Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Surface characteristics of and in vitro behavior of osteoblast-like cells on titanium with nanotopography prepared by high-energy shot peening
Authors Deng Z, Yin B, Li W, Liu J, Yang J, Zheng T, Zhang D, Yu H, Liu X, Ma J
Received 23 July 2014
Accepted for publication 25 October 2014
Published 28 November 2014 Volume 2014:9(1) Pages 5565—5573
DOI https://doi.org/10.2147/IJN.S71625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Zhennan Deng,1,2 Baodi Yin,1 Weihong Li,1 Jinsong Liu,1 Jingyuan Yang,1 Tieli Zheng,1 Dafeng Zhang,1 Haiyang Yu,2 Xiaoguang Liu,3 Jianfeng Ma1
1School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, 3National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan, People’s Republic of China
Background and methods: Commercial pure titanium with nanotopography was prepared via a high-energy shot-peening (HESP) technique. The surface characteristics were evaluated, and the preliminary cell responses to the nanotopographical surface were investigated.
Results: The nanotopographical surface layer on titanium was successfully processed by HESP. The average nanoscale grains were approximately 60 nm in diameter and they were nonhomogeneously distributed on the surface. MG-63 cells with an osteogenic phenotype were well adhered and well spread on the nanostructured surface. Compared to the original polished control, the nanotopographical surface highly improved the adhesion, viability, and differentiation of MG-63 cells.
Conclusion: Titanium with nanotopography achieved by HESP has good cytocompatibility and shows promise for dental implant applications.
Keywords: nanotopography, cytocompatibility, titanium, high-energy shot peening
Introduction
Osseointegration-based dental implants comprise a well-accepted treatment method for complete and partial edentulism. The success rate of this treatment has been reported to be between 90% and 95% at 10 years.1 However, implant failures still remain in some cases, varying in terms of implantation sites and patients.2 The general consensus is that more biocompatible and osteoconductive implants are required 1) in regions of either poor bone quality or lesser bone quantity and 2) for more rapid implant loading.3–6
The surface properties of implants, such as their topography, chemistry, and wettability, can directly influence their interactions with cells.7,8 The interactions of cells and the extracellular matrix would then directly affect cell responses including adhesion, proliferation, and differentiation.9 In this way, the surface properties of implants play an essential role in terms of the cell responses at the bone–implant interface, affecting the osseointegration process. Various surface modification techniques have been developed to improve the implants’ biological interface properties, which favor the bioactivity and bioconductivity of implants.10–12
Recently, increasing attention has been focused on the topography characteristics of implant materials, especially with respect to changes in nanotopography, due to their clearly dynamic effects on cell interactions at the surfaces and because they alter cell behavior by direct (cell–surface interactions) and indirect (protein–surface interactions) mechanisms when compared to materials with conventional-sized topography.3,4,13 At the nanoscale level, the nanotopography could increase the surface energy, thus improving surface wettability to blood, as well as the spreading and binding of fibrin and matrix proteins; this results in the favoring of bone cell attachment and proliferation, especially following implantation, which is an important point in the osseointegration process.14 However, current surface nanotechnologies are known to be very expensive, technically sensitive, and time consuming. Therefore, alternative methods of creating nanotopographical surfaces are highly desirable.15,16
The high-energy shot-peening (HESP) technique is a cold working process used in industry for surface treatment. Peening a surface spreads it plastically, creating plastic deformation and causing changes in the mechanical properties of the surface. Its main application is to avoid the propagation of microcracks across a surface. It has been recently shown that the microstructure of conventional titanium can be significantly refined to yield ultrafine-grained titanium via severe plastic deformation techniques.17 Hence, HESP holds potential as a new method that can be applied to prepare nanoscale surfaces for implants at both the laboratory and the industry levels, without using any additional chemicals or complicated synthesis routes.
