Back to Journals » International Journal of Nanomedicine » Volume 15
Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review
Authors Tortorella S , Vetri Buratti V , Maturi M , Sambri L , Comes Franchini M , Locatelli E
Received 18 June 2020
Accepted for publication 7 August 2020
Published 10 December 2020 Volume 2020:15 Pages 9909—9937
DOI https://doi.org/10.2147/IJN.S266103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Silvia Tortorella, Veronica Vetri Buratti, Mirko Maturi, Letizia Sambri, Mauro Comes Franchini, Erica Locatelli
Department of Industrial Chemistry “Toso Montanari”, Alma Mater Studiorum – University of Bologna, Bologna 40136, Italy
Correspondence: Erica Locatelli Email [email protected]
Abstract: Presently, a plenty of concerns related to the environment are due to the overuse of petroleum-based chemicals and products; the synthesis of functional materials, starting from the natural sources, is the current trend in research. The interest for nanocellulose has recently increased in a huge range of fields, from the material science to the biomedical engineering. Nanocellulose gained this leading role because of several reasons: its natural abundance on this planet, the excellent mechanical and optical features, the good biocompatibility and the attractive capability of undergoing surface chemical modifications. Nanocellulose surface tuning techniques are adopted by the high reactivity of the hydroxyl groups available; the chemical modifications are mainly performed to introduce either charged or hydrophobic moieties that include amination, esterification, oxidation, silylation, carboxymethylation, epoxidation, sulfonation, thiol- and azido-functional capability. Despite the several already published papers regarding nanocellulose, the aim of this review involves discussing the surface chemical functional capability of nanocellulose and the subsequent applications in the main areas of nanocellulose research, such as drug delivery, biosensing/bioimaging, tissue regeneration and bioprinting, according to these modifications. The final goal of this review is to provide a novel and unusual overview on this topic that is continuously under expansion for its intrinsic sophisticated properties.
Keywords: nanocelluse, surface modification, drug delivery, scaffold, tissue engineering
Introduction
At present, resorting to non-fossil derivatives as the starting materials for modern research in industries and academia, is becoming essential. Among sustainable resources, biopolymers represent a valuable alternative. In particular, cellulose is the most abundant natural biopolymer on earth, and it is an excellent biomass for developing new and more sustainable materials from renewable resources. Natural cellulose continues to be a macroscopic material widely employed for textile and paper industrial uses, but notably, the mechanical or chemical treatment of cellulose accounts for its easy separation to more organized sub-unit of nanoscale cellulose that can satisfy the requirements for engineered bottom-up applications.1 Nanofibrillated cellulose (NFCs) and Cellulose Nanocrystals (CNCs), also called cellulose nanowhiskers, possess many interesting features typical for nanomaterials in general, such as high surface area to volume ratio, enhanced and tuneable chemical reactivity, high mechanical durability, as well as biocompatibility, biodegradability and non-toxicity. All these properties make it an excellent candidate for a huge variety of applications in almost all the material science fields. Currently, great attention has been dedicated to paper-based (bio-) sensors since they offer low-cost, portable and disposable platforms, mainly as a replacement for the plastic and glass substrates in high-performance (opto-) electronic devices such as electrodes, sensors, actuators, electrochromic devices and so forth. The use of nanocellulose for these purposes is becoming an emerging field of research.
Anyway, a particular attracting field arose to be the biomedical applications, such as tissue engineering, cartilage replacement, bone repair, medical implants and wound dressings, which gained increasing attention exponentially during the last 10 years.2 Again, regarding the biomedical field in recent years, nanocellulose has also been investigated as drug delivery carrier.3 The number of publications on this specific topic remains low, with studies mostly focused on adsorption of drugs or medication onto the surface of the nanocrystals.4 Nevertheless, specific advantages of using nanocellulose amongst other possible nanoparticles have been recently elucidated and the research in this field is gaining attention.5
A major advantage in the use of a polysaccharide such as cellulose is represented by the chance of surface modification, which in other nanostructures, is not easy neither is it always possible. Indeed, the polysaccharide nanocrystals are characterized by a reactive surface covered by numerous active hydroxyl groups, which provide the possibility of modification and exploiting chemical reaction strategies. During the last 15 years, hundreds of scientific articles have been published, which focused on the chemical modifications of nanocellulose for adjusting the surface properties or tuning its hydrophilic–hydrophobic balance.6 Indeed, the use of pristine nanocellulose without any kind of surface modification is commonly limited to the applications involving hydrophilic or polar media, because of its poor dispersibility in non-polar organic solvent and non-polar polymer matrix. This aspect is a strong limitation, in particular for polymer science, where nanocrystals are frequently used as reinforcing additives, thus this field drove the research in surface modification of nanocellulose for obtaining hydrophobilization and better mixing of nanocrystals with polymer matrix.7
The nanomedicine field has surely preferred this approach, trying not only to use pristine nanocellulose for all the above-cited applications but also to modify the surface of the nanocrystals in order to open a plethora of novel possibilities. Indeed, by applying appropriately developed reactions, it is possible to introduce functional groups or moieties on the surface of the nanocellulose contributing to specific properties, thereby, extending its use in more widely sophisticated applications.8,9
Considering the extremely wide number of articles and review on nanocellulose, this study only focus attention on the opportunities for chemical functionality of nanocellulose to obtain proper materials to be used in drug delivery, in biosensors, bioimaging and bioprinting applications. The paper is structured in four main sections (Figure 1): the first section accounts for the current methods of surface functionality of nanocellulose; the second section deals with the applications of functionalized nanocellulose in drug delivery; the third section reports on its applications in biosensing and bioimaging; finally, the last section discusses the current uses of functionalized nanocellulose in tissue regeneration and bioprinting, which is one of the most promising application for nanomaterial.
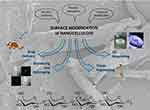 |
Figure 1 The surface modification of nanocellulose can be performed either via CNCs synthesis, physical adsorption or chemical modification, leading to advanced nanomaterials with a wide variety of possible applications. At the bottom: chemical structure of the polymeric backbone of CNCs; the common numbering of the glucopyranose ring is reported on one of the monomers. In the background: SEM image of cellulose nanocrystals. Bioimaging and bioprinting images have been reprinted from Eur J Pharm Sci, 50(1), Valo H, Arola S, Laaksonen P, et al. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. 69–77, Copyright (2013), with permission from Elsevier60 and Martínez Ávila H, Schwarz S, Feldmann E-M, et al. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl Microbiol Biotechnol. 2014;98(17):7423–7435. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode95, respectively. |
Surface Chemistry of Nanocellulose
Several reactions have been tested or specifically developed for surface modification of nanocellulose. The purpose of surface modification of a nanomaterial always remains the introduction of novel functional groups or of important biological components onto the nanostructure, avoiding any possible damage to the nanoparticle itself. In the case of nanocellulose modification, this is an aspect of utmost importance, since the core of the material presents exactly the same functional groups of the surface (hydroxyl groups) and every conversion should be limited to the ones presented on the surface only by controlling the reaction parameters.
The approach for surface modification of nanocellulose greatly relies on the reactivity of the hydroxyl groups, which are the only functional group naturally present in this polysaccharide. It has been reported that the hydroxyl group at position 6 is 10 times more reactive than the other OH groups, while the reactivity of the hydroxy group in position 2 is found to be twice that of the OH group in position 3.10 Simultaneously, chemical modification should be mild in order to preserve the other useful properties of pristine nanocellulose; enhancements of the substitution degree and/or the grafting efficiency of the material should not affected its structure, morphology and crystalline properties.11 Chemical modification of CNCs can be classified into three groups: (1) native surface chemistry during the isolation/purification process or as a result of similar methods of surface treatment, (2) physical adsorption to the surface and (3) covalent bond formation or derivatization of the surface. In recent decades, the chemical modification strategies of nanocellulose gained a lot of attention in studies,12,13 but the aim of this review is to describe those that are involved in the biomedical applications. In addition, recently, the fabrication of high-performance polymer materials using nanocellulose has received significant interest,14 but this work is focused on reporting all those applications where chemical modifications were involved. Each strategy is thoroughly described in the following sections, Table 1 provides a summary of the most widespread functionality.
 |
Table 1 Main Approaches for Surface Functionalization of Nanocellulose in the Recent Literature |
Functionalization Using CNCs Synthesis
There are few methods that lead to CNCs but the most popular approach among all of them, is the acidic hydrolysis. The strongly acidic environment leads to dissolution of the amorphous parts in cellulose microfibrils, leaving the crystalline region unaffected.15 The selection of the acid plays an important role, as it determines the properties of the final material, as well as the additional synthetic path for grafting of particular molecules. The degradation performed with sulfuric acid-catalysed hydrolysis allows the formation of sulphate esters, characterized by a different degree of sulfonation depending on the acid concentration, temperature and hydrolysis time16,17
Sulfonation results in a negative net surface charge which is able to stabilize nanocrystal suspensions, useful for a wide range of applications. A less common synthetic method is hydrochloric acid degradation to obtain hydroxylated surfaces with a lower charge density and, as result, poorer dispersibility in water.9 Habibi et al showed other investigations that exploit less conventional strategies such as phosphoric and hydrobromic acids.18,19
Furthermore, Chen et al demonstrated that the organic acid-mediated hydrolysis, with maleic and oxalic acids, results in formation of CNCs with moderate dispersibility in aqueous media and significantly higher thermal stability in comparison to that produced through sulfuric acid.20 Simultaneously, esterification of hydroxyl group with the hydrolysis of amorphous cellulose chains has been described by Dorgan et al as a viable one-pot reaction that allows the isolation of acetylated CNCs in a single step process. Fischer-Spicer esterification was carried out, exploiting the acetic acid both as acid for digestion and as catalyst, to achieve the acetylated surface.21 In addition, this study group recently reported the one-step esterification of cellulose in Brønsted-acid ionic liquids, such as, N-methyl pyrrolidinium hydrogensulfate (NMP-HSO4), which enabled the covalent linking of chlorotoxin as the active targeting agent for glioblastoma multiforme (Figure 2).22 Another method that is becoming increasingly common is TEMPO-mediated oxidation coupled with mild mechanical treatment.23,24 The use of this technique has been the focus for hundreds of works since its first introduction by Nooy et al who presented the regioselective oxidation of the primary hydroxy groups to carboxylic acids mediated by 2.2,6,6 tetramethyl-1-piperidinyloxy radical (TEMPO), leaving the secondary hydroxy moieties unaffected.25 The oxidation of cellulose occurs in the presence of NaClO, a catalytic amount of TEMPO and NaBr at temperatures ranging between 0°C and 25°C and pH of 10, to ensure high oxidation efficiency. The presence of carboxylic group provides negative charges to the CNCs surface, thus inducing electrostatic stabilization.
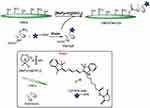 |
Figure 2 Surface esterification of nanocellulose with cyanine- (Cy5) conjugated chlorotoxin (Cltx) in in Brønsted-acid ionic liquid. Reproduced by permission of The Royal Society of Chemistry (RSC) on behalf of the Centre National de la Recherche Scientifique (CNRS) and the RSC. Cellante L, Costa R, Monaco I, Cenacchi G, Locatelli E. One-step esterification of nanocellulose in a Brønsted acid ionic liquid for delivery to glioblastoma cancer cells. New J Chem. 2018;42(7):5237–5242.22 |
Functionalization Through Physical Adsorption
The second subset of the modification procedures involves the adsorption to surfactants, polyelectrolytes or generally, opposite charged entities. Simple addition of surfactant is an interesting alternative to the chemical modification with different possible approaches. In such cases, several factors like charge-charge interactions, hydrogen bonds, Van der Waals forces, solvency and association forces between the hydrophobic groups are involved.26 As a disadvantage, the lack of a covalent strong bond may be an issue for what concerns the possible release of surfactant molecules.27 Sulfuric acid derived CNC has a charged surface, characterized by the presence of sulfate esters, that may interact with cationic surfactants like tetraalkyl ammonium halides (mostly chloride and bromide).28 In this regard, Jackson et al compared the release of hydrophobic drugs encapsulated into pristine CNCs or surface-modified CNCs by the action of cetyl trimethylammonium bromide (CTAB).29 Similarly, Trabelsi et al observed the aggregation of carboxymethylcellulose in aqueous solution in the presence of various cationic surfactants, varying in their aliphatic chain length.30
As reported by Wang et al chloride anions have been found to increase the interaction between cationic surfactants towards the non-ionic cellulose derivatives.31 With regards to the polar head-groups, the substituents on nitrogen, may influence the affinity between the surfactant itself and the cellulosic material; for instance, in case of poorly anionic cellulosic materials, surfactants bearing ammonium head-groups show a higher interaction than those carrying trimethylammonium, because of the higher hydrophobic nature of the latter.
