Back to Journals » International Journal of Nanomedicine » Volume 14
Surface Treatment Of Nanozirconia Fillers To Strengthen Dental Bisphenol A–Glycidyl Methacrylate–Based Resin Composites
Authors Dai S , Chen Y, Yang J , He F, Chen C , Xie H
Received 17 July 2019
Accepted for publication 31 October 2019
Published 26 November 2019 Volume 2019:14 Pages 9185—9197
DOI https://doi.org/10.2147/IJN.S223392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Shiqi Dai,1 Ying Chen,1 Jiaxue Yang,1 Feng He,1 Chen Chen,2 Haifeng Xie1
1Department of Prosthodontics, Jiangsu Key Laboratory of Oral Diseases, Affiliated Hospital of Stomatology, Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Endodontics, Jiangsu Key Laboratory of Oral Diseases, Affiliated Hospital of Stomatology, Nanjing Medical University, Nanjing, People’s Republic of China
Correspondence: Haifeng Xie; Chen Chen
Han-Zhong Road 136th, Stomatological Hospital of Jiangsu Province, Nanjing 210029, People’s Republic of China
Tel +86 25 8503 1831
; +86 25 8503 1822
Fax +86 25 8651 6414
Email [email protected]; [email protected]
Purpose: To investigate the effects of nanozirconia fillers conditioned with 10-methacryloyloxydecyl dihydrogen phosphate (MDP) with or without zirconium hydroxide precoating on bending strength, Vickers hardness, and translucence of dental resin composites.
Methods: We obtained nanozirconia fillers coated with different concentrations of Zr(OH)4 using wet-chemical synthesis. We analyzed coating quality by observing electron-diffraction patterns using transmission electron microscopy. We conditioned zirconia fillers, with or without prior Zr(OH)4-coating, using MDP-containing primers and evaluated the formation of chemical bonds using nuclear magnetic resonance (NMR) and X-ray photoelectron spectroscopy (XPS). We then performed three-point bending-strength tests, Weibull analysis, Vickers hardness, and translucence-parameter analysis with or without addition of different concentrations of zirconia using untreated zirconia fillers as controls.
Results: We achieved desirable Zr(OH)4 coating using 5 mmol/L zirconium chloride. NMR and XPS analysis detected stronger Zr–O–P peaks on MDP-conditioned zirconia fillers with prior Zr(OH)4-coating compared with MDP-conditioned fillers alone, suggesting that MDP bonding with zirconia was enhanced by zirconium hydroxide. Our three-point bending-strength tests revealed that increasing levels of untreated zirconia fillers decreased the three-point bending strength of the resin composites, while MDP-conditioned zirconia fillers with or without prior Zr(OH)4 coating improved three-point bending strengths. Adding 5 wt% and 7.5 wt% MDP-conditioned zirconia fillers with prior Zr(OH)4 coating achieved the highest three-point bending strength. Furthermore, addition of zirconia fillers decreased the translucence of silica-based resin composites.
Conclusion: MDP conditioning with prior Zr(OH)4 coating is recommended for treating nanozirconia fillers of resin composites.
Keywords: resin composite, zirconia, surface treatment, phosphate ester monomer, MDP, filler
Introduction
Dental filling materials require good mechanical properties to withstand long-term and complex occlusal loading. Resin composites have been widely used in dentistry, owing to their enhanced mechanical properties, aesthetic features, and biosafety. However, resin composites have a failure rate of 10%,1,2 with one of the most important reasons for failure being fracture, especially in posterior teeth.1,2
To combat resin-composite fractures, various methods have been applied to improve the mechanical properties of dental resin composites, including changing the particle size,3–5 shape,5,6 and content of the inorganic fillers.7 Silica–resin composites are the most commonly used among commercial dental resin composites.8 Other fillers can be added to silica–resin composites to further improve their mechanical properties, such as zirconium oxide,9 titanium dioxide,10 and zinc oxide.11 Most notably, however, zirconia has been added to silica-based filler systems since the 1990s,12 owing to its high strength, fracture toughness, hardness, and chemical stability.13 Commercial products, such as the Filtek Z250 and Harvard ZirkonCore, are typical products composed of zirconia fillers.
The contents of zirconia fillers can determine the mechanical properties of resin composites.14 In a recent study, optimum tensile/bending strength, elastic/bending modulus, strain, and toughness were obtained using glass fiber–epoxy composites with 1 wt% silanized zirconia particles,15 and decreased when zirconia concentrations were increased to 2 wt%.15 Similarly, increased flexural strength and fracture toughness of glass-based resin composites have been reported with 2.5–5.0 wt% silanized zirconia–silica/zirconia–yttria–silica nanofibers, which also decreased when zirconia–silica concentrations were increased to 7.5%.9 This decrease may have been due to excessive zirconia fillers failing to disperse uniformly in the matrix, which can affect bonding of the resin matrix to the fillers.9 Moreover, agglomerated zirconia can create cracks and microcracks at the initiation-site interface between the matrix and the filler,16 impacting mechanical properties. Finally, the enhanced stiffness of bare zirconia can promote stress concentration at the resin matrix–zirconia interface, which also accelerates the propagation of microcracks.17
Surface treatment of zirconia can improve the interfacial adhesion strength between zirconia filler and resin matrix and reduce aggregation.15,18 The majority of zirconia-filled resin-composite products form a complex with silica fillers, which then undergo silanization to improve their mechanical properties (such as flexural strength and fracture toughness).9 However, as zirconia-filler surfaces lack polar bonds and cannot form chemical bonds with silane hydroxyl groups, efficient surface treatment is needed to improve zirconia bonding to the resin matrix.