In the current study, a nanoscale surface of titanium was prepared by HESP to explore the feasibility of using this method for the surface treatment of dental implants. The obtained surface nanostructures of titanium were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), and contact angle measurements; then bone cell responses (using a human MG-63 osteosarcoma cell line) on these nanoscale surfaces were evaluated.
Materials and methods
Materials
The raw material used was a commercial pure titanium (grade 2) plate 10 mm in thickness. A disk of 14 mm in diameter and 1 mm in height was obtained from the plate. One side of the disk was mechanically ground using silicon carbide (SiC) paper from P300 to P1200 and was ultrasonically cleaned with a succession of acetone, ethyl alcohol, and deionized water.
Surface preparation
The polished surface of the disk was processed for HESP in a vibrator generator (Rösler C2*4S-RH; Untermerzbach, Bavaria, Germany), which is commonly used in industry.
The samples were divided into one of two groups, shot peened for 3 minutes or 5 minutes, respectively, with glass beads of 1-mm diameter, in an effort to achieve an optimum combination of nanosized surface roughness, surface area, and associated energy. Following the peening process, it was expected that a very thin region of a microscale – or even nanoscale – structure would be created at the surface of the titanium samples. The obtained samples were named HESP3 and HESP5. Untreated polished titanium samples (p-Ti) were used as control.
Surface microstructure characterization
The surface of the disks was observed by SEM (JSM-5900LV; JEOL, Tokyo, Japan) and AFM (SPM-9600; Shimadzu Corporation, Kyoto, Japan). Observations were made at three different points on the disk surfaces, and average values were calculated.
Surface wettability
The wettability of the different surfaces was assessed by measuring the water contact angles using a contact angle instrument (SL200B; Solon, Shanghai, People’s Republic of China). Three samples from each group were assessed, and two measurements were performed on each sample to evaluate the average contact angle.
Cell culture and morphology
The MG-63 cells used in this experiment were from a lineage derived from human osteosarcoma. The cells were purchased from the Cell Bank at the Chinese Academy of Sciences, Beijing, People’s Republic of China. MG-63 cells were routinely cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% fetal bovine serum (FBS); (Thermo Fisher Scientific), and cultured in 25-mL culture bottles in a humidified 5% CO2 atmosphere at 37°C. The culture medium was refreshed every 3 days. Before seeding of the cells, the samples containing surface nanostructures and the control samples were sterilized with ethylene oxide at the West China Hospital Affiliated to Sichuan University.
After being cultured on the samples for 1 day, the cells that adhered to the samples were gently washed with phosphate-buffered saline three times, fixed with 2.5% glutaraldehyde solution for 2 hours at 4°C, dehydrated through a series of graded ethanol solutions, critical point dried, and then sputter gold coated in vacuum. The samples were observed by SEM to assess the morphologic changes associated with cell attachment and spreading.
Initial cell adhesion and cell viability
Cells were cultured for 4 hours in 24-well culture plates at an initial seeding density of 5×104 cells per well to evaluate the initial cell adhesion. After trypsinization of the attached cells, the cell numbers were counted using a hemocytometer. Then, the cell viability was investigated following culture periods of 1 day, 3 days, 5 days, and 7 days using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay. In the MTT assay, the mitochondrial dehydrogenases of viable cells cleaved the tetrazolium ring of the substrate to yield purple formazan crystals.18 The resulting purple solution was spectrophotometrically measured at 570 nm using a Bio Assay Reader (HTS7000 Plus; PerkinElmer, Inc, Waltham, MA, USA), and the relative intensities were plotted.
Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity is one of the most widely used markers for the early osteodifferentiation of osteoblasts.19 After culture periods of 1 day, 4 days, and 7 days, ALP activity was measured to characterize the normal osteoblast phenotypic response to nanostructured Ti. Adherent cells on the Ti plates were lysed with 0.05% Triton X-100 (Amresco, Solon, OH, USA) to release intracellular ALP. The lysed solution was incubated with 500 μL of ALP substrate solution for 30 minutes at 37°C. The reaction was terminated by the addition of 250 μL of 0.2 mol/L NaOH. A colorimetric assay was used to measure the absorbance of the solution at 405 nm. The total protein concentration of the samples was then evaluated using a bicinchoninic acid protein assay kit (Thermo Scientific, Waltham, MA, USA), and the absorbance in each sample was normalized based on the protein content.