Moreover, inspired by the industrial paper manufacturing process, adsorption modification has been promoted by electrostatic interactions between macromolecules. Weishaupt et al revealed how attractive interactions between negatively charged TEMPO-oxidized Nanofibrillar Cellulose (ToNFC) and positively charged antimicrobial peptides are responsible for the integration of nisin.32
Most commonly, electrostatic layer-by-layer (LbL) deposition is used: A charged solid substrate is exposed to a solution of oppositely charged polyelectrolyte, followed by rinsing.33 This leads to the adhesion of the polymeric material in greater amount than that required to achieve neutrality, reversing the net charge. Repeating this process, it is possible to obtain structured LbL films with potential applications as bio/optical sensors as well as drug delivery systems.34,35
Functionalization Through Chemical Modification
The last approach of surface chemistry modification is by the use of direct chemical modifications through covalent bond formation. CNCs are characterized by the presence of a primary hydroxy group, thus, the direct functionality is the possible exploiting of all the functional groups that are able to react with alcohols. These reactions can be used to form a host of alternative surface chemistries as summarized in Figure 3 and described in the following examples.
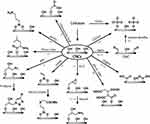 |
Figure 3 Main surface chemical modifications of CNCs for biomedical applications discussed in this review. References are reported on the arrows. |
Nonetheless, sulfuric acid hydrolysis still remains the most commonly employed degradation strategy, because of its ability to provide highly charged surface, but it is also worth mentioning that the determination of sulfonation degree remains still challenging.36 To solve this issue, post-treatment of CNCs obtained by hydrochloric acid hydrolysis with sulfuric acid has been suggested to introduce sulphate moieties in a controlled fashion.37 This method leads to the formation of particles with a size comparable to those obtained from sulfuric acid hydrolysis, but with different morphology. The combination of both sulfuric and hydrochloric acids appears to generate spherical CNCs with a high thermal stability, instead of the more common rod-like crystals.38
Besides the direct sulfonation, Lin and Dufresne investigated a post-sulfonation modification strategy, involving the treatment of surface hydroxy groups with sulfonating agents such as, chlorosulfuric acid, in order to get a tuneable sulfonation degree.39
Finally, a last approach for the introduction of sulphate groups was performed by Liimatainen et al involving a two-step process: Firstly, the vicinal diol bond of the glucose monomers on CNCs surface is cleaved by sodium or potassium periodate into the corresponding acyclic dialdehyde, followed by the sulfonation through the sodium bisulfite (Figure 4).40 When a glucopyranose ring on CNCs surface is converted into the dialdehyde, the neighbouring groups become more susceptible to oxidant attack because of the local loss of crystalline order.41,42 Consequently, it is possible to further use 2.3-dialdehyde cellulose (DAC) with active molecules for drug delivery.43
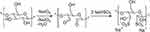 |
Figure 4 Periodate oxidation and further sulfonation of CNCs surface as reported by Liimatainen et al. Reproduced from Liimatainen H, Visanko M, Sirviö J, Hormi O, Niinimäki J. Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose. 2013;20(2):741–749. focus. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.40 |
Dash et al inserted DAC into a spacer molecule, γ-aminobutyric acid (GABA), by the Schiff’s base condensation reaction. After the reduction of the resulting imine intermediate with NaCNBH4, they obtained a controlled and rapid delivery of syringyl alcohol, that acts as a targeting moiety.44
Eyholzer et al showed how it was possible to obtain biocomposite hydrogels with carboxymethylated nanofibrillar cellulose for the replacement of native human nucleus pulposus (NP) in intervertebral disks.45
Carboxymethylation of NFC is commonly carried out in the presence of chloroacetic acid, which is nucleophically attacked by the primary OH group of cellulose surface monomers leading to its 6-carboxymethylated derivative. This process produces negatively charged surfaces and it is frequently performed with the help of mechanical treatments.46
Several works demonstrated how reacting CNCs with carboxylic acid halides or anhydrides is possible to create ester linkages.47 However, this approach usually does not allow for reactions in water suspension and it requires dispersion of CNCs into anhydrous and non-protic organic solvents such as acetonitrile, acetone or DMF. In 2015, Galkina et al investigated the esterification of NFC with 1,2,3,4-butanetetracarboxylic acid (BTCA), in the presence of sodium hypophosphite (SHP) acting as a base, followed by the attachment with TiO2 (Figure 5).48 The purpose of the chemical modification was to synthesize a novel transdermal drug delivery system by binding a model molecule, as it is explained in following section.49 With a similar approach, silylation reaction can be performed with 3-aminopropyltrimethoxysilane (APTES). Reactive terminal amino group activate cellulose toward further functionalization.50
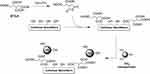 |
Figure 5 Schematics of the chemical conjugation of TiO2 nanoparticles on cellulose nanofibers by exploiting the reactivity of BTCA and its anhydride derivatives, as described in Galkina et al.48 |
Another well-known strategy to add a spacer for binding active species is the functionality of amino acids.51 In 2011, Cateto et al modified cellulose nanowhiskers with L-leucine by a two-step process involving the reaction between CNCs and Fmoc-protected L-leucine followed by the removal of the Fmoc protecting group.52 The reaction was performed with 4-dimethylaminopyridine (DMAP) as a base catalyst and N-ethyl-N’-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC HCl), as a coupling agent.
Likewise, Akhlaghi et al understood a novel drug delivery system based on TEMPO-oxidized Cellulose nanocrystals and chitosan oligosaccharide (CSOS). The primary alcohol moieties and the amino groups of CSOS were reacted with the carboxylic acid groups on oxidized CNC using EDC HCl facilitated by N-hydroxysuccinimide (NHS) as the coupling agents.53
An alternative approach was proposed by Pahimanolis et al who developed amino functional NFC, prepared using click-chemistry in aqueous solution (Figure 6).54 Reactive azido groups were introduced on the surface of NFC employing 1-azido-2,3-epoxypropane (AEP); subsequently, the azido groups were coupled to propargyl amine through the copper-catalysed azido-alkyne cycloaddition.
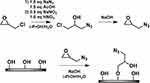 |
Figure 6 Synthetic path for the preparation of AEP and its further coupling on CNCs surface. Reproduced from Pahimanolis N, Hippi U, Johansson L-S, et al. Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose. 2011;18(5):1201. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.54 |
Feese et al described the synthesis and characterization of CNCs surface-modified with a cationic porphyrin.55 The latter was appended onto the cellulose surface through the click reaction which occurred between the azido groups on the cellulosic surface and the porphyrinic alkynes. The resulting nanostructure showed excellent efficacy toward photodynamic bacterial inactivation.
Another challenging chemical pathway is to utilize the solid cellulose substrates involved in alkoxysilane chemistry coupled with a photocatalysed thiol-ene click chemistry. In particular, Tingaut et al have focused on the development of a two-step synthetic strategy, where a silylation reaction is firstly performed with vinyltrimethoxysilane on the CNCs, followed by its photochemical coupling with methyl thioglycolate.56
Applying the same principles, in 2016, Guo et al obtained superhydrophobic flexible nanocellulose films by reacting vinyl-functionalized nanocellulose with various thiol-containing molecules such as, fluorinated long-chain thiols or small functional molecules.57 Flexibility, transparency and perfect super hydrophobicity of the produced nanocellulose substrates guarantees their application in biosensing, biomedical and diagnostic devices.
Nanocellulose as Drug Delivery Carrier
The high surface area-to-volume ratio of nanocellulose, as well as, of whatever nanoparticle, allows for a high level of drug adsorption at the surface. Nevertheless, specific advantages of using nanocellulose amongst other possible nanoparticles, have been recently elucidated and the research in this field has gained attention over the years.
Several attempts of using nanocellulose as a drug carrier, rely on the use of pristine material without any kind of surface modification. Amongst these cases, nanocellulose have been used as excipients or as real carrier through both physical adsorption and ionic interaction with the drug involved. Anyway the loading and release profile of such construct is generally poorly controllable. These examples are well summarized in the review of Salimi et al.3
One of the first studies, involving some kind of relationship between nanocellulose and drug, was provided by Jackson et al,58 who investigated the possibility to use pristine CNCs to bind and release water soluble drugs such as, doxorubicin and tetracycline: even if the amount of both drugs incorporated was significant, 80% of these were released in the first minute and only the remaining 20% accounted for a sustained release in the next 4 days. In the same paper, the authors faced difficulties of incorporating hydrophobic drugs onto CNCs surface.
To overcome this limitation, they provide an efficient CTAB surface modification of CNCs, as highlighted in this review, thus, obtaining an amphiphilic nanostructure with a hydrophilic core and a lipophilic surface, was able to accommodate common medications such as, docetaxel, paclitaxel, and etoposide. Also, in this case, a burst release was present in the first few hours of releasing the test, but it accounted for less than 40% of the incorporated drugs, whereas the remaining amount was released in 2–4 days in a controlled manner. Moreover, fluorescein was tagged to the nanocarrier and internalization of KU-7 cell line was shown (Figure 7).
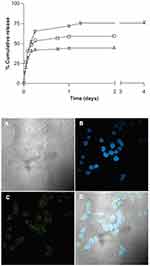 |
Figure 7 Release kinetics of the different drugs loaded in CNCs/CTAB complexes at 37°C (top); etoposide, docetaxel, paclitaxel (from the top curve to bottom); confocal microscopy images showing internalization of fluorescent CNCs/CTAB: (A) white light image, (B) DAPI staining of cell nuclei, (C) fluorescein in the cytoplasm, (D) overlay of images (B and C) (bottom). Copyright |
Another interesting approach, that is based on the CNCs surface modification, but maintained an electrostatic interaction with the drug was proposed by Akhlaghi et al,53 who oxidized CNCs with TEMPO, in order to obtain a free carboxylic group that is used for covalent binding with amino groups of chitosan, the second most abundant biopolymer on earth, by intervention of the coupling agent, EDC. Drug loading of procaine hydrochloride, a local anesthetic used in dental surgery, was determined to be 14% w/w at pH 8. Indeed, it was well explained that insignificant interaction between CNCs-Chitosan and procaine was observed at pH 6, due to the electrostatic repulsion between the positively charged samples of both counterparts, whereas the highest degree of interaction was shown at pH 8, therefore, this pH was preferred for drug loading experiments. Due to the ionic nature of the interaction, released profile presents an intense fast release of the drug, in the first 10 min and a slower release of the remaining drug in about 1 h.
In a series of studies, Valo et al described a linkage between the drug (Itraconazole) and nanocellulose, obtained via enzymatic recognition.59,60 Although, chemical modification of nanocellulose was not properly described. In the first study, drug nanoparticles were coated with an hydrophobins protein that was genetically linked with cellulose binding domains (CBD) to obtain the drug nanoparticles that could bind to cellulose. Strong affinity between CBDs and cellulose, guarantees the binding of hydrophobins coated with drug nanoparticles, to the cellulose network, which in the proposed study, was used as a protective agent during the storage and the formulation processes.