Currently, 10-methacryloyloxydecyl-dihydrogen-phosphate (MDP) is the most widely used phosphate ester monomer and provides excellent bonding outcomes between zirconia restorations and methacrylate-based resin composites.19–21 However, MDP is rarely used in zirconia fillers containing resin composites. Our recent study evaluated MDP treatment of zirconia fillers on the mechanical properties of resin composites, and found MDP-conditioned zirconia treatment improved bending strength.22 Since concentrations of zirconia fillers can affect the final mechanical properties of resin composites,9,15 the effect of adding different concentrations of MDP-conditioned zirconia fillers with or without prior Zr(OH)4-coating on the mechanical properties of resin composites needs further investigation.
In this study, we used MDP surface treatment of zirconia conditioning with or without prior Zr(OH)4 coating and investigated the correlation between concentrations of MDP-conditioned zirconia fillers with or without precoating with Zr(OH)4 on bending strength, Vickers hardness, and translucence of dental resin composites. Our null hypotheses were that MDP-conditioned zirconia fillers with or without Zr(OH)4 coating would not enhance the mechanical properties of the composite resin and that concentrations of zirconia filler would have no effect on mechanical properties or translucence.
Methods
Zirconium Hydroxide Coating And Transmission Electron Microscopy Observations
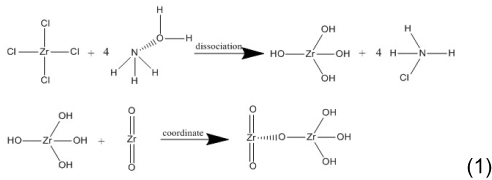
We obtained zirconia fillers with different concentrations of Zr(OH)4 coating using wet-chemical synthesis. We prepared ZrO2·ZrCl4 suspensions by mixing 0.5 g zirconia particles (average particle size 50 nm; McLin, China) and 0.5/2/5/10/100 mmol/L zirconium chloride aqueous solution (McLin). After thorough stirring with a magnetic stirrer, we adjusted the pH to 10–12 by adding NH3·H2O (McLin) and stirred this for 2 hours before leaving mixture to stand for 1 hour. We next removed the supernatant, washed the mixture with distilled water, and centrifuged and dried it to obtain Zr(OH)4-coated zirconia fillers.
The chemical reaction of Zr(OH)4-coated zirconia is shown in formula 1:
We observed zirconia fillers with or without different concentrations of Zr(OH)4 coating using transmission electron microscopy (TEM; 2100F; JEOL, Japan) at 200 kV and a dot resolution of 0.24 nm. Before observation, we ultrasonically dispersed the samples with absolute ethanol for 30 minutes. We then placed a few microliter droplets of suspension on a holey carbon film, which was left to dry at room temperature. We then observed the electron-diffraction pattern of the samples using TEM. We screened the process according to the TEM results. Based on our morphological analysis, we used Zr(OH)4-coated zirconia particles (obtained by adding 5 mmol/L of zirconium chloride) in our next experiments.
MDP Conditioning
We prepared MDP primers using MDP (DM Healthcare Products, USA):acetone:camphorquinone (Aladdin, China):4-dimethylamino-benzoic acid ethyl ester (Aladdin) at 10:88.8:0.3:0.9.23 We placed zirconia fillers with or without Zr(OH)4 coating in MDP solution at 1 g/1 mL for 12 hours. We washed the fillers with acetone to remove excess unreacted MDP, before centrifuging and drying them. We analyzed MDP-conditioned zirconia fillers with or without Zr(OH)4 coating using X-ray photoelectron spectroscopy (XPS; Escalab 250xi; Thermo Fisher Scientific, USA) with monochromatized A1Ka radiation (1,486.6 eV photo energy, energy step size 0.05 eV). We analyzed O1s spectra using XPSPeak 4.1 software to determine Zr–O–P content. We fixed the Lorentz–Gauss ratio at 80% and set untreated zirconia fillers as the control.
We mixed 1 g MDP-conditioned zirconia particles with or without Zr(OH)4 coating with a 10 mL solution of 10 wt% MDP in acetone for 40 minutes using ultrasonic stirring, before leaving the mixture to rest for 20 minutes, washing with acetone, and centrifuging to remove excess unbonded functional monomer. We examined the local structure around H and P in the two samples via solid-state magic-angle spinning (MAS) nuclear magnetic resonance (NMR) using NMR spectrometry (400 MHz, Avance III HD NMR; Bruker, Germany). The spinning frequency of the zirconia rotor was 5 kHz. We took 31P MAS-NMR spectra at 202.3 MHz with pulse lengths of 2.8 μs (pulse angle π/2) and 90-second recycle delays. We accumulated signals of 790 pulses using NH4H2PO4 as an external reference (1 ppm vs 0 ppm 85% H3PO4). We took 1H MAS-NMR spectra at 499.8 MHz with pulse lengths of 1.15 μs (pulse angle π/4) and 120-second recycle delays. We accumulated signals of four pulses using adamantane (C10H16) as an external reference (1.91 ppm vs 0 ppm TMS). We performed NMR analysis twice to ensure repeatability.
