, sensory nerve conductive velocities (SCVs), and sensory nerve amplitude were measured. Diagnosis of axonal or demyelinating neuropathy was based on the electrophysiological criteria proposed by Hadden and colleagues.11
Statistical Analysis
Data are presented as number (%), median (range), or mean ± standard deviation and they were compared between the data of post-surgical and non-surgical patients. For quantitative data, comparison between two groups was undertaken using the Student’s t-test. The differences in the categorical variables were compared using the Fisher exact test. All analyses were performed using GraphPad Prism software (La Jolla, CA), statistical significance was set at p < 0.05.
Results
Demographic Characteristics and Details of Surgeries
In 17 patients developed post-surgical GBS, there were 11 males and 6 females; The gender ratio between male and female patients was 1.83 in our study, which showed a male preponderance. The mean age of patients with post-surgical GBS was 56.2 years. The median duration between surgery and onset of GBS symptoms was 16 days (range 4–36 days). The main types of surgeries preceding GBS were orthopedic, gastrointestinal and neurosurgery. Details of the surgeries are included in Table 1.
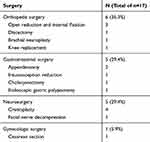 |
Table 1 Details of the Surgeries |
Clinical Characteristics and Neuropathy Disability Score
Table 2 contains the details of clinical presentation, CSF findings and total neuropathy scores from 17 post-surgical GBS patients and 66 non-surgical GBS patients. Symmetrical weakness in the lower and upper extremities with prominent distal limbs involvement was present in all 17 post-surgical GBS patients and 11 of them presented muscle atrophy during hospitalization. The frequency of respiratory weakness, autonomic dysfunction and muscle atrophy was significantly higher than that in non-surgical GBS patients. 8 (47.1%) patients with post-surgical GBS were transferred to intensive care unit (ICU) for acute respiratory failure and required mechanical ventilatory support. The incidence of acute respiratory failure and mechanical ventilatory support in post-surgical GBS patients was also significantly higher than non-surgical GBS patients (P < 0.01). Three patients with post-surgical GBS died during hospitalization and 2 patients died after discharge. Death resulted from ventilator-associated pneumonia, pulmonary embolism and one patient died of cardiac severe bradycardia and cardiac arrest attributable to autonomic dysfunction.
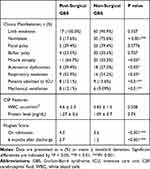 |
Table 2 Clinical Characteristics and Neuropathy Disability Score |
All patients in this study underwent lumbar puncture with albumino-cytological dissociation. The mean CSF protein content was 1.27 g/L in post-surgical GBS patients. There was no difference in CSF protein level between the post-surgical GBS patients and non-surgical GBS patients (P > 0.05).
HFGS scores at the time of peak disease and 6 months after discharge in the post-surgical GBS group were 4.0 and 2.7, respectively. These scores in the non-surgical GBS group were 3.6 and 1.2, respectively. HFGS scores were higher in the post-surgical GBS group than in the non-surgical GBS group (P < 0.001).
Electrophysiological Features
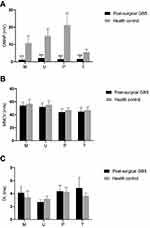
Mean values of motor nerve conduction parameters in post-surgical GBS group and health control group are given in Figure 1. The mean CMAP amplitude in median, ulnar, and sural nerve was significantly reduced when compared with health control group (P < 0.001). We also observed a slight prolonged distal latency and motor nerve conductive velocities slowing in all extremity nerves, although there was no significant difference between the health control and post-surgical GBS groups. Figure 2 shows the sensory nerve conduction studies results. SNAP amplitude in median, ulnar, and sural nerve showed a slightly decreasing trend but with no significance, when compared with health control group (P > 0.05). Sensory nerve conduction velocities were relatively preserved in all extremity nerves and these were not significantly different from the healthy control group.
Table 3 depicts the electrophysiological subtypes of post-surgical GBS and non-surgical GBS patients on the basis of the electrodiagnostic criteria reported by Hadden and colleagues. Fifteen of the 17 post-surgical GBS were finally classified with AMAN and 2 with AMSAN. None of these patients were classified with AIDP. All 17 post-surgical GBS patients exhibited an axonal subtype GBS. In contrast, among 66 non-surgical patients, 38 (57.6%) had an axonal subtype (including 30 AMAN and 8 AMSAN). Twenty-four (36.4%) had a demyelinating subtype (AIDP); The other 4 patients (6.0%) only exhibited mildly abnormal findings and were not classified. The proportion of axonal subtype GBS in post-surgical patients was higher than that in non-surgical patients (***P < 0.001).
 |
Table 3 Electrophysiological Subtypes of Post-Surgical GBS and Non-Surgical GBS Patients |
Anti-Ganglioside Antibodies
In this study, anti-ganglioside antibodies were detected in only 3 of 17 patients with post-surgical GBS. Two of them showed seropositive for GD1a antibody and one patient with severe muscle weakness and paresthesia showed sulfatide antibody positive.