In the second paper, the same approach was used for binding of Beclomethasone dipropionate nanoparticles onto the aerogels, based on different cellulosic sources such as, nanofibrillar celluloses, bacterial cellulose, cellulose extracted from red pepper and quince seeds as well as TEMPO-oxidized nanofibrillar cellulose, that remains the only chemical modification attempted. The nanocellulose aerogel scaffolds made from bacterial cellulose, quince seed and TEMPO-oxidized cellulose aerogels showed sustained drug release (60% in 600 min), whereas, the others released BDP immediately (70% in the first 5–10 min). Therefore, by the modulation of the nanocarriers, the drug could be released in a controlled manner.
Remarkably, Dash and Ragauskas (2012) developed the first synthetic modification of nanocellulose, aimed at the covalent linkage of a drug onto its surface.44
The proposed strategy involved many reaction steps with the purpose of covalent linkage of amino-bearing drugs, but also of effective release of the unaltered drug under enzymatic or plasma cleavage of a linker or spacer arm between nanocellulose and drug.
In particular, as shown in Figure 8, γ-aminobutyric acid, a spacer molecule, was embedded into the nanocellulose surface, previously oxidized with sodium periodate, in order to generate aldehydes groups. Then, syringyl alcohol was linked to the carboxylic group of the amino acid. With this approach, the authors examined the use of an aromatic linker, known as a benzyl elimination linker, in designing the delivery system, since it relies on a classic and rapid 1.6-benzyl elimination reaction and molecular decomposition, to regenerate the amine group of the conjugated drug/biomolecule.
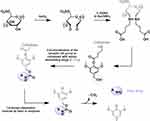 |
Figure 8 Functionalizaton of cellulose with amino-terminating drugs. The active pharmaceutical ingredient is then enzymatically released through 1,6-benzyl elimination as suggested by Dash et al.44 |
Pickering emulsion based on aminated nanocellulose has been prepared by Ngwabebhoh et al and used as stabilizing carrier for lipophilic drugs, such as, curcumin and coumarin (Figure 9).61 For the preparation of the nanosystem, oil-in-water approach were applied and aminated nanocellulose particles stabilized the different composition of oil phase, with medium-chain triglyceride and Tween 80. Aminated nanocellulose was obtained by the reaction with epichlorohydrin (ECH), isolation of the epoxide-modified cellulose and subsequent addition of ammonium hydroxide in order to open the epoxide and obtain aminated nanocellulose. After the examination of the optimal oil phase amount and composition, fine droplet with size of 84 nm were prepared and stabilized, by adding aminated nanocellulose, thus obtaining a final size of 325 nm. Encapsulation efficiencies for curcumin and coumarin were calculated as 93.1% and 96.2%, respectively. The high encapsulation efficiency achieved, was attributed to the hydrogen bond interaction between the polar groups of cellulose, curcumin and coumarin, signifying the importance of modified cellulose for the attainment of significant drug setup. The delivery percentage of coumarin and curcumin, after 24 h in pH 7.4 media was estimated at 25% and 10%, respectively; with a gradual increase over a period of 8 days giving rise to a maximum delivery of 76% and 41%, respectively. A significant anticancer activity was confirmed in human fibroblastic cells (L929) and breast cancer cells (MCF-7).
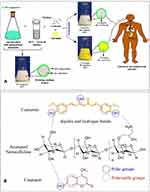 |
Figure 9 (A) preparation of drug-encapsulated nanocellulose pickering emulsion; (B) main interactions taking place between the drugs and the aminated CNCs surface. Reproduced from Carbohydr Polym. 201 (August), Asabuwa Ngwabebhoh F, Ilkar Erdagi S, Yildiz U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: in vitro release, anticancer and antimicrobial activities, 317–328, copyright (2018), with permission from Elsevier.61 |
Besides drug loading onto the nanocellulose surface, incorporation of metal nanoparticles, with peculiar biological properties have been recently attempted. In particular, Diez et al, for the first time, were able to bind silver nanoclusters, which represent well known fluorescent and antimicrobial properties, on the native cellulose nanofibers.62 Firstly, pink fluorescent silver nanoclusters, smaller than 2 nm, were synthesized in the presence of poly (methacrylic acid) (PMAA) and then linked to nanofibrillated cellulose.
The possible reaction mechanism was supposed to involve a migration of fluorescent silver cluster in solution, from one support to another. In this case, from PMAA to nanocellulose or the H-bonding between the PMAA containing –COOH groups and nanocellulose containing –OH groups.
Although, this cannot be considered as a proper nanocellulose chemical modification, it represents a first proof-of-concept for the linkage of metal object to nanocellulose for biomedical purposes. In this light, the work of Galkina et al (2015) was more complete and describes the binding via ester bond of titania nanoparticles, loaded with three different model drugs, to nanocellulose.48 In particular, in order to crosslink titania nanoparticles with cellulose nanofibers, butanetetracarboxylic acid was used as a spacer in the presence of sodium hypophosphite. This allows the introduction of several carboxylic acid onto nanocellulose surface and the subsequent esterification with titania nanosol. Titania was previously loaded with different drugs such as, diclofenac sodium salt, penicillamine D or phosphomycin disodium salt as well as, with different adsorption methods. Thus, comprehensive release studies were reported.
 |
Figure 10 Preparation of fluorescent carbohydrate-conjugated CNCs. Reprinted with permission from Zhou J, Butchosa N, Jayawardena HSN, et al. Synthesis of multifunctional cellulose nanocrystals for lectin recognition and bacterial imaging. Biomacromolecules. 2015;16(4):1426–1432. Copyright (2015) American Chemical Society.67 |
Nanocellulose in Biosensing and Bioimaging
It is currently known that biosensors are a very remarkable novel technology with many advantageous choices, such as simplicity, time and cost efficiency, suitable for a wide range of applications. This thriving technology, surely revolutionized the capability of measuring important parameters, above all those that are related to medical diagnostics. Biomaterials could clearly represent a big advantage for biosensor development, in order to simplify this technology and improve the overall performance, as well as the main features such as, sensitivity, specificity and replicability.
Recently, great attention has been dedicated to paper-based biosensors, mainly as a replacement for plastic and glass substrates in high-performance (opto) electronic devices such as electrodes, sensors, actuators, electrochromic devices and so forth. Precisely, the use of ‘nanopaper’ could also overcome some regular paper’s shortcomings such as large surface roughness, optical opaqueness, low mechanical strength and low stability in water, which may hinder its integration into (opto) electronic devices and sensors.
Many efforts have already been committed to the fabrication of CNCs-based substrates, such as conductive/transparent and flexible platforms, that are expected to have potential applications in flexible/portable electronics devices, such as displays, sensors and actuators. Furthermore, several fluorescently labeled CNCs have been synthesized, for application in the fields of (bio) sensing, bioimaging and optical labeling. Beside medical diagnostic purposes, theranostic approaches could also take advantages from CNCs-based hybrid materials. There is a very high and interesting probability that, in a very short time, CNCs would be revolutionizing the field of the (bio) sensing technology.
In this study, we highlighted the state-of-the-art on the use of CNCs in biosensing and bioimaging, as well as the possibility of adapting and applying CNCs for this purpose along with the future perspectives.
CNCs in Optical Biosensing
Surface Enhanced Raman Scattering (SERS) has been regarded recently, as one of the most powerful tools for (bio) chemical detection, its high sensitivity and selectivity is also commended. Basically, it is a surface-sensitive technique that enhances Raman scattering by molecules adsorbed on the rough metal surfaces or by nanostructures. The signal enhancement factor can be as high as 1010 to 1011, meaning that single molecules could be detected. The first application of CNCs in optical sensing has been reported by Marques et al.63 They fabricated nanocomposites based on silver nanoparticles (AgNP) and bacterial cellulose (BC) through in situ chemical reduction of adsorbed silver ions onto the BC by citrate method. In this work, BC acted as a biotemplate for in situ growth of AgNPs. The fabricated AgNP/BC nanocomposites were then used as an active surface enhanced Raman scattering (SERS) substrate for detecting the thiosalicylic acid, 2,2-dithiodipyridine and amino acids such as, L-phenylalanine, L-glutamine and L-histidine in aqueous solutions. AuNP/BC nanocomposite as a sensing platform for sensitive and reproducible SERS detection of carbamazepine (CBZ) and atrazine (ATZ), has also been used by Wei et al.64
CNCs labeled with pH-responsive fluorescent dyes such as isothiocyanate and succinimidyl ester dyes, could be successfully used for pH sensing. For example, Davarayn and Kim, developed an eco-friendly pH sensor by simply immobilizing a natural pigment (from red cabbage) onto the electrospun NFC, the adsorption was followed by a chemical crosslinking reaction using a bifunctional cross-linker reagent (hexamethylene diisocyanate).65 The biocomposite-based pH sensor has many advantages such as: is reversible, recyclable, stable (under different temperatures at three different pH values of 2, 5 and 10, and for over 1 month at room temperature) and it is able to display different colors for each pH value in the range of 1−14. Due to these interesting properties, the authors suggested that, it could be employed for health monitoring and diagnosis assessment; such as to evaluate certain illnesses for example, infectious and digestive diseases and some types of cancer, or even for the diagnosis of alcoholism, etc; because, the status of many biological systems and processes could be determined by checking the pH changes of human biological fluids such as blood, urine, saliva, etc.
Edwards et al developed a colorimetric and fluorimetric method for the detection of human neutrophil elastase (HNE), secreted by neutrophils and macrophages during inflammation, by using peptide-linked cotton CNCs.66 The sequence, n-Succinyl-Alanine-Alanine-Valine-paranitroanilide (Suc-Ala-Ala-Val-pNA) has been covalently immobilized on glycine esterified CNC as an HNE tripeptide. In presence of HNE, the chromogen para-nitroaniline is enzymatically released from the peptide-conjugated CNCs; its reaction with color amplified reagents enhanced by the visible absorption of the chromogen, has then been employed as the sensing strategy for colorimetric/fluorimetric detection of HNE. The same author also reported the synthesis, characterization of fluorescent analogs and structure/function analysis of peptide biosensors, conjugated to cellulosic and nanocellulosic materials that is applied for fluorescence detection of HNE, as low as 0.05 U/mL in chronic wounds. It has been reported that, among all the cellulosic and nanocellulosic HNE biosensor, the CNC-based presented the highest sensitivity toward HNE due to their high surface area.
Zhou et al have developed a multifunctional glyconanomaterial CNCs-based platform, for the detection of lectin: in particular, the carboxylated CNCs (CCNCs) with double functionality with a fluorescent dye (quinolone) and two carbohydrate ligands (Figure 10).67 The fluorescence intensity of the multifunctional fluorescent CCNCs was significantly reduced in the presence of lectin. Furthermore, the authors showed that the multifunctional fluorescent CCNCs could be employed as bacterial affinity probes for Escherichia coli imaging by targeting lectin receptors at the surface of the bacterial cells.
In 2015, Morales-Narvaez et al have developed different nanopaper-based nanocomposites by incorporating different plasmonic and photoluminescent NPs within the support of BC nanopaper.68 The plasmonic and photoluminescence nanopaper-based nanocomposites were fabricated by different methods such as, the hydroxyl reactive groups of the BC, which have been exploited as a reducing agent for the in situ chemical synthesis of AgNPs within transparent BC nanopaper; BCs acted as a biotemplate/support to embed the AuNPs, using a reducing agent during their synthesis. Alternatively, the attachment of protein/amino-functional photoluminescent NPs and other aminated photoluminescent (nano)-materials, such as, amino-functional carbon dots, photoluminescent graphene oxide, trypan blue and rhodamine, has been achieved onto BCs that were previously carboxylated using a TEMPO-mediated oxidation (Figure 11). The authors also revealed how BC nanopaper could be easily shaped and patterned, by using a wax printing machine or punch tools, in order to generate useful devices, as 2D cuvettes, 2D microwell plates, and spots for single assays. In addition, the authors described the decoration of quantum dots QD/BC with antibodies for targeting a large sized analyte, that is, E. coli, so as to use graphene-oxide (GO) as a pathogen-revealing agent through fluorescence resonance energy transfer (FRET). Because FRET strongly depends on the distance between the photoexcited donors (antibody-decorated QD/BC) and the FRET acceptor (GO), upon addition of GO, the donors will exhibit a strong photoluminescence emission in the presence of the large-sized analyte, whereas, without the large-sized analyte, the donors will be quenched.