Preparation Of Resin Composites
To compose the resin matrix, we mixed bisphenol A–glycidyl dimethacrylate (Aladdin) and triethylene glycol dimethacrylate (Aladdin) at a ratio of 7:3. We added 0.5 wt% photosensitizer camphorquinone (Aladdin) and 1 wt% ethyl-4-dimethylaminobenzoate (Aladdin) and stirred the mixture in the dark. We next added uniformly ground silica and zirconia (ZrO2) to the mixtures and stored the resin composites in the dark. The formulations used are shown in Table 1.
 |
Table 1 Contents Of Resin Composites (wt%) |
Three-Point Bending-Strength Tests And Weibull Analysis
We prepared ten groups of resin-composite samples (n=15, 25×2×2 mm3) according to International Organization for Standardization (ISO) 4049–2009,24 which were light-cured for 40 seconds from each side. We controlled the size of the samples at ±0.02 mm by grinding and polishing. We stored samples in 37°C water for 24 hours before measuring using a universal testing machine (3365 ElectroPuls; Instron, USA) with 20 mm span and 0.5 mm/min crosshead speed. We recorded fracture load values F(N) and calculated three-point bending according to the formula 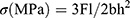 , where l is the distance between supports, b the width and h the thickness of the sample (all in mm), and F(N) is the maximum force sustained before failure.
, where l is the distance between supports, b the width and h the thickness of the sample (all in mm), and F(N) is the maximum force sustained before failure.
We arranged the three-point bending-strength values of each group in ascending order (using labels i=1, 2, 3, etc, N, n=15). The minimum value of each group was recorded as i = 1 and the maximum value i = N. We calculated a ranked probability of failure (Pf) according to the following formula: 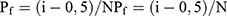 . We calculated the proportional parameter σθ and the Weibull modulus m (two-parameter Weibull distribution) according to the formula
. We calculated the proportional parameter σθ and the Weibull modulus m (two-parameter Weibull distribution) according to the formula 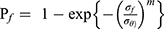 , where Pf is the probability of failure at or below the bending stress σf. Following calculations were linearly transformed from the aforementioned equation using the least-squares method:
, where Pf is the probability of failure at or below the bending stress σf. Following calculations were linearly transformed from the aforementioned equation using the least-squares method: 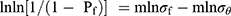 , where m is the slope and m/σθ the intercept.
, where m is the slope and m/σθ the intercept.
Vickers Hardness
We prepared ten groups of resin-composite samples (n=5, diameter 6 mm, thickness 2 mm), which were light-cured for 40 seconds from each side. We polished the samples to a size of 2±0.02 mm using a PG1 metallographic polishing machine (Shanghai Standard Precision Instrument, China) and 3,000-grit sandpaper. The samples were then stored in a 37°C water bath for 24 hours. We used a Vickers hardness tester (FM700, Future-Tech Corp, Japan) to measure microhardness values at room temperature (23°C) under a load of 1 kg and 10-second dwell time with a diamond pyramid microindenter. We observed indentation on the surface of the samples using optical microscopy under 40× magnification. We measured microhardness by measuring the lengths of the x-axis and y-axis following the formula 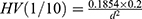 , where d (mm) is the diagonal of the indentation. To reduce variation, we repeated the measurements three times and obtained average microhardness values.
, where d (mm) is the diagonal of the indentation. To reduce variation, we repeated the measurements three times and obtained average microhardness values.
Translucence Parameter
We prepared ten groups of resin composite samples (n=5, diameter 6 mm, thickness 2 mm), which we light-cured for 40 seconds from each side. We polished the samples to a size of 2±0.02 mm using the PG1 metallographic polishing machine and 3,000-grit sandpaper, which we then stored in a 37°C water bath for 24 hours. We used a dental colorimeter (ShadeEye NCC; Shofu, Japan) to measure color parameters (L*, lightness; a*, redness–greenness; b*, yellowness–blueness) of each sample under black (B) and white (W) backgrounds three times. We used an average of three readings. The colorimeter probe was perpendicular to the surface of the sample and the dental colorimeter recalibrated after every five readings. We calculated the translucence parameter (TP): 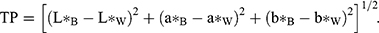
Statistical Analysis
We separately analyzed three-point bending strength, Vickers hardness, and TP values of the resin-composite samples using SPSS 25. After verifying normal distribution and homogeneity of the variance via Levene’s test, we used multivariate ANOVA and least-significant-difference to compare differences between groups. We considered P 0.05 statistically significant.