Discussion
In the present study, all 17 patients had received a surgery before the onset of GBS. The main types of surgeries preceding GBS were orthopedic, gastrointestinal and neurosurgery. Our results were consistent with recent French nationwide data, which showed GBS was moderately associated with any type of recent surgery but was more strongly associated with bone and digestive organ surgery.12 Another large consecutive series study showed post-surgical GBS was more common in patients with pre-existing autoimmune disorders or active malignancies.13 However, neither autoimmune diseases nor malignancies were found in this study. Prospective studies are still needed to determine whether autoimmune disorder and active malignancy can increase the risk of post-surgical GBS.
Generally, GBS is a typical post-infectious disorder and two-thirds of adult patients report preceding symptoms of a respiratory or gastrointestinal tract infection within 4 weeks of onset of weakness.1 GBS is believed to be mediated by antibodies stimulated by pathogen antigens that can crosstalk with molecules like gangliosides in the peripheral nervous tissue and, therefore, the immune responses cross-react with the nerves causing axonal degeneration.14 However, the underlying pathophysiological mechanisms of GBS triggered by a surgery are not well understood so far. It has been hypothesized that clinical and subclinical infections secondary to the transient immunosuppressed state after surgery, rather than the surgery itself, induce the risk of GBS.15,16 This hypothesis is promoted by the fact that patients with immunosuppression caused by HIV infection or organ transplantation are associated with more frequent development of GBS.17,18 Another hypothesis contributing to the development of GBS after surgery in the literature is that surgery (especially cervical, lumbar, limbs, and brain surgeries) can cause varying degrees of impairment of blood-nerve barrier. Destruction of this innate protective barrier enables the entry of antigens intraoperatively from the blood into the nervous system, where they can induce subsequent autoimmunization responses.19 This may explain why orthopedic surgery and neurosurgery were more prone to develop GBS in our present study.
About 20–30% of GBS patients have respiratory failure requiring invasive mechanical ventilation.20 In this study, nearly half of the post-surgical GBS patients were intubated and mechanically ventilated. The incidence of acute respiratory failure and mechanical ventilatory support in post-surgical GBS patients was significantly higher than non-surgical GBS patients. Thus, frequent evaluations of pulmonary function parameters should be performed on post-surgical GBS patients to monitor respiratory status and the need for ventilatory assistance.
Guillain-Barré syndrome is a potentially life-threatening disease with frequent morbidities, even with the best treatment available. Mortality in GBS varies between 3% and 7% and it doubles in patients who have been mechanically ventilated for months. It may even approach 10% to 20% in patients with severe comorbidities.20 In the post-surgical GBS group, 3 patients died during hospitalization and 2 patients died after discharge. Death resulted from ventilator-associated pneumonia, pulmonary embolism and one patient died of cardiac severe bradycardia and cardiac arrest attributable to autonomic dysfunction. These results indicated marked increases in disease severity and poor prognosis of post-surgical GBS. Therefore, when unexplained progressive limb weakness occurs after surgery, Guillain-Barré syndrome should be highly suspected and quick and effective measures should be taken to reduce morbidity and improve prognosis.
All 17 post-surgical GBS patients exhibited severe motor dysfunction, 11 patients had severe muscle atrophy, while few of them had the symptom of paresthesia. The clinical manifestations matched the acute motor axonal neuropathy (AMAN). AMAN, also known as Chinese paralytic syndrome, is characterized by the rapid progressive ascending tetraparesis, frequent respiratory system involvement and ventilator dependence, sensory nerves are rarely or only slightly involved. It often progresses more rapidly than AIDP.21 Electrophysiological study is helpful for assigning the subtypes of GBS. Based on the Hadden criteria,11 15 of the 17 post-surgical GBS patients were diagnosed with AMAN and the other 2 with AMSAN. Electrophysiological findings showed an axonal rather than demyelinating form of neuropathy in all post-surgical GBS patients. We systematically searched for previous case reports of GBS in the context of surgery and found that the axonal subtype of GBS is more common than the demyelinating subtype.22–25 Based on current researches, the electrodiagnosis of post-surgical GBS is supportive of acute axonal neuropathy (AMAN and AMSAN).
Conclusion
In conclusion, post-surgical GBS should be considered in patients with rapidly progressive muscle weakness after surgery. Corresponding measures should be immediately taken, as in such GBS cases, patients often have severely motor dysfunction, a high risk of respiratory failure and a poor prognosis. Electrophysiological study reveals that damage occurs predominantly in axons, suggesting AMAN or AMSAN subtype of GBS.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) NO. 81771282 and The Natural Science Foundation of the Jiangsu Province (Grant No: BK20180991).
Disclosure
The authors declare no competing financial interests in this work.