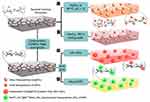 |
Figure 11 Nanopaper-based composites proposed by Morales-Narvaez. Reproduced with permission from Morales-Narváez E, Golmohammadi H, Naghdi T, et al. Nanopaper as an optical sensing platform. ACS Nano. 2015;9(7):7296–7305. Copyright (2015) American Chemical Society.68 |
They confirmed that BC nanopaper, compared to other conventional paper substrates, could provide advantageous preconcentration platform/membrane that facilitates the analysis of small volumes (~4 μL) of optically active materials. It has been reported that the photoluminescent or plasmonic properties of the fabricated nanopaper-based nanocomposites could be tuned using different analytes to perform analytical tasks, including the detection of 2-mercaptobenzothiazole (MBT), cyanide, iodide, thiourea, methimazole, E. coli and ammonia.69
Ruiz-Palomero et al have developed a ß-cyclodextrin-decorated NC as a supermolecular sorbent in a solid phase microextraction (SPME) method for the selective fluorimetric detection of danofloxacin (DAN, a fluoroquinolone antibiotic).70 An amidation reaction was performed to covalently attach ß-cyclodextrin to amine-functional CNCs. This system, adequately reproducible, reusable and selective, was also successfully employed in the fluorimetric recognition of DAN in milk samples.
CNCs in Bioimaging
On account of their cyto- and biocompatibility, biodegradability and strong ability to be functional, fluorescently labeled CNCs have been used to develop emergent diagnostic tools.
Dong and Roman71 were the first authors that reported the potential application of CNCs as bioimaging and bioassay resource. By a covalent bond of fluorescein-5-isothiocyanate (FITC) to the surface of CNCs through a three-step reaction pathway they used for studying the interaction of CNCs with cells and the biodistribution of CNCs in vivo, a crucial point for biomedical applications of nanocellulose. They also synthesized folic acid-conjugated CNCs and their application in cancer targeting: the goal was the folate receptor-positive human and rat brain tumor cells.72 Furthermore, Grate et al and Colombo et al have covalently attached Alexa Fluor dyes onto the surface of CNCs for bioimaging applications in living animals.73,74 In 2016, Navarro et al labeled a graft block copolymer-modified NFC with a lucifer yellow derivative for fabricating a luminescent platform and used this material for fluorescence-based optical sensing (Figure 12). The NFC uptake and biodistribution in living organisms were followed through optical imaging.75
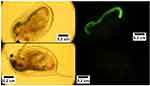 |
Figure 12 Bright field (left) and fluorescence (right) imaging of Daphnia magna fed with control NCFs (bottom) and exposed to lucifer yellow-conjugated NCF (top). Reprinted with permission from Navarro JRG, Wennmalm S, Godfrey J, Breitholtz M, Edlund U. Luminescent nanocellulose platform: from controlled graft block copolymerization to biomarker sensing. Biomacromolecules. 2016;17(3):1101–1109. Copyright (2016) American Chemical Society.75 |
CNCs in Electrical Biosensing
CNC-based platforms could be conductive in electrical sensing devices, giving an electrical signal response. CNCs are essentially nonconductive, for this reason, many modifying agents have been employed to make them conductive; for example, by introducing conducting electroactive (nano) materials into their network.76 The application of cellulose nanomaterials for electrical sensing targets has been revealed for the first time by Bonne et al (2008).77 They used thin films of reconstituted NFCs for coating the surface of glassy carbon electrodes (GCE), which were modified with embedded receptors to introduce selective binding sites for the transport and accumulation of anions and particularly hydrophobic anions. The same research group coated the surface of GCE with a thin film of NFC/chitosan composite (chitosan was employed as a molecular binding site or “receptor”). The relatively high affinity of sodium dodecyl sulfate (SDS) to the NFC/chitosan composite was commended and has suggested that the NFC/chitosan-modified electrode could be potentially used for the accumulation and electrochemical detection of SDS and other anionic surfactants.78
Hydrogen peroxide and glucose could also be detected using hybrid materials such as AuNPs/CNCs. Zhang et al studied the fabrication of AuNP/BC nanocomposites: they described the synthesis of AuNPs within the support of BC by a one-step biotemplated method using poly (ethylenimine) (PEI) as a linking and reducing agent.79 The fabricated AuNP/BC nanocomposites were found to be very good supports for enzyme immobilization. Therefore, the prepared AuNP/BC nanocomposites, coated on the surface of GCE, were functional with horseradish peroxidase (HRP): The resultant HRP/AuNP/BC/GCE exhibited remarkable bioactivity in the reduction of H2O2 and was applied for amperometric detection with a LOD lower than 1 μM.
In 2010, Wang et al fabricated a biosensor for glucose based on the immobilization of two enzymes, glucose oxidase (GOx) and HRP, on the surface of the fabricated AuNP/BC/GCE while retaining their bioactivities. The pretreated GCE was coated by AuNP/BC spread in isopropyl alcohol and then dried in air at room temperature. The modified electrode (AuNP/BC/GCE) was cast with a phosphate buffer solution containing HRP, GOx and poly (diallyldimethylammonium chloride) (PDDAC) at 4°C for 12 h. The constructed bi-enzymatic glucose biosensor showed good stability, reproducibility, selectivity and linearity in the concentration range from 10 to 400 μM with a LOD of 2.3 μM and was successfully applied in the determination of glucose in human blood samples with satisfactory results.80 The same group also gave an important effort in the development of an amperometric biosensor for the detection of H2O2 (evaluated in the presence of hydroquinone (HQ) as an electron mediator), which embedded HRP, myoglobin and hemoglobin into the AuNP/BC/GCE matrix.81 Similarly, Dong et al placed AuNPs on positively charged functional groups of PDDAC/CNCs.82 The surface of GCEs was then modified with the explored AuNP/PDDA/CNC nanohybrids. Subsequently, the modified electrodes were then employed as an electrochemical biosensor for non-enzymatic glucose sensing, which showed a high linear amperometric response, a high sensitivity (ca. 62.8 μA mM−1) and a LOD of 2.4 μM glucose.
Another kind of glucose biosensor has been fabricated via covalent immobilization of GOx enzyme onto a polypyrrole/cellulose nanocrystal (PPy/CNC) membrane by Esmaeili et al (2015).83 Nanocomposites for DNA detection has been investigated by Liu et al, they fabricated AgNP-carboxylated cellulose nanocrystals-based nanocomposites (AgNP/CCNC) by the chemical reduction (using NaBH4) of adsorbed Ag+ onto CCNCs.84 Then, a DNA probe was spliced on the fabricated AgNP/CCNC by the amide linkage in order to achieve sensitive and selective detection of the complementary target DNA sequence: The identification of DNA hybridization was revealed by the electroreduction of Ag+ ions. Similarly, this strategy was employed for creating Ag-Pd NPs alloy/CCNC nanocomposites and investigating their application for electrochemical detection of phosphoenolpyruvate (PEP) gene sequence.85
Mechanical deformations could easily be transduced/transformed into electrical signals (changes of resistance or capacitance) by using strain sensors: These devices have found a wide variety of applications for their promising potentials in interactive electronics, robotic systems, implantable medical devices, human motion detection, human-machine interfaces beside others.86–88 The strain sensitivity of the flexible and electrically conductive nanocomposites has been investigated by Farjana et al who worked on the treatment of BCs with double-walled carbon nanotubes (DWCNTs) and multiwalled carbon nanotubes (MWCNTs), and found out that the latter showed higher strain sensitivity.89
Taking advantage of the extraordinary mechanical properties, easy processing and the abundant availability of NFCs and combining that with the attractive electrical features of CNTs aerogels in a synergistic way, Wang et al invented hybrid nanocomposite aerogels (Figure 13).90 Basically, an aqueous dispersion of functional CNT was first added into the prepared NFC hydrogel and then mixed using a high-speed mixer and ultrasonic bath. Freeze-drying of the hydrogel was eventually performed, tweaking the hydrogel morphology by controlling and modifying the freeze-drying process. Owing to the sponge-like framework of fabricated conductive CNT/NFC aerogels, their electrical properties (resistance or conductivity) could be changed immediately after a pressure is imposed. Therefore, the elastic mechanical properties and electrically reversible compressional behavior of the hybrid conductive nanocomposites were used for mechano-responsive conductivity and pressure sensing.
 |
Figure 13 Schematic representation of the NFC-CNTs nanocomposite aerogels preparation and characterization. (A) Nanofibrillated cellulose (NFC) forms strong physical gels in aqueous medium due to the long and entangled hydrogen bonded native cellulose nanofibers of diameters in the nanometer range. (B) Few-walled carbon nanotubes (FWCNTs) are modified to allow dispersion in the aqueous medium, mixed in the NFC hydrogel and homogenized by ultrasound treatment. (C) The hybrid NFC/FWCNT hydrogel is inserted in a mold, and cooled by plunging into liquid propane or liquid nitrogen and freeze-dried to allow aerogels. (D) Freeze-drying from liquid nitrogen leads to slow cooling and to sheet-like morphology due to aggregation of NFC and FWCNT, whereas freeze-drying from liquid propane leads to quicker cooling and to fibrillar morphology. (E) Cryo-TEM images of NFC/FWCNT 75/25 w/w aerogel; at high magnifi cation the FWCNT (black arrow) can be distinguished from NFC (red arrow). It is clear that NFC and FWCNT are in close contact. (F) Conductivity of NFC/FWCNT aerogels. Reproduced from Wang M, Anoshkin IV, Nasibulin AG, et al. Modifying native nanocellulose aerogels with carbon nanotubes for mechanoresponsive conductivity and pressure sensing. Adv Mater. 2013;25(17):2428–2432. Copyright © 2013 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.90 |
Another advantageous material investigated for (bio) sensing applications are the mesoporous nanopaper, composed of a thin layer of nanopaper film with a remarkable surface roughness and a thick layer of hardwood fibrous network with extraordinary liquid absorption ability. Bao et al developed a high-performance liquid electrolyte-based transistor using a bilayer mesoporous nanopaper via deposition of 2D Van der Waals materials (MoS2, graphene).91 The source, drain and gate contacts were deposited onto the smooth side of the nanopaper, while the electrolyte was absorbed on the mesoporous side of the bilayer nanopaper. The fabricated liquid-gated transistor was then applied for electrical pH sensing, but the authors also suggested that could be potentially used for many different (bio) sensing applications.
Other electrochemical strategies have been developed by Shahrokhian et al who suggested the immobilization of cellulose nanofibers/carbon nanoparticles (NFC/CNPs) on a glassy carbon electrode (CNFs/CNPs/GCE) for voltammetric detection of clonazepam (CLNP) and metoclopramide (MCP).92 The results of this voltammetric analysis showed that the peak current of CLNP and MCP onto the surface of CNFs/CNPs/GCE have been remarkably enhanced (up to 60 times and 49 times for CLNP and MCP, respectively) due to the increase of the electroactive surface area, compared to the bare GCE. Moreover, this approach was successfully employed for performing quantitative analysis of CLNP and MCP in pharmaceutical and clinical preparations.93
CNCs multifunctional behavior and unique features have been successfully employed to produce novel hybrid materials and devices exhibiting excellent electrical character: the optical transparency of NCs has barely been employed with the aim of building innovative devices, taking advantage of photocurrent phenomena or showing electrochromic or electroluminescent properties.