Results
TEM Observations
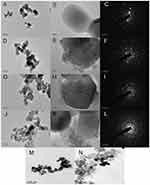
TEM images of untreated zirconia and zirconia coated with different concentrations of Zr(OH)4 are shown in Figure 1. Untreated zirconia particles had a smooth surface (Figure 1, A and B). Changes in zirconium chloride concentrations (0.5/2/5/10/100 mmol/L) created different forms of lower electrodensity coating on the zirconia particle surface or amorphous floccules around the zirconia particles (Figure 1, D, E, G, H, J, K, M and N). We observed minimal zirconium hydroxide formation when 0.5 mmol/L zirconium chloride (Figure 1M) was added and excessive floccule agglomeration when 100 mmol/L zirconium chloride (Figure 1N) was added. Contrastingly, we also observed limited amorphous structure at 2 mmol/L zirconium chloride (Figure 1, D and E), which failed to completely coat the zirconia surface. Figure 1H shows the amorphous structure was smoothly coated on the surface of zirconia at 5 mmol/L zirconium chloride. Figure 1, J and K shows the amorphous structure had not only formed a thick deposit on the zirconia surface but had also agglomerated, which then dispersed. Electron-diffraction patterns of untreated zirconia particles and zirconia particles coated with different concentrations of Zr(OH)4 are shown in Figure 1, C, F, I and L. According to our TEM observations and electron diffraction–pattern analysis, we recommend using 5 mmol/L zirconium chloride for wet-chemical synthesis.
XPS Analysis
XPS spectra of untreated zirconia and MDP-conditioned zirconia with or without Zr(OH)4 coating are shown in Figure 2. We failed to detect a P spectrum in the untreated zirconia group (Figure 2A). In the MDP-conditioned zirconia group (Figure 2C), we divided peak decomposition of the O1s region of the narrow-scan spectrum into four contributions: the peak located at 533.3 eV was attributed to the C–O bond,25 that at 532.6 eV to the OH– bond,25,26 that at 531.7 eV to the Zr–O–P bond,18 and that at 530.0 eV to the Zr–O–Zr bond.25,27 Similarly, binding energy of the C–O bond in the MDP-conditioned zirconia with Zr(OH)4-coating group (Figure 2D) was 533.2 eV, binding energy of the P–O–H bond 532.6 eV, binding energy of the Zr–O–P bond 531.7 eV, and binding energy of the Zr–O–Zr bond 529.9 eV. The spectrum for the untreated zirconia group (Figure 2B) was different from the others. We did not observe a Zr–O–P bond peak in the untreated zirconia group. Binding energy observed at 532.8 eV, 532.4 eV, and 529.5 eV was attributed to the C–O bond, OH− bond, and Zr–O–Zr bond, respectively.25,26 The component peak positions of the O1s region and the relative percentages of the Zr–O–P bond (III) in each group are shown in Table 2 and Figure 2, C and D, wherein the Zr–O–P bonds in the MDP-conditioned zirconia group with or without Zr(OH)4 coating were 52.93% and 35.89%, respectively.
 |
Table 2 Binding Energy Of OC–O, OZr–O–P, OZr–O–Zr, OOH– And Relative Percentages Of Zr–O–P Bond |
NMR Analysis
1H and 31P MAS NMR spectra of MDP-conditioned zirconia particles with or without Zr(OH)4 coating are shown in Figure 3. 1H MAS NMR spectra (Figure 3A and C) showed sharp narrow resonance at 6.1 ppm, 5.5 ppm, 4.2 ppm, 1.9 ppm, 1.7 ppm, and 1.4 ppm, attributed to the methacryloyloxy groups of the MDP monomer. The broad resonance at 5.0 ppm was attributed to the Zr–OH groups, which adsorbed water molecules from the hydrated surface layer around the zirconia particles.28 The weak broad resonance around 6.0 ppm was attributed to OH– groups.29 31P MAS NMR spectra of MDP-conditioned zirconia particles with or without Zr(OH)4 coating (Figure 3B and D) showed broad resonance deconvoluted into four peaks around 0.3 ppm, −2.6 ppm, −7.7 ppm, and −13.7 ppm, which were attributed to MDP monomer and dimer. Peaks at 0.3 ppm and −13.7 ppm could not cause deprotonation of P–OH groups, while the peaks at −2.6 ppm and −7.7 ppm were attributed to chemisorbed MDP monomer. Curve-fitting analysis showed that the peak areas for 0.3 ppm, −2.6 ppm, −7.7 ppm, and −13.7 ppm were 0.85%, 1.3%, 12.39%, and 85.46% in the MDP-conditioned group and 1.57%, 2.39%, 32.04%, 64.00% in the MDP-conditioned Zr(OH)4-coated group, respectively (Figure 3, E and F).
Three-Point Bending-Strength Test And Weibull Analysis
Figure 4 shows mean values and standard deviations from our three-point bending strength tests. All three-point bending-strength values conformed to homogeneity of variance and normal distribution (P=0.405), and ANOVA and least significant–difference analyses revealed statistical differences among the groups. Three-point bending-strength values for untreated zirconia were statistically different from the control group. Moreover, the higher the zirconia content, the lower the three-point bending strength. MDP-conditioned zirconia with or without Zr(OH)4 coating exhibited statistically higher three-point bending-strength values than the control group. However, we did not observe any statistical differences between the 7.5 wt% MDP-conditioned zirconia group and the control group.
 |
Figure 4 Mean and SD three-point bending strength values of different resin composites groups.Notes: Letter superscripts (a–e) above columns indicate no significant difference between groups. |
We failed to observe any statistical differences between the 2.5 wt% untreated zirconia group and the 2.5 wt% MDP-conditioned zirconia group with or without Zr(OH)4 coating. We observed statistical differences between the three 5 wt% and three 7.5 wt% zirconia-filled groups, revealing that three-point bending strength (at both 5 wt% and 7.5 wt% concentrations) decreased: MDP-conditioned Zr(OH)4-coated zirconia group > MDP-conditioned zirconia group > untreated zirconia group.