References
1. Kaida K. Guillain-Barre syndrome. Adv Exp Med Biol. 2019;1190:323–331.
2. Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med. 2012;366(24):2294–2304. doi:10.1056/NEJMra1114525
3. Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain-Barre syndrome. Expert Rev Clin Immunol. 2013;9(7):627–639. doi:10.1586/1744666X.2013.811119
4. Yang B, Lian Y, Liu Y, Wu BY, Duan RS. A retrospective analysis of possible triggers of Guillain-Barre syndrome. J Neuroimmunol. 2016;293:17–21. doi:10.1016/j.jneuroim.2016.02.003
5. Arnason BG, Asbury AK. Idiopathic polyneuritis after surgery. Arch Neurol. 1968;18(5):500–507. doi:10.1001/archneur.1968.00470350058005
6. Wiederholt WC, Mulder DW, Lambert EH. The Landry-Guillain-Barr’e-Strohl syndrome or polyradiculoneuropathy: historical review, report on 97 patients, and present concepts. Mayo Clin Proc. 1964;39:427–451.
7. Gensicke H, Datta AN, Dill P, Schindler C, Fischer D. Increased incidence of Guillain-Barre syndrome after surgery. Eur J Neurol. 2012;19(9):1239–1244. doi:10.1111/j.1468-1331.2012.03730.x
8. Sipila JO, Soilu-Hanninen M. The incidence and triggers of adult-onset Guillain-Barre syndrome in southwestern Finland 2004–2013. Eur J Neurol. 2015;22(2):292–298. doi:10.1111/ene.12565
9. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barr- syndrome. Ann Neurol. 1990;27(S1):S21–S24. doi:10.1002/ana.410270707
10. Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2(8093):750–753.
11. Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. plasma exchange/sandoglobulin Guillain-Barre syndrome Trial Group. Ann Neurol. 1998;44(5):780–788. doi:10.1002/ana.410440512
12. Rudant J, Dupont A, Mikaeloff Y, Bolgert F, Coste J, Weill A. Surgery and risk of Guillain-Barre syndrome: a French nationwide epidemiologic study. Neurology. 2018;91(13):e1220–e1227. doi:10.1212/WNL.0000000000006246
13. Hocker S, Nagarajan E, Rubin M, Wijdicks EFM. Clinical factors associated with Guillain-Barre syndrome following surgery. Neurol Clin Pract. 2018;8(3):201–206. doi:10.1212/CPJ.0000000000000451
14. Nobile-Orazio E. The complement story in Guillain-Barre syndrome: from pathogenesis to therapy. Lancet Neurol. 2018;17(6):483–485. doi:10.1016/S1474-4422(18)30144-3
15. Rashid A, Kurra S, Lavelle W. Guillain-Barre syndrome after revision lumbar surgery: a case report. Cureus. 2017;9(6):e1393.
16. Wakerley BR, Yuki N. Surgery itself does not trigger Guillain-Barre syndrome. Eur J Neurol. 2013;20(3):e40. doi:10.1111/ene.12065
17. Cools P, van de Wijgert J, Jespers V, et al. Role of HIV exposure and infection in relation to neonatal GBS disease and rectovaginal GBS carriage: a systematic review and meta-analysis. Sci Rep. 2017;7(1):13820. doi:10.1038/s41598-017-13218-1
18. Zhang L, Arrington S, Keung YK. Guillain-Barre syndrome after transplantation. Leuk Lymphoma. 2008;49(2):291–297. doi:10.1080/10428190701760003
19. Zhong YX, Lu GF, Chen XL, Cao F. Postoperative Guillain-Barre syndrome, a neurologic complication that must not be overlooked: a literature review. World Neurosurg. 2019;128:347–353. doi:10.1016/j.wneu.2019.04.239
20. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388(10045):717–727. doi:10.1016/S0140-6736(16)00339-1
21. Dimachkie MM, Barohn RJ. Guillain-Barre syndrome and variants. Neurol Clin. 2013;31(2):491–510. doi:10.1016/j.ncl.2013.01.005
22. Xu S, Wang YS, Li S, Liu HY. Guillain-Barre syndrome complicated on post-operation with renal carcinoma and meningioma: a case report. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51(4):775–777. doi:10.19723/j.issn.1671-167X.2019.04.032
23. Nadia B, Nouha F, Salma S, Mariem D, Salah BM, Chokri M. Acute motor axonal neuropathy form of the Guillain Barre syndrome two months after bariatric surgery. Presse Med. 2019;48(6):725–727. doi:10.1016/j.lpm.2019.05.008
24. Han J, Xie Y, Yan H, Cao Y, HongmeiWang CD. A case of surgically-associated anti GQ1b antibody syndrome accompanied by saccadic ping pong gaze. BMC Neurol. 2019;19(1):28. doi:10.1186/s12883-019-1258-x
25. Sunbol AH, Almaghrabi S, Al Aslany SJ, et al. Delayed Guillain-Barre syndrome after bariatric surgery: a report of three cases. Case Rep Surg. 2018;2018:8413206.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.