Nanocellulose in Tissue Regeneration and Bioprinting
CNCs for Tissue Engineering
Nanocellulose represents a promising biomaterial for tissue regeneration thanks to its good biocompatibility,94,95 and relatively low toxicity96,98 as well as distinct geometry, tunable surface chemistry, rheology, crystallinity and self-assembly behavior.99,100 All these properties of nanocellulose are useful in scaffold design to enhance structural mechanical properties,101,102 cell adhesion, proliferation and differentiation,103,104 as well as cellular patterning.105,106 Nanocellulose is inherently suitable for tissue engineering supports and allows to create different fabrication shapes, such as membrane-like structures,107,108 hydrogels102,109 and electrospun fibers110 all having controllable porosities and surface chemistries.
Nanocellulose as a matter of fact, does not own properties for tissue regeneration and healing. However, it could provide a versatile platform when blended with other biomaterials to support and promote cellular activities for tissue regeneration and repair. Many examples are found for both hard111,112 and soft113,114 tissue regeneration, although skin and wound dressing remains the most explored and clinically advanced in this field.
Several researches have shown the suitability of nanocellulose hydrogels in biomedical application. Chemical modification that enabled covalent crosslinking of nanocellulose fibers, enhances the mechanical properties of the pristine gel. Prakobna and Kasinee described the addition of galactoglucomannan (GGM) in NFC that significantly enhances the Young’s Modulus of NFC hydrogel from 4.1 MPa to 28.8 MPa for CNF/GGM 13.4 wt%. This strategy also helped in increasing tensile strength from 0.11 MPa (NFC) to 0.65 MPa (CNF/GGM).115 McKee et al established that the storage modulus (G′) could be controlled over an order of magnitude, by adding different concentrations of CNCs to a methylcellulose (MC) network. The hydrogel displayed a large range of elastic modulus (G′) varying from 110 to 900 Pa for concentration of CNC/MC = 3.5/1.0 wt%; this is due to physical crosslinking.116
An interesting work by Eyholzer (2011) reported the production of biocomposite hydrogels with carboxymethylated nanofibrillated cellulose (c-NFC) powdered by UV polymerization of N-vinyl-2-pyrrolidone (NVP) with Tween 20 trimethacrylate as a cross-linking agent with the aim of replacing the native, human nucleus pulposus (NP) in intervertebral disks.45 By varying the amount of c-NFC and its degrees of substitution, it was possible to alter the swelling behavior and compression moduli of the developed biocomposite hydrogels (Figure 14).
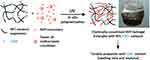 |
Figure 14 Graphical representation of the formation of photopolymerized NFC with NVp monomers and photocurable Tween 20 developed by Eyholzer et al (2011) to replace human NP in intervertebral disks. Reprinted with permission from Eyholzer C, Borges de Couraça A, Duc F, et al. Biocomposite hydrogels with carboxymethylated, nanofibrillated cellulose powder for replacement of the nucleus pulposus. Biomacromolecules. 2011;12(5):1419–1427, copyright (2011) American Chemical Society.45 |
Tuning gel pores or mesh dimensions range into the nanocellulose network could help control the biomolecules diffusion within the hydrogel matrix.117,118 Johns et al (2017) investigated hydrogels regenerated from an ionic liquid/co-solvent mixture (organic electrolyte solution). The effect of the cellulose degree of polymerization (DP) on the resulting pore structure was evaluated using a-cellulose (AC), DP: 500–1300 and bacterial cellulose (BC), DP: 2000–6000. They finally demonstrated that the crystallinity, hydrophobicity and tortuosity of the regenerated hydrogel are dependent on both the type of cellulose and the anti-solvent used (methanol or water) to regenerate the hydrogel, which, in turn affects the affinity of the carbohydrate-binding modules (CBM) for the material and also modulates the rate of CBM migration within the hydrogel.119 The macromolecules diffusion related to the structure architecture has also been studied for hemicellulose composites: it has shown that the effect of model hemicelluloses in the mass transport properties of cellulose networks in highly hydrated environments, is relevant to understanding the role of hemicelluloses in the permeability of plant cell walls and aided the design of plant based materials with tailored properties.120
Membranes-like structures could be formed using different surface modification of nanocellulose and coating with other biomolecules. Innala et al (2014) developed a new in vitro model based on a bacterial nanocellulose (BNC) framework that supports the 3D culturing of neuronal cells, mimicking the complexity of nerve tissue.121 To improve cell adhesion and cell proliferation, the surface of the BNC was modified using trimethyl ammonium betahydroxypropyl (TMAHP) to induce a positive electric charge, followed by coating with collagen I. Cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) was used as alternative surface modifier, covalently binding the proteins such as collagen to the surface. A very recent study by Huang et al described the assembly of chitosan and hyaluronic acid (HA) on a CNCs/HAP matrix, synthesized by a facile in situ HAP coating on the CNCs fibers.108 Due to the CNCs that are tightly coated with HAP particles, the composites show improved mechanical property. The establishment of LbL with bioactive chitosan and hyaluronic acid on the hard CNCs/HAP matrix, also improves its biocompatibility, possessing much potential to be used in the bone tissue engineering.
Recently, Yang et al showed that the incorporation of metal cations (Ce3+) to carboxylated cellulose nanofibres produced stiff NFC-polyacrylamide hydrogels with Young’s modulus of 257 kPa and fracture toughness of 386 kJ/m3; this is 8.5 times higher than the pristine gels (Figure 15).122
 |
Figure 15 (A) Formation of nanocellulose-based ionic hydrogel: a dispersion of CNCs containing acrylamide and photoinitiators is subjected to UV light to polymerize acrylamide, then it is immersed in an aqueous solution of a cation (generally Ce3+) to obtain ionic coordination. (B) The ionic coordination resulted in an increased opacity of the hydrogel, as shown with the corresponding UV-VIS spectra (C). Reprinted with permission from Yang J, Xu F, Han C-R. Metal ion mediated cellulose nanofibrils transient network in covalently cross-linked hydrogels: mechanistic insight into morphology and dynamics. Biomacromolecules. 2017;18(3):1019–1028. Copyright (2017) American Chemical Society.122 |
To recreate the complexity of the in vivo nanoenvironment, electrospinning is another technique employed for nanocellulose production support.101,123,124 This technique enables the creation of 3D porous matrices that mimic the natural structure of the skin. The porosity, stiffness, hydrophilicity, fluid uptake and diffusion, as well as surface area could be changed by modifying the ratio of the constituents in the composites.
A flexible porous nanocomposite fiber mats, based on chitosan–polyethyleneoxide-cellulose nanocrystals were successfully produced using electrospinning by Naseri et al (2015).125 The crosslinked mats reinforced with 50 wt% CNCs produced that formed HCl hydrolysis, had a high tensile strength of 58 MPa and modulus of 3.1 GPa due to good integration of CNCs into the matrix. Moreover, the mats showed permeability to O2 and CO2 as well as compatibility towards the adipose derived stem cells after 7 days; thus, they represent a potential candidate as wound dressing material.
Conversion to cellulose acetate is known to enhance the ability of cellulose of being electrospun, was demonstrated in cellulose extracted from sugar cane bagasse: the electrospun fibrous frameworks then supported the adhesion and growth of mouse subcutaneous fibroblasts of the line L929. The cell behavior was further improved by blending the cellulose with poly (L-lactide) or with polydioxanone.126 Other cellulose acetate-based supports with potential for skin tissue engineering include composite 3D electrospun cellulose acetate/pullulan supports, which promoted the adhesion and growth of mouse L929 fibroblasts, and composite biomimetic nanofibrous gelatin/cellulose acetate/elastin supports, which promoted the adhesion and growth of human gingival fibroblasts.127,128
3D-Bioprinting of Nanocellulose
The field of the 3D bioprinting of nanocellulose is still at the commencement, although in the last decades, the number of researches has exponentially increased. Most of the published papers concerns nanocellulose-reinforced bioinks, where nanocellulose acted as a filler agent since nanocellulose-based structures are able to entangle with each other to form hydrogel networks that have crucial properties of a 3D printable ink, such as shear-thinning (non-Newtonian behavior of fluids whose viscosity decreases under shear strain), strong thickening and sufficiently high yield stress.129 Furthermore, the introduction of charged functional groups to the CNF interface highly increases the colloidal stability of these hydrogels and keeps inks viable for a long time.130 On other hand, CNCs-reinforced inks, designed for 3D printing, offer advantages over the semi-crystalline NFC because higher solid loadings may be reached at a given viscosity and storage modulus due to the absence of physical entanglements.131
Blends with other polymers represent the best choice, in order to improve the printability (in terms of rheological properties) of nanocellulose-based inks. Auxiliary materials such as alginate,132–135 hyaluronic acid136,137 and gelatin138,139 have been explored.
With the aim of adjusting the ink flow and elastic properties, carboxymethylcellulose (CMC) could be used depending on the molecular weight: neat CMC with a MW of 35 kDa acts as a dispersing/gelling agent, enhancing the stiffness of the gel network upon adding amounts up to 2 wt %. Surface modification of CMC in hydrogel form would further favor its applicability. Aldehyde-modified CMC was mixed with hydrazide-modified gelatin, which then readily and rapidly forms a cross-linked hydrogel through the hydrazide/aldehyde coupling reaction during extrusion-based 3D printing. Altering the concentrations of the two components is possible to modify the hydrogel stiffness. The cytocompatibility of the obtained biomaterial has been assessed for vascular endothelial cells, providing a suitable microenvironment for angiogenesis (Figure 16).140
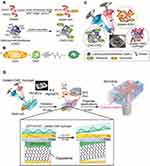 |
Figure 16 (A–C) Preparation of the gelatin-CMC hydrogel suitable for 3D bioprinting, (D) fabrication of vasculature employing HUVEC cells through electrochemical cell transfer from a gold-coated rod. Reprinted with permission from Kageyama T, Osaki T, Enomoto J, et al. In situ cross-linkable gelatin-CMC hydrogels designed for rapid engineering of perfusable vasculatures. ACS Biomater Sci Eng. 2016;2(6):1059–1066. Copyright (2016) American Chemical Society.140 |
The abundant hydroxyl groups in cellulose and the aldehyde and carboxylate groups introduced on the surface of CNCs by TEMPO oxidation provide feasible routes for various chemical cross-linking strategies, which could be directly applied to print NC or indirectly to the auxiliary material. One of the successful methods of nanocellulose 3D-printing involves TEMPO-oxidized NFC hydrogel fabrication based on the double network cross-linking; the first is an in situ CaCl2 cross-linking, the second is a post-printing chemical cross-linking with 1.4-butanediol diglycidyl ether.141 Scaffolds have been successfully printed from 1 wt% NFC ink, with the possibility of tuning mechanical strength of the 3D-printed hydrogels in the range of 3 to 8 kPa. Cytocompatibility tests highlighted human dermal fibroblast cells proliferation on scaffolds, with a remarkable growth improvement with increasing scaffold rigidity. TEMPO oxidation strategy has been adopted also by Leppiniemi et al (2017) for the production of a nanocellulose-alginate hydrogel, suitable for 3D printing.142 The material has also been biofunctional by covalent coupling of an enhanced avidin protein to the nanofibrils of cellulose, providing a generic platform for the immobilization of bioactive components via biotin−avidin interaction, envisioning a potential use in the development of portable biosensors. Rees et al have also successfully printed carboxymethylated periodate-oxidized nanocellulose (C-periodate nanocellulose) on a TEMPO-mediated oxidized nanocellulose film. C-periodate nanocellulose showed a pronounced shear thinning and thixotropic behaviors, enabling the printing process, when used with a high consistency (3.9 wt%).143 However, TEMPO mediated oxidized nanocellulose with a low consistency (0.9 wt%) tends to collapse after drying and leads to failure of the printing. Blending can help to improve the ink printability and structure fidelity using the auxiliary materials, as mentioned earlier: water-soluble lignosulfonate has been applied to adjust the rheological properties of 2 wt% nanocellulose obtained through mechano-enzymatic hydrolysis.144
Inspired by the roles of cellulose and hemicellulose have in the plant cell wall, an interesting result has been obtained by Markstedt et al with tyramine-modified xylan used as both a fiber surface modifier and a biodegradable cross-linker in the formulation of an all wood-based bioink.145 The presence of hydroxyl and carboxylic groups on hemicelluloses makes them suitable for being chemically modified to introduce new functionalities. Several chemical modifications have been studied in order to obtain hydrogels from hemicelluloses, introducing cross-linking such as thiol-functional,146,147 methacrylate derivatives,148 and grafting of acrylic acid.149 It has been previously shown that, hemicelluloses extracted from spruce, such as xylan and galactoglucomannan, could be modified with tyramine, a molecule similar to lignin, which enable the enzymatic cross-linking using horseradish peroxidase.150,151 The enzymatic crosslinking is a fast process and starts within seconds. It is also an irreversible reaction and results in a stable hydrogel (Figure 17).