In the MDP-conditioned zirconia group, we obtained the highest three-point bending strength for the 5 wt% group, and differences were not statistically significant from the 2.5 wt% or 7.5 wt% MDP-conditioned zirconia groups (P=0.267). Similarly, in the MDP-conditioned Zr(OH)4-coated zirconia group, we obtained the highest three-point bending strength for the 5 wt% group; however, differences were statistically significant from the 2.5 wt% and 7.5 wt% MDP-conditioned Zr(OH)4-coated zirconia groups (P=0.030).
Table 3 and Figure 5, A–J show our Weibull distribution analysis with 95% confidence. A high slope corresponds to high homogeneity and small errors within the group and a more reliable material structure. In all our experiments, we noted a Weibull modulus of >8, indicating a reliable resin-composite material structure.
 |
Table 3 Three-Point Bending Strength And Parameters Of Different Resin Composites |
Vickers Hardness
Table 4 shows Vickers hardness values for each group, which conformed to homogeneity of variance and normal distribution (P=0.389). We did not find any significant differences between the experimental and control groups, except for the 7.5 wt% untreated zirconia group (P=0.012) and the 2.5 wt% MDP-conditioned Zr(OH)4-coated zirconia group (P=0.001).
 |
Table 4 Vickers Hardness Of Different Resin Composites |
Translucence Parameter
Figure 6 shows TP values for the different groups, which conformed to homogeneity of variance and normal distribution (P=0.244). Overall, we noted significant differences between the experimental and control groups (P=0), whereby control-group TP values were significantly higher than zirconia-filled TP values.
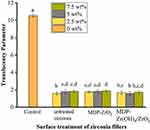 |
Figure 6 Mean and SD translucency parameter (TP) values of different resin composites groups.Notes: Letter superscripts (a–d) above columns indicate no significant difference between groups. |
Discussion
The flexural strength of resin-composite materials should be >80 MPa to withstand long-term occlusal loading, according to the ISO 4049.30 In our study, all three-point bending strengths met this standard, suggesting its potential for clinical application. Moreover, our Weibull analysis revealed the Weibull modulus of each group was >8, indicating that the resin-composite materials in all groups were homogeneous and reliable.31
The addition of 2.5 wt% untreated zirconia to pure silica-filled resin composite improved its three-point bending strength. Interestingly, increasing levels to 5 wt% and 7.5 wt% zirconia revealed resin composites with similar or even lower three-point bending strength than pure silica-filled resin composites. This is consistent with a previous study suggesting that while surface treatment is necessary for the bonding of matrix and fillers, few zirconia particles are needed in the matrix to withstand high strength: an excess of bare zirconia causes agglomeration, which creates crack-initiation sites in the interfaces between fillers and matrix.16 The high hardness of bare zirconia particles has also been shown to promote stress concentration and accelerate the generation of microcracks.17
In our study, we found that MDP-conditioned zirconia fillers improved three-point bending strength of the composite resins and prior Zr(OH)4 coating of the zirconia fillers further enhanced this improvement. TEM observations reveal spot electron-diffraction patterns in Figure 1C, indicating pure single-crystalline zirconia. The hazy dotted ring and spot electron-diffraction patterns in Figure 1, F, J, and L suggest both zirconium hydroxide and crystalline zirconia existed in the samples. Moreover, the hazy dotted-ring patterns strengthened as the concentration of zirconium chloride increased, indicating that zirconium hydroxide can be synthetized via wet-chemical synthesis routes and successfully form zirconia-surface coating. MDP has two functional groups that impact the bonding efficiency of zirconia to resin matrix.32 At one terminus, the phosphoric acid functional group promotes adhesion to zirconia, and at the other terminus an ethylene group promotes cross-linking with unsaturated carbon bonds of an organic resin.30 However, bonding of MDP with zirconia is affected by pH:33 alkaline environments improve the bonding of MDP-conditioned zirconia fillers and resin matrix, while acidic environments reduce bonding efficiency.34 Zirconium hydroxide is an insoluble alkali and adheres to the surface of zirconia by van der Waals force. It creates an alkaline microenvironment that promotes the bonding of zirconia to matrix.35 Therefore, we coated Zr(OH)4 on the surface of zirconia in our experiments. We first verified that Zr–O–P bonds existed on the surface of MDP-conditioned zirconia fillers using XPS, suggesting chemical affinity of MDP to the zirconia filler surface, which provided zirconia fillers the capacity to be polymerized with bisphenol A–glycidyl dimethacrylate. Next, we reported increased Zr–O–P signals on the surface of MDP-conditioned Zr(OH)4-coated zirconia fillers (52.93%) compared with MDP-conditioned zirconia fillers (35.89%). The increase in hydroxyl groups provided by zirconium hydroxide contributed to the improved chemical affinity of MDP for zirconia.35 These findings are consistent with previous studies, suggesting that bonding of MDP and zirconia is enhanced by zirconium hydroxide.29
Our NMR analysis also showed that prior Zr(OH)4 coating positively impacted MDP bonding with zirconia. The 1H MAS NMR spectrum (Figure 3A and C) showed sharp narrow resonance at 6.1 ppm, 5.5 ppm, 4.2 ppm, 1.9 ppm, 1.7 ppm, and 1.4 ppm, attributed to the methacryloyloxy group of the MDP monomer.35 The broad resonance at 5.0 ppm was attributed to the Zr–OH groups, which adsorbed water molecules from the hydrated surface layer around the zirconia particles.28 Weak broad resonance around 6.0 ppm was attributed to OH– groups, including the P–OH bonds of MDP,29 indicating that zirconia strongly interacts with MDP, even after acetone washes. The 31P MAS NMR spectrum of MDP-conditioned zirconia particles with or without Zr(OH)4 coating (Figure 3, B and D) showed broad resonance at −30 to 0 ppm, indicating that MDP reacted on the surface of zirconia. The peak at 0.3 ppm was attributed to an MDP monomer, indicating physical adsorption and hydrogen bonding. The peak at −13.7 ppm was attributed to an MDP dimer, indicating physical adsorption. Both physical adsorption and hydrogen bonding failed to cause deprotonation of P–OH groups. The peak at −2.6 ppm was attributed to Zr–O–P, indicating MDP reacted with zirconia via ionic bonds. The peak at −7.7 ppm was attributed to hydrogen bonding and the Zr–O–P bond.29 The peaks at −2.6 ppm and −7.7 ppm indicated chemisorbed MDP monomer. Consistent with our XPS results, the peak areas at −2.6 ppm and −7.7 ppm in the MDP-conditioned Zr(OH)4-coated zirconia group were larger than in the MDP-conditioned zirconia group.
Our NMR and XPS analyses indicated that Zr(OH)4 enhanced bonding of MDP to zirconia, which is consistent with previous findings.35 Moreover, previous experiments have shown that the addition of 10 wt% MDP-conditioned Zr(OH)4-coated zirconia does not significantly improve the flexural strength of resin composites.22 In our study, we controlled the concentration of zirconium chloride used to avoid the influence of excess zirconium hydroxide on the properties of resin composites. For analysis and quantification of surface treatment, it is necessary to observe the result microscopically.36,37 We added zirconium chloride 0.5–100 mmol/L and found that using 5 mmol/L zirconium chloride demonstrated an even zirconium hydroxide coating on the zirconia filler surface. At this concentration, precoated zirconium hydroxide formed an alkaline environment, improving the bonding effect of MDP to zirconia and matrix and reducing the weakening effects caused by loosely structured zirconium hydroxide.
Noteworthily, we showed that MDP conditioning achieved maximum three-point bending strength at 5 wt% zirconia filler concentrations, which then decreased at 7.5 wt%. Similarly, 7.5 wt% Zr(OH)4-coated zirconia fillers did not increase three-point bending strength compared to 5 wt% Zr(OH)4-coated zirconia fillers. These findings suggest that following Zr(OH)4-coating and MDP conditioning, excessive zirconia still fails to improve the mechanical properties of resin composites. Additionally, our Vickers hardness tests showed that resin composites containing zirconia fillers remained unimproved by Zr(OH)4 coating or MDP conditioning. Previous studies have confirmed that multimodal resin composites have different packing structures, which affect their mechanical properties:8 the tighter the fillers, the better the mechanical properties.7 In our study, we observed different packing structures for different nanozirconia–microsilica resin composites, which explains why zirconia content and three-point bending strengths were not linearly related. We also determined that zirconia has low translucence and a higher refractive index than silica (2.22 versus 1.46, respectively). An increase in zirconia content results in increased light reflection and refraction and a decrease in polymerization of resin composites.9 This may also contribute to decreased mechanical properties.38 These observations explain why Vickers hardness of resin composites does not significantly increase with MDP conditioning.
For silica-based resin composites with zirconia fillers, lower translucence not only has the potential to affect the degree of polymerization but also affect aesthetic outcomes. We showed that zirconia fillers significantly reduced TP, though not linearly, as has been reported in previous research.39 Instead, translucence of resin composites is affected by multiple factors, including particle size, filler type, surface treatment (which all affect light absorbance),40 and differences in refractive indices between the matrix and filler particles (which affect light scattering). Noteworthily, a decrease in translucence means an increase in masking ability, which is beneficial for discolored teeth. Nevertheless, further research is needed to investigate the use of various translucent resin composites and zirconia fillers for clinical selection.