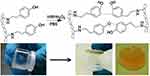 |
Figure 17 Chemical crosslinking of tyramine residues on xylan polymeric chains initiated by H2O2 (top) and tilt-test. Reproduced from Kuzmenko V, Hägg D, Toriz G, Gatenholm P. In situ forming spruce xylan-based hydrogel for cell immobilization. Carbohydr Polym. 2014;102(1):862–868.150 |
Conclusions
In conclusion, this review article has highlighted the opportunities arising from a proper chemical modification of the surface of nanocellulose in order to obtain a material with specific and desired properties for application in the complex and demanding field of nanomedicine. After a rich, yet specific, paragraph on chemical reactions for surface modification methodologies, a special focus have been posed on those applications that gained particular benefit from the surface modification and on the future perspectives that may arise in all the fields of innovative medicine. This specific focus may guide and elucidate the role and the advantages that easy reactions could be allowed to obtain, if used for surface modification of an already promising nanomaterial such as nanocellulose, and at the same time may highlight the future possibilities that could still be explored in this fascinating world.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chin K-M, Sung Ting S, Ong HL, Omar M. Surface functionalized nanocellulose as a veritable inclusionary material in contemporary bioinspired applications: A review. J Appl Polym Sci. 2018;135(13):46065. doi:10.1002/app.46065
2. Gorgieva T. Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials. 2019;9(10):1352. doi:10.3390/nano9101352
3. Salimi S, Sotudeh-Gharebagh R, Zarghami R, Chan SY, Yuen KH. Production of nanocellulose and its applications in drug delivery: a critical review. ACS Sustain Chem Eng. 2019;7(19):15800–15827. doi:10.1021/acssuschemeng.9b02744
4. Huang L, Chen X, Nguyen TX, Tang H, Zhang L, Yang G. Nano-cellulose 3D-networks as controlled-release drug carriers. J Mater Chem B. 2013;1(23):2976–2984. doi:10.1039/c3tb20149j
5. Pachuau L. Application of nanocellulose for controlled drug delivery. In: Jawaid M and Mohammad F, eds. Nanocellulose and Nanohydrogel Matrices. Weinheim, Germany. Wiley-VCH Verlag GmbH & Co. KGaA. 2017. 1–19. doi:10.1002/9783527803835.ch1
6. Zhu G, Lin N. Surface chemistry of nanocellulose. In: Huang J, Dufresne A and Lin N, eds. Nanocellulose. Weinheim: Germany: Wiley-VCH Verlag GmbH & Co. KGaA;2019:115–153. doi:10.1002/9783527807437.ch5
7. Kovalenko A. Predictive multiscale modeling of nanocellulose based materials and systems. IOP Conf Ser Mater Sci Eng. 2014;64(1):012040. doi:10.1088/1757-899X/64/1/012040
8. Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev. 2010;110(6):3479–3500. doi:10.1021/cr900339w
9. Klemm D, Kramer F, Moritz S, et al. Nanocelluloses: a new family of nature-based materials. Angew Chemie Int Ed. 2011;50(24):5438–5466. doi:10.1002/anie.201001273
10. Hebeish A, Guthrie JT The Chemistry and Technology of Cellulosic Copolymers. Vol 53. (Intergovernmental Panel on Climate Change, ed). Berlin, Heidelberg: Springer Berlin Heidelberg; 1981. doi:10.1007/978-3-642-67707-6
11. Çetin NS, Tingaut P, Özmen N, et al. Acetylation of cellulose nanowhiskers with vinyl acetate under moderate conditions. Macromol Biosci. 2009;9(10):997–1003. doi:10.1002/mabi.200900073
12. Lin N, Huang J, Dufresne A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale. 2012;4(11):3274. doi:10.1039/c2nr30260h
13. Lewandowska-Łańcucka J, Karewicz A, Wolski K, Zapotoczny S. Surface functionalization of nanocellulose-based hydrogels. In: Mondal MK, editor. Cellulose-Based Superabsorbent Hydrogels. Springer; 2019:705–733. doi:10.1007/978-3-319-77830-3_24
14. Patel DK, Dutta SD, Lim K-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019;9(33):19143–19162. doi:10.1039/C9RA03261D
15. George J, S N S. Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl. 2015;8(45). doi:10.2147/NSA.S64386
16. Beck-Candanedo S, Roman M, Gray DG. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules. 2005;6(2):1048–1054. doi:10.1021/bm049300p
17. Trache D, Hussin MH, Haafiz MKM, Thakur VK. Recent progress in cellulose nanocrystals: sources and production. Nanoscale. 2017;9(5):1763–1786. doi:10.1039/C6NR09494E
18. Sucaldito MR, Camacho DH. Characteristics of unique HBr-hydrolyzed cellulose nanocrystals from freshwater green algae (Cladophora rupestris) and its reinforcement in starch-based film. Carbohydr Polym. 2017;169:315–323. doi:10.1016/j.carbpol.2017.04.031
19. Camarero Espinosa S, Kuhnt T, Foster EJ, Weder C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules. 2013;14(4):1223–1230. doi:10.1021/bm400219u
20. Chen L, Zhu JY, Baez C, Kitin P, Elder T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016;18(13):3835–3843. doi:10.1039/C6GC00687F
21. Braun B, Dorgan JR. Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules. 2009;10(2):334–341. doi:10.1021/bm8011117
22. Cellante L, Costa R, Monaco I, Cenacchi G, Locatelli E. One-step esterification of nanocellulose in a Brønsted acid ionic liquid for delivery to glioblastoma cancer cells. New J Chem. 2018;42(7):5237–5242. doi:10.1039/C7NJ04633B
23. Saito T, Nishiyama Y, Putaux J, Vignon M, Isogai A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules. 2006;7(6):1687–1691. doi:10.1021/bm060154s
24. Habibi Y, Chanzy H, Vignon MR. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose. 2006;13(6):679–687. doi:10.1007/s10570-006-9075-y
25. de Nooy AEJ, Besemer AC, van Bekkum H. Highly selective tempo mediated oxidation of primary alcohol groups in polysaccharides. Recl des Trav Chim des Pays-Bas. 2010;113(3):165–166. doi:10.1002/recl.19941130307
26. Tardy BL, Yokota S, Ago M, et al. Nanocellulose–surfactant interactions. Curr Opin Colloid Interface Sci. 2017;29:57–67. doi:10.1016/j.cocis.2017.02.004
27. Hubbe MA, Rojas OJ, Lucia LA. Green modification of surface characteristics of cellulosic materials at the molecular or nano scale: a review. Bio Resources. 2015;10(3):6095–6206. doi:10.15376/biores.10.3.Hubbe
28. Aloulou F, Boufi S, Belgacem N, Gandini A. Adsorption of cationic surfactants and subsequent adsolubilization of organic compounds onto cellulose fibers. Colloid Polym Sci. 2004;283(3):344–350. doi:10.1007/s00396-004-1143-y
29. Letchford J, Wasserman B, Ye HW, Burt H. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int J Nanomedicine. 2011;6:321. doi:10.2147/IJN.S16749
30. Trabelsi S, Raspaud E, Langevin D. Aggregate formation in aqueous solutions of carboxymethylcellulose and cationic surfactants. Langmuir. 2007;23(20):10053–10062. doi:10.1021/la7016177
31. Wang G, Olofsson G. Ethyl hydroxyethyl cellulose and ionic surfactants in dilute solution. Calorimetric and viscosity study of the interaction with sodium dodecyl sulfate and some cationic surfactants. J Phys Chem. 1995;99(15):5588–5596. doi:10.1021/j100015a049
32. Weishaupt R, Heuberger L, Siqueira G, et al. Enhanced antimicrobial activity and structural transitions of a nanofibrillated cellulose–nisin biocomposite suspension. ACS Appl Mater Interfaces. 2018;10(23):20170–20181. doi:10.1021/acsami.8b04470
33. Cranston ED, Gray DG. Morphological and optical characterization of polyelectrolyte multilayers incorporating nanocrystalline cellulose. Biomacromolecules. 2006;7(9):2522–2530. doi:10.1021/bm0602886
34. Jiang C, Markutsya S, Pikus Y, Tsukruk VV. Freely suspended nanocomposite membranes as highly sensitive sensors. Nat Mater. 2004;3(10):721–728. doi:10.1038/nmat1212
35. Utsel S, Malmström EE, Carlmark A, Wågberg L. Thermoresponsive nanocomposites from multilayers of nanofibrillated cellulose and specially designed N-isopropylacrylamide based polymers. Soft Matter. 2010;6(2):342–352. doi:10.1039/B910481J
36. Abitbol T, Rivkin A, Cao Y, et al. Nanocellulose, a tiny fiber with huge applications. Curr Opin Biotechnol. 2016;39(I):76–88. doi:10.1016/j.copbio.2016.01.002
37. Wang N, Ding E, Cheng R. Preparation and liquid crystalline properties of spherical cellulose nanocrystals. Langmuir. 2008;24(1):5–8. doi:10.1021/la702923w
38. Araki J, Wada M, Kuga S, Okano T. Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. J Wood Sci. 1999;45(3):258–261. doi:10.1007/BF01177736
39. Lin N, Dufresne A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale. 2014;6(10):5384–5393. doi:10.1039/C3NR06761K
40. Liimatainen H, Visanko M, Sirviö J, Hormi O, Niinimäki J. Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose. 2013;20(2):741–749. doi:10.1007/s10570-013-9865-y
41. Kim U-J, Kuga S, Wada M, Okano T, Kondo T. Periodate oxidation of crystalline cellulose. Biomacromolecules. 2000;1(3):488–492. doi:10.1021/bm0000337
42. Sun B, Hou Q, Liu Z, Ni Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose. 2015;22(2):1135–1146. doi:10.1007/s10570-015-0575-5
43. Volkert B, Wolf B, Fischer S, Li N, Lou C. Application of modified bead cellulose as a carrier of active ingredients. Macromol Symp. 2009;280(1):130–135. doi:10.1002/masy.200950615
44. Dash R, Ragauskas AJ. Synthesis of a novel cellulose nanowhisker-based drug delivery system. RSC Adv. 2012;2(8):3403–3409. doi:10.1039/c2ra01071b
45. Eyholzer C, Borges de Couraça A, Duc F, et al. Biocomposite hydrogels with carboxymethylated, nanofibrillated cellulose powder for replacement of the nucleus pulposus. Biomacromolecules. 2011;12(5):1419–1427. doi:10.1021/bm101131b
46. Eyholzer C, Bordeanu N, Lopez-Suevos F, Rentsch D, Zimmermann T, Oksman K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose. 2010;17(1):19–30. doi:10.1007/s10570-009-9372-3
47. Wang Y, Wang X, Xie Y, Zhang K. Functional nanomaterials through esterification of cellulose: a review of chemistry and application. Cellulose. 2018;25(7):3703–3731. doi:10.1007/s10570-018-1830-3
48. Galkina OL, Ivanov VK, Agafonov AV, Seisenbaeva GA, Kessler VG. Cellulose nanofiber–titania nanocomposites as potential drug delivery systems for dermal applications. J Mater Chem B. 2015;3(8):1688–1698. doi:10.1039/C4TB01823K
49. Evdokimova O, Svensson F, Agafonov A, Håkansson S, Seisenbaeva G, Kessler V. Hybrid drug delivery patches based on spherical cellulose nanocrystals and colloid titania—synthesis and antibacterial properties. Nanomaterials. 2018;8(4):228. doi:10.3390/nano8040228
50. Yang Q, Pan X. A facile approach for fabricating fluorescent cellulose. J Appl Polym Sci. 2010;116(5):
51. Kalaskar DM, Ulijn RV, Gough JE, et al. Characterisation of amino acid modified cellulose surfaces using ToF-SIMS and XPS. Cellulose. 2010;17(4):747–756. doi:10.1007/s10570-010-9413-y
52. Cateto CA, Ragauskas A. Amino acid modified cellulose whiskers. RSC Adv. 2011;1(9):1695. doi:10.1039/c1ra00647a