Conclusion
In this study, we surface-treated nanozirconia fillers to strengthen dental bisphenol A–glycidyl methacrylate–based resin composites. Based on limited experimental materials and instruments, our null hypotheses were accepted and the following conclusions drawn. First, prior Zr(OH)4 coating (obtained by adding 0.5 g zirconia particles to 5 mmol/L zirconium chloride) and MDP conditioning is recommended for treating zirconia fillers of resin composites, and resin composites with 5 wt% MDP-conditioned zirconia fillers with or without prior Zr(OH)4 coating achieved the best three-point bending strength. Second, Zr–O–P bonds between MDP and zirconia improved bonding between zirconia fillers and resin matrix. Finally, zirconia fillers decreased the translucence of silica-based resin composites. Altogether, these findings highlight the potential of MDP-conditioned zirconia fillers with prior Zr(OH)4 coating for clinical applications.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grants 81970927 and 81400539), the Natural Science Foundation of Jiangsu Province of China (BK20191348), Jiangsu Higher Education Institutions (grant 2018-87), and the Qing Lan Project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bernardo M, Luis H, Martin MD, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138(6):775–783. doi:10.14219/jada.archive.2007.0265
2. Beck F, Lettner S, Graf A, et al. Survival of direct resin restorations in posterior teeth within a 19-year period (1996–2015): a meta-analysis of prospective studies. Dent Mater. 2015;31(8):958–985. doi:10.1016/j.dental.2015.05.004
3. Rodríguez HA, Kriven WM, Casanova H. Development of mechanical properties in dental resin composite: effect of filler size and filler aggregation state. Mater Sci Eng C Mater Biol Appl. 2019;101:274–282. doi:10.1016/j.msec.2019.03.090
4. Habib E, Wang RL, Zhu XX. Monodisperse silica-filled composite restoratives mechanical and light transmission properties. Dent Mater. 2017;33(3):280–287. doi:10.1016/j.dental.2016.12.008
5. Satterthwaite JD, Maisuria A, Vogel K, Watts DC. Effect of resin-composite filler particle size and shape on shrinkage-stress. Dent Mater. 2012;28(6):609–614. doi:10.1016/j.dental.2012.01.007
6. Lassila L, Keulemans F, Säilynoja E, et al. Mechanical properties and fracture behavior of flowable fiber reinforced composite restorations. Dent Mater. 2018;34(4):598–606. doi:10.1016/j.dental.2018.01.002
7. Randolph LD, Palin WM, Leloup G, Leprince JG. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent Mater. 2016;32(12):1586–1599. doi:10.1016/j.dental.2016.09.034
8. Wang R, Habib E, Zhu XX. Evaluation of the filler packing structures in dental resin composites: from theory to practice. Dent Mater. 2018;34(7):1014–1023. doi:10.1016/j.dental.2018.03.022
9. Guo G, Fan Y, Zhang JF, Hagan JL, Xu X. Novel dental composites reinforced with zirconia-silica ceramic nanofibers. Dent Mater. 2012;28(4):360–368. doi:10.1016/j.dental.2011.11.006
10. Raorane DV, Chaughule RS, Pednekar SR, Lokur A. Experimental synthesis of size-controlled TiO2 nanofillers and their possible use as composites in restorative dentistry. Saudi Dent J. 2019;31(2):194–203. doi:10.1016/j.sdentj.2019.01.008
11. Chen H, Wang R, Zhang J, Hua H, Zhu M. Synthesis of core-shell structured ZnO@m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent Mater. 2018;34(12):1846–1855. doi:10.1016/j.dental.2018.10.002
12. Mehmet Ç, Murat Ş. Synthesis and characterization of a Zr-containing silicate-based epoxy-functional polymer nanocomposite system. Polym Eng Sci. 2015;55(4):792–798. doi:10.1002/pen.23946
13. Taira M, Toyooka H, Miyawaki H, Yamaki M. Studies on radiopaque composites containing ZrO2-SiO2 fillers prepared by the sol-gel process. Dent Mater. 1993;9(3):167–171. doi:10.1016/0109-5641(93)90115-7
14. Umesh VH, Vipin KT. Optimisation of compressive strength in zirconia nanoclusters of the Bis-GMA & TEGDMA based dental composites. Procedia Eng. 2013;51(9):494–500. doi:10.1016/j.proeng.2013.01.070
15. Halder S, Ahmed S, Das S, Wang J. Epoxy/glass fiber laminated composites integrated with amino functionalized ZrO2 for advanced structural applications. ACS Appl Mater Interfaces. 2016;8(3):1695–1706. doi:10.1021/acsami.5b09149
16. Zaman I, Kuan HC, Dai J, et al. From Carbon Nanotubes and Silicate Layers to Graphene Platelets for Polymer Nanocomposites. Nanoscale. 2012;4(15):4578–4586. doi:10.1039/c2nr30837a
17. Godara A, Mezzo L, Luizi F, et al. Influence of carbon nanotube reinforcement on the processing and the mechanical behaviour of carbon fiber/epoxy composites. Carbon. 2009;47(12):2914–2923. doi:10.1016/j.carbon.2009.06.039