53. Akhlaghi SP, Berry RC, Tam KC. Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose. 2013;20(4):1747–1764. doi:10.1007/s10570-013-9954-y
54. Pahimanolis N, Hippi U, Johansson L-S, et al. Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose. 2011;18(5):1201. doi:10.1007/s10570-011-9573-4
55. Feese E, Sadeghifar H, Gracz HS, Argyropoulos DS, Ghiladi RA. Photobactericidal porphyrin-cellulose nanocrystals: synthesis, characterization, and antimicrobial properties. Biomacromolecules. 2011;12(10):3528–3539. doi:10.1021/bm200718s
56. Tingaut P, Hauert R, Zimmermann T. Highly efficient and straightforward functionalization of cellulose films with thiol-ene click chemistry. J Mater Chem. 2011;21(40):16066. doi:10.1039/c1jm11620g
57. Guo J, Fang W, Welle A, et al. Superhydrophobic and slippery lubricant-infused flexible transparent nanocellulose films by photoinduced thiol–ene functionalization. ACS Appl Mater Interfaces. 2016;8(49):34115–34122. doi:10.1021/acsami.6b11741
58. Jackson JK, Letchford K, Wasserman BZ, et al. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int J Nanomed. 2011;6:321–330. doi:10.2147/IJN.S16749
59. Valo H, Kovalainen M, Laaksonen P, et al. Immobilization of protein-coated drug nanoparticles in nanofibrillar cellulose matrices-Enhanced stability and release. J Control Release. 2011;156(3):390–397. doi:10.1016/j.jconrel.2011.07.016
60. Valo H, Arola S, Laaksonen P, et al. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur J Pharm Sci. 2013;50(1):69–77. doi:10.1016/j.ejps.2013.02.023
61. Asabuwa Ngwabebhoh F, Ilkar Erdagi S, Yildiz U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: in vitro release, anticancer and antimicrobial activities. Carbohydr Polym. 2018;201(August):317–328. doi:10.1016/j.carbpol.2018.08.079
62. Díez I, Eronen P, Österberg M, Linder MB, Ikkala O, Ras RHA. Functionalization of nanofibrillated cellulose with silver nanoclusters: fluorescence and antibacterial activity. Macromol Biosci. 2011;11(9):1185–1191. doi:10.1002/mabi.201100099
63. Marques PAAP, Nogueira HIS, Pinto RJB, Neto CP, Trindade T. Silver-bacterial cellulosic sponges as active SERS substrates. J Raman Spectrosc. 2008;39(4):439–443. doi:10.1002/jrs.1853
64. Wei H, Vikesland PJ. pH-triggered molecular alignment for reproducible SERS detection via an AuNP/nanocellulose platform. Sci Rep. 2015;5(1):18131. doi:10.1038/srep18131
65. Devarayan K, Kim B-S. Reversible and universal pH sensing cellulose nanofibers for health monitor. Sensors Actuators B Chem. 2015;209:281–286. doi:10.1016/j.snb.2014.11.120
66. Edwards JV, Prevost N, Sethumadhavan K, Ullah A, Condon B. Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose. 2013;20(3):1223–1235. doi:10.1007/s10570-013-9901-y
67. Zhou J, Butchosa N, Jayawardena HSN, et al. Synthesis of multifunctional cellulose nanocrystals for lectin recognition and bacterial imaging. Biomacromolecules. 2015;16(4):1426–1432. doi:10.1021/acs.biomac.5b00227
68. Morales-Narváez E, Golmohammadi H, Naghdi T, et al. Nanopaper as an optical sensing platform. ACS Nano. 2015;9(7):7296–7305. doi:10.1021/acsnano.5b03097
69. Pourreza N, Golmohammadi H, Naghdi T, Yousefi H. Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens Bioelectron. 2015;74:353–359. doi:10.1016/j.bios.2015.06.041
70. Ruiz-Palomero C, Soriano ML, Valcárcel M. β-Cyclodextrin decorated nanocellulose: a smart approach towards the selective fluorimetric determination of danofloxacin in milk samples. Analyst. 2015;140(10):3431–3438. doi:10.1039/C4AN01967A
71. Dong S, Roman M. Fluorescently labeled cellulose nanocrystals for bioimaging applications. J Am Chem Soc. 2007;129(45):13810–13811. doi:10.1021/ja076196l
72. Dong S, Cho HJ, Lee YW, Roman M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules. 2014;15(5):1560–1567. doi:10.1021/bm401593n
73. Grate JW, Mo K-F, Shin Y, et al. Alexa fluor-labeled fluorescent cellulose nanocrystals for bioimaging solid cellulose in spatially structured microenvironments. Bioconjug Chem. 2015;26(3):593–601. doi:10.1021/acs.bioconjchem.5b00048
74. Colombo L, Zoia L, Violatto MB, et al. Organ distribution and bone tropism of cellulose nanocrystals in living mice. Biomacromolecules. 2015;16(9):2862–2871. doi:10.1021/acs.biomac.5b00805
75. Navarro JRG, Wennmalm S, Godfrey J, Breitholtz M, Edlund U. Luminescent nanocellulose platform: from controlled graft block copolymerization to biomarker sensing. Biomacromolecules. 2016;17(3):1101–1109. doi:10.1021/acs.biomac.5b01716
76. Shi Z, Phillips GO, Yang G. Nanocellulose electroconductive composites. Nanoscale. 2013;5(8):3194. doi:10.1039/c3nr00408b
77. Bonné MJ, Edler KJ, Buchanan JG, et al. Thin-film modified electrodes with reconstituted cellulose−PDDAC films for the accumulation and detection of triclosan. J Phys Chem C. 2008;112(7):2660–2666. doi:10.1021/jp709783k
78. Tsourounaki K, Bonné MJ, Thielemans W, et al. Nanofibrillar cellulose-chitosan composite film electrodes: competitive binding of triclosan, Fe(CN) 6 3−/4−, and SDS surfactant. Electroanalysis. 2008;20(22):2395–2402. doi:10.1002/elan.200804338
79. Zhang T, Wang W, Zhang D, et al. Biotemplated synthesis of gold nanoparticle-bacteria cellulose nanofiber nanocomposites and their application in biosensing. Adv Funct Mater. 2010;20(7):1152–1160. doi:10.1002/adfm.200902104
80. Wang W, Li H-Y, Zhang D-W, et al. Fabrication of bienzymatic glucose biosensor based on novel gold nanoparticles-bacteria cellulose nanofibers nanocomposite. Electroanalysis. 2010;22(21):2543–2550. doi:10.1002/elan.201000235
81. Wang W, Zhang T-J, Zhang D-W, et al. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta. 2011;84(1):71–77. doi:10.1016/j.talanta.2010.12.015
82. Dong L, Zhang X, Ren S, et al. Poly(diallyldimethylammonium chloride)–cellulose nanocrystals supported Au nanoparticles for nonenzymatic glucose sensing. RSC Adv. 2016;6(8):6436–6442. doi:10.1039/C5RA23935D
83. Esmaeili C, Abdi M, Mathew A, Jonoobi M, Oksman K, Rezayi M. Synergy effect of nanocrystalline cellulose for the biosensing detection of glucose. Sensors. 2015;15(10):24681–24697. doi:10.3390/s151024681
84. Liu H, Wang D, Song Z, Shang S. Preparation of silver nanoparticles on cellulose nanocrystals and the application in electrochemical detection of DNA hybridization. Cellulose. 2011;18(1):67–74. doi:10.1007/s10570-010-9464-0
85. Liu H, Wang D, Shang S, Song Z. Synthesis and characterization of Ag–Pd alloy nanoparticles/carboxylated cellulose nanocrystals nanocomposites. Carbohydr Polym. 2011;83(1):38–43. doi:10.1016/j.carbpol.2010.07.019
86. Amjadi M, Kyung K-U, Park I, Sitti M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: a review. Adv Funct Mater. 2016;26(11):1678–1698. doi:10.1002/adfm.201504755
87. Li X, Ballerini DR, Shen W. A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics. 2012;6(1):011301. doi:10.1063/1.3687398
88. Tang W, Yan T, Ping J, Wu J, Ying Y. Rapid fabrication of flexible and stretchable strain sensor by chitosan-based water ink for plants growth monitoring. Adv Mater Technol. 2017;2(7):1700021. doi:10.1002/admt.201700021
89. Farjana S, Toomadj F, Lundgren P, Sanz-Velasco A, Naboka O, Enoksson P. Conductivity-dependent strain response of carbon nanotube treated bacterial nanocellulose. J Sensors. 2013;2013:1–7. doi:10.1155/2013/741248
90. Wang M, Anoshkin IV, Nasibulin AG, et al. Modifying native nanocellulose aerogels with carbon nanotubes for mechanoresponsive conductivity and pressure sensing. Adv Mater. 2013;25(17):2428–2432. doi:10.1002/adma.201300256
91. Bao W, Fang Z, Wan J, et al. Aqueous gating of van der waals materials on bilayer nanopaper. ACS Nano. 2014;8(10):10606–10612. doi:10.1021/nn504125b
92. Shahrokhian S, Balotf H, Ghalkhani M. Nano composite coating based on cellulose nanofibers/carbon nanoparticles: application to voltammetric determination of clonazepam. J Solid State Electrochem. 2015;19(1):251–260. doi:10.1007/s10008-014-2597-6
93. Shahrokhian S, Naderi L, Ghalkhani M. Nanocellulose/carbon nanoparticles nanocomposite film modified electrode for durable and sensitive electrochemical determination of metoclopramide. Electroanalysis. 2015;27(11):2637–2644. doi:10.1002/elan.201500266
94. Helenius G, Bäckdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res Part A. 2006;76A(2):431–438. doi:10.1002/jbm.a.30570
95. Martínez Ávila H, Schwarz S, Feldmann E-M, et al. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl Microbiol Biotechnol. 2014;98(17):7423–7435. doi:10.1007/s00253-014-5819-z
96. Roman M. Toxicity of cellulose nanocrystals: a review. Ind Biotechnol. 2015;11(1):25–33. doi:10.1089/ind.2014.0024
97. Alexandrescu L, Syverud K, Gatti A, Chinga-Carrasco G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose. 2013;20(4):1765–1775. doi:10.1007/s10570-013-9948-9
98. Pereira MM, Raposo NRB, Brayner R, et al. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology. 2013;24(7):075103. doi:10.1088/0957-4484/24/7/075103
99. Courtenay JC, Deneke C, Lanzoni EM, et al. Modulating cell response on cellulose surfaces; tunable attachment and scaffold mechanics. Cellulose. 2018;25(2):925–940. doi:10.1007/s10570-017-1612-3
100. Fontana G, Gershlak J, Adamski M, et al. Biofunctionalized plants as diverse biomaterials for human cell culture. Adv Healthc Mater. 2017;6(8):1601225. doi:10.1002/adhm.201601225
101. He X, Xiao Q, Lu C, et al. Uniaxially aligned electrospun all-cellulose nanocomposite nanofibers reinforced with cellulose nanocrystals: scaffold for tissue engineering. Biomacromolecules. 2014;15(2):618–627. doi:10.1021/bm401656a
102. Domingues RMAA, Silva M, Gershovich P, et al. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjug Chem. 2015;26(8):1571–1581. doi:10.1021/acs.bioconjchem.5b00209
103. Martínez Ávila H, Feldmann E-M, Pleumeekers MM, et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials. 2015;44:122–133. doi:10.1016/j.biomaterials.2014.12.025
104. Jonsson M, Brackmann C, Puchades M, et al. Neuronal networks on nanocellulose scaffolds. Tissue Eng Part C Methods. 2015;21(11):1162–1170. doi:10.1089/ten.tec.2014.0602
105. Dugan JM, Gough JE, Eichhorn SJ. Directing the morphology and differentiation of skeletal muscle cells using oriented cellulose nanowhiskers. Biomacromolecules. 2010;11(9):2498–2504. doi:10.1021/bm100684k
106. Berti FV, Rambo CR, Dias PF, Porto LM. Nanofiber density determines endothelial cell behavior on hydrogel matrix. Mater Sci Eng C. 2013;33(8):4684–4691. doi:10.1016/j.msec.2013.07.029
107. de Oliveira CR. Bacterial cellulose membranes constitute biocompatible biomaterials for mesenchymal and induced pluripotent stem cell culture and tissue engineering. J Tissue Sci Eng. 2012;S11. 10.4172/2157-7552.S11-005.