18. Lü Q, Guo F, Sun L, Li A, Zhao L. Surface modification of ZrO2: er3+ nanoparticles to attenuate aggregation and enhance upconversion fluorescence. J Phys Chem C. 2008;112(8):2836–2844. doi:10.1016/j.dental.2018.03.022
19. Thompson JY, Stoner BR, Piascik JR, Smith R. Adhesion/cementation to zirconia and other non-silicateceramics: where are we now? Dent Mater. 2011;27(1):71–82. doi:10.1016/j.dental.2010.10.022
20. Inokoshi M, De Munck J, Minakuchi S, Van Meerbeek B. Meta-analysis of bonding effectiveness to zirconia ceramics. J Dent Res. 2014;93(4):329–334. doi:10.1177/0022034514524228
21. Ozcan M, Bernasconi M. Adhesion to zirconia used fordental restorations: a systematic review and meta-analysis. J Adhes Dent. 2015;17(1):7–26. doi:10.3290/j.jad.a33525
22. Wu X, Dai S, Chen Y, He F, Xie H, Chen C. Reinforcement of dental resin composite via zirconium hydroxide coating and phosphate ester monomer conditioning of nano-zirconia fillers. J Mech Behav Biomed Mater. 2019;94:32–41. doi:10.1016/j.jmbbm.2019.03.002
23. Chen Y, Lu Z, Qian M, et al. Chemical affinity of 10-methacryloyloxydecyl dihydrogen phosphate to dental zirconia: effects of molecular structure and solvents. Dent Mater. 2017;33(12):e415–e427. doi:10.1016/j.dental.2017.09.013
24. Gavranović-Glamoč A, Ajanović M, Korać S, et al. Evaluation of the water sorption of luting cements in different solutions. Acta Med Acad. 2017;46(2):124–132. doi:10.5644/ama2006-124.197
25. Lin XZ, Yuan ZY. Synthesis of amorphous porous zirconium phosphonate materials: tuneable from micropore to mesopore sizes. RSC Adv. 2014;4:32443–32450. doi:10.1039/C4RA03970J
26. Adolphi B, Jähne E, Busch G, Cai X. Characterization of the adsorption of omega-(thiophene-3-yl alkyl) phosphonic acid on metal oxides with AR-XPS. Anal Bioanal Chem. 2004;379(4):646–652. doi:10.1007/s00216-004-2634-x
27. Dupin JC, Gonbeau D, Vinatier P, Levasseur A, Levasseur A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys Chem Chem Phys. 2000;2:1319–1324. doi:10.1039/a908800h
28. Chadwick AV, Mountjoy G, Nield VM, et al. Solid-state NMR and X-ray studies of the structural evolution of nanocrystalline zirconia. Chem Mater. 2001;13(4):1219–1229. doi:10.1021/cm001152w
29. Yoshida Y, Yoshihara K, Hayakawa S, et al. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J Dent Res. 2012;91(11):1060–1065. doi:10.1177/0022034512460396
30. Goracci C, Cadenaro M, Fontanive L, et al. Polymerization efficiency and flexural strength of low-stress restorative composites. Dent Mater. 2014;30(6):688–694. doi:10.1016/j.dental.2014.03.006
31. Rodrigues SA, Scherrer SS, Ferracane JL, Della Bona A. Microstructural characterization and fracture behavior of a microhybrid and a nanofill composite. Dent Mater. 2008;24(9):1281–1298. doi:10.1016/j.dental.2008.02.006
32. Tian FC, Wang XY, Huang Q, et al. Effect of nanolayering of calcium salts of phosphoricacid ester monomers on the durability of resin-dentinbonds. Acta Biomater. 2016;38:190–200. doi:10.1016/j.actbio.2016.04.034
33. Chen C, Chen Y, Lu Z, et al. The effects of water on degradation of the zirconia-resin bond. J Dent. 2017;64:23–29. doi:10.1016/j.jdent.2017.04.004
34. Xie H, Tay FR, Zhang F, et al. Coupling of 10-methacryloyloxydecyldihydrogenphosphate to tetragonal zirconia: effect of pH reaction conditions on coordinate bonding. Dent Mater. 2015;31(10):e218–e225. doi:10.1016/j.dental.2015.06.014
35. Qian M, Lu Z, Chen C, Zhang H, Xie H. Alkaline nanoparticle coatings improve resin bonding of 10-methacryloyloxydecyldihydrogenphosphate-conditioned zirconia. Int J Nanomedicine. 2016;11:5057–5066. doi:10.2147/IJN.S116006
36. Cervino G, Fiorillo L, Spagnuolo G, et al. Interface between MTA and dental bonding agents: scanning electron microscope evaluation. J Int Soc Prev Community Dent. 2017;7(1):64–68. doi:10.4103/jispcd.JISPCD_521_16
37. Lo Giudice G, Cicciù M, Cervino G, Lizio A, Visco AM. Flowable resin and marginal gap on tooth third medial cavity involving enamel and radicular cementum: a SEM evaluation of two restoration techniques. Indian J Dent Res. 2012;23(6):763–769. doi:10.4103/0970-9290.111256
38. Akiba S, Takamizawa T, Tsujimoto A, et al. Influence of different curing modes on flexural properties, fracture toughness, and wear behavior of dual-cure provisional resin-based composites. Dent Mater J. Epub 2019 Jun 21.
39. Haas K, Azhar G, Wood DJ, Moharamzadeh K, van Noort R. The effects of different opacifiers on the translucency of experimental dental composite resins. Dent Mater. 2017;33(8):e310–e316. doi:10.1016/j.dental.2017.04.026
40. Emami N, Sjodahl M, Soderholm KJ. How filler properties, filler fraction, sample thickness and light source affect light attenuation in particulate filled resin composites. Dent Mater. 2005;21(8):721–730. doi:10.1016/j.dental.2005.01.002
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.