108. Huang C, Fang G, Zhao Y, et al. Bio-inspired nanocomposite by layer-by-layer coating of chitosan/hyaluronic acid multilayers on a hard nanocellulose-hydroxyapatite matrix. Carbohydr Polym. 2019;222:
109. Luo H, Xiong G, Hu D, et al. Characterization of TEMPO-oxidized bacterial cellulose scaffolds for tissue engineering applications. Mater Chem Phys. 2013;143(1):373–379. doi:10.1016/j.matchemphys.2013.09.012
110. Hsieh Y-L. Cellulose nanofibers: electrospinning and nanocellulose self-assemblies. In: Netravali A, editor. Advanced Green Composites. Vol. 16. Hoboken, NJ, USA: John Wiley & Sons, Inc.;2018:67–95. doi:10.1002/9781119323327.ch4
111. Kim DS, Jung S-M, Yoon GH, Lee HC, Shin HS. Development of a complex bone tissue culture system based on cellulose nanowhisker mechanical strain. Colloids Surfaces B Biointerfaces. 2014;123:838–844. doi:10.1016/j.colsurfb.2014.10.031
112. Torgbo S, Sukyai P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl Mater Today. 2018;11:34–49. doi:10.1016/j.apmt.2018.01.004
113. Mathew AP, Oksman K, Pierron D, Harmand M-F. Fibrous cellulose nanocomposite scaffolds prepared by partial dissolution for potential use as ligament or tendon substitutes. Carbohydr Polym. 2012;87(3):2291–2298. doi:10.1016/j.carbpol.2011.10.063
114. Mathew AP, Oksman K, Pierron D, Harmad M-F. Crosslinked fibrous composites based on cellulose nanofibers and collagen with in situ pH induced fibrillation. Cellulose. 2012;19(1):139–150. doi:10.1007/s10570-011-9624-x
115. Prakobna K, Kisonen V, Xu C, Berglund LA. Strong reinforcing effects from galactoglucomannan hemicellulose on mechanical behavior of wet cellulose nanofiber gels. J Mater Sci. 2015;50(22):7413–7423. doi:10.1007/s10853-015-9299-z
116. McKee JR, Hietala S, Seitsonen J, Laine J, Kontturi E, Ikkala O. Thermoresponsive nanocellulose hydrogels with tunable mechanical properties. ACS Macro Lett. 2014;3(3):266–270. doi:10.1021/mz400596g
117. Hickey RJ, Modulevsky DJ, Cuerrier CM, Pelling AE. Customizing the shape and microenvironment biochemistry of biocompatible macroscopic plant-derived cellulose scaffolds. ACS Biomater Sci Eng. 2018;4(11):3726–3736. doi:10.1021/acsbiomaterials.8b00178
118. Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8(5):607–626. doi:10.1586/erd.11.27
119. Johns MA, Bernardes A, De Azevêdo ER, et al. On the subtle tuneability of cellulose hydrogels: implications for binding of biomolecules demonstrated for CBM 1. J Mater Chem B. 2017;5(21):3879–3887. doi:10.1039/C7TB00176B
120. Lopez-Sanchez P, Schuster E, Wang D, Gidley MJ, Strom A. Diffusion of macromolecules in self-assembled cellulose/hemicellulose hydrogels. Soft Matter. 2015;11(20):4002–4010. doi:10.1039/C5SM00103J
121. Innala M, Riebe I, Kuzmenko V, et al. 3D Culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif Cells, Nanomed, Biotechnol. 2014;42(5):302–308. doi:10.3109/21691401.2013.821410
122. Yang J, Xu F, Han C-R. Metal ion mediated cellulose nanofibrils transient network in covalently cross-linked hydrogels: mechanistic insight into morphology and dynamics. Biomacromolecules. 2017;18(3):1019–1028. doi:10.1021/acs.biomac.6b01915
123. Liu X, Lin T, Gao Y, et al. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J Biomed Mater Res Part B Appl Biomater. 2012;100B(6):1556–1565. doi:10.1002/jbm.b.32724
124. Vatankhah E, Prabhakaran MP, Jin G, Mobarakeh LG, Ramakrishna S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J Biomater Appl. 2014;28(6):909–921. doi:10.1177/0885328213486527
125. Naseri N, Mathew AP, Girandon L, Fröhlich M, Oksman K. Porous electrospun nanocomposite mats based on chitosan–cellulose nanocrystals for wound dressing: effect of surface characteristics of nanocrystals. Cellulose. 2015;22(1):521–534. doi:10.1007/s10570-014-0493-y
126. Ramphul H, Bhaw-Luximon A, Jhurry D. Sugar-cane bagasse derived cellulose enhances performance of polylactide and polydioxanone electrospun scaffold for tissue engineering. Carbohydr Polym. 2017;178(September):238–250. doi:10.1016/j.carbpol.2017.09.046
127. Atila D, Keskin D, Tezcaner A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr Polym. 2015;133:251–261. doi:10.1016/j.carbpol.2015.06.109
128. Khalili S, Nouri Khorasani S, Razavi M, Hashemi Beni B, Heydari F, Tamayol A. Nanofibrous scaffolds with biomimetic structure. J Biomed Mater Res Part A. 2018;106(2):370–376. doi:10.1002/jbm.a.36246
129. Kuzmenko V, Karabulut E, Pernevik E, Enoksson P, Gatenholm P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr Polym. 2018;189(February):22–30. doi:10.1016/j.carbpol.2018.01.097
130. Fall AB, Lindström SB, Sundman O, Ödberg L, Wågberg L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir. 2011;27(18):11332–11338. doi:10.1021/la201947x
131. Siqueira G, Kokkinis D, Libanori R, et al. Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv Funct Mater. 2017;27(12):1604619. doi:10.1002/adfm.201604619
132. Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–1496. doi:10.1021/acs.biomac.5b00188
133. Martínez Ávila H, Schwarz S, Rotter N, Gatenholm P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting. 2016;12:22–35. doi:10.1016/j.bprint.2016.08.003
134. Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng. 2017;45(1):210–223. doi:10.1007/s10439-016-1704-5
135. Jessop ZM, Al-Sabah A, Gao N, et al. Printability of pulp derived crystal, fibril and blend nanocellulose-alginate bioinks for extrusion 3D bioprinting. Biofabrication. 2019;11(4):045006. doi:10.1088/1758-5090/ab0631
136. Henriksson I, Gatenholm P, Hägg DA. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication. 2017;9(1):015022. doi:10.1088/1758-5090/aa5c1c
137. Nguyen D, Hägg DA, Forsman A, et al. Cartilage tissue engineering by the 3D BIoprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep. 2017;7(1):658. doi:10.1038/s41598-017-00690-y
138. Xu W, Molino BZ, Cheng F, et al. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing toward wound healing application. ACS Appl Mater Interfaces. 2019;11(9):8838–8848. doi:10.1021/acsami.8b21268
139. Xu X, Zhou J, Jiang Y, Zhang Q, Shi H, Liu D. 3D printing process of oxidized nanocellulose and gelatin scaffold. J Biomater Sci Polym Ed. 2018;29(12):1498–1513. doi:10.1080/09205063.2018.1472450
140. Kageyama T, Osaki T, Enomoto J, et al. In situ cross-linkable gelatin-CMC hydrogels designed for rapid engineering of perfusable vasculatures. ACS Biomater Sci Eng. 2016;2(6):1059–1066. doi:10.1021/acsbiomaterials.6b00203
141. Xu C, Zhang Molino B, Wang X, et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J Mater Chem B. 2018;6(43):7066–7075. doi:10.1039/C8TB01757C
142. Leppiniemi J, Lahtinen P, Paajanen A, et al. 3D-printable bioactivated nanocellulose–alginate hydrogels. ACS Appl Mater Interfaces. 2017;9(26):21959–21970. doi:10.1021/acsami.7b02756
143. Rees A, Powell LC, Chinga-Carrasco G, et al. 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. Biomed Res Int. 2015;2015:1–7. doi:10.1155/2015/925757
144. Shao Y, Chaussy D, Grosseau P, Beneventi D. Use of microfibrillated cellulose/lignosulfonate blends as carbon precursors: impact of hydrogel rheology on 3D printing. Ind Eng Chem Res. 2015;54(43):10575–10582. doi:10.1021/acs.iecr.5b02763
145. Markstedt K, Escalante A, Toriz G, Gatenholm P. Biomimetic inks based on cellulose nanofibrils and cross-linkable xylans for 3D printing. ACS Appl Mater Interfaces. 2017;9(46):40878–40886. doi:10.1021/acsami.7b13400
146. Pahimanolis N, Kilpeläinen P, Master E, Ilvesniemi H, Seppälä J. Novel thiol- amine- and amino acid functional xylan derivatives synthesized by thiol–ene reaction. Carbohydr Polym. 2015;131:392–398. doi:10.1016/j.carbpol.2015.06.007
147. Maleki L, Edlund U, Albertsson A-C. Thiolated hemicellulose as a versatile platform for one-pot click-type hydrogel synthesis. Biomacromolecules. 2015;16(2):667–674. doi:10.1021/bm5018468
148. Dax D, Chávez MS, Xu C, Willför S, Mendonça RT, Sánchez J. Cationic hemicellulose-based hydrogels for arsenic and chromium removal from aqueous solutions. Carbohydr Polym. 2014;111:797–805. doi:10.1016/j.carbpol.2014.05.045
149. Sun X-F, Wang H, Jing Z, Mohanathas R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr Polym. 2013;92(2):1357–1366. doi:10.1016/j.carbpol.2012.10.032
150. Kuzmenko V, Hägg D, Toriz G, Gatenholm P. In situ forming spruce xylan-based hydrogel for cell immobilization. Carbohydr Polym. 2014;102(1):862–868. doi:10.1016/j.carbpol.2013.10.077
151. Markstedt K, Xu W, Liu J, Xu C, Gatenholm P. Synthesis of tunable hydrogels based on O-acetyl-galactoglucomannans from spruce. Carbohydr Polym. 2017;157:1349–1357. doi:10.1016/j.carbpol.2016.11.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