Statistical analysis
The results were expressed as the means ± standard deviations. For statistical analysis, one-way analysis of variance was performed, followed by the Student–Newman–Keuls posttest using SPSS l6.0 statistical software. A value of P<0.05 was considered statistically significant.
Results
Surface analysis
As shown in Figure 1, the gross appearance of the samples was observed. The HESP3 and HESP5 samples were rough and very rough, respectively, whereas the surface of p-Ti was flat and smooth. The microstructures of HESP3, HESP5, and p-Ti, as observed by SEM, are displayed in Figure 2. It can be seen that p-Ti showed a smooth surface (Figure 2A). In contrast, many spherical crown-shaped pits were observed on HESP3 (Figure 2B) and HESP5 (Figure 2C). Moreover, at higher magnifications of SEM, the microstructure of p-Ti clearly showed that it was featureless and it contained small shallow pits and microscratches that followed a relatively identical direction generated by the polishing process, as was expected (Figure 2D). Conversely, the HESP3 samples showed manifested grain refinement and unique micron-scale structures (Figure 2E). Few nanofeatures can be found in the HESP3 group. With the increase in peening time and plastic deformation, a large number of distinct nanocrystals with an average grain size of approximately 60 nm were observed; the nanocrystals exhibited an inhomogeneous distribution and a clear grain boundary in HESP5 samples, indicating that a nanotopographical surface on titanium was successfully achieved by HESP after a 5-minute shot-peening process (Figure 2F–H). The in vitro cytocompatibility of HESP5 samples with nanotopography was then investigated and compared with that of the p-Ti samples.
Surface roughness parameters were obtained from the AFM analysis (Figure 3), and are described in Table 1. The HESP5 samples showed significantly increasing roughness values (Ra and Rz), compared with both the p-Ti and HESP3 samples (P<0.05; Table 1). Meanwhile, the water contact angle of HESP5 surfaces significantly decreased from 61.6°±2.9° to 40.8°±1.7° compared with that of p-Ti (P<0.05; Figure 4). There were no significant differences in surface roughness or water contact angle between p-Ti and HESP3 surfaces (P>0.05; Table 1 and Figure 4).
Cell morphology
The morphology of the MG-63 cells cultured on HESP5 and p-Ti for 1 day is shown in Figure 5. The cells were well spread on both HESP5 and p-Ti, and they exhibited a spindle-shaped morphology. Moreover, the cells on HESP5 displayed a more widely spread morphology (Figure 5B, D–F) that was highly connected at the surface, and the filopodia were much more discernible and longer when compared with those on p-Ti (Figure 5A and C).
Cell adhesion and viability
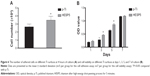
After 4 hours of culture, there were significantly more cells adhered on the HESP5 surface than on the p-Ti surface (P<0.05; Figure 6A). Figure 6B shows the absorbance of formazan produced by viable cells adhered to both the titanium with nanotopography (HESP5) and the control samples (p-Ti) at days 1, 3, 5, and 7 of culture. The MTT test results showed that the number of cells was not significantly different between the two groups at days 1 and 7 of culture, but a quicker increase in cell quantity on the nanostructured titanium surfaces was evident when compared to the cell quantity on the control samples at days 3 and 5 of culture (P<0.05), suggesting that nanotopography obtained by HESP could be more favorable for the adhesion of cells.
Alkaline phosphatase activity
The ability of cell differentiation can be estimated by the ALP activity assay. Figure 7 shows that MG-63 cells on HESP5 showed higher ALP levels when compared with those on the polished p-Ti surfaces after 4 days and 7 days of culture, and they exhibited a stronger propensity to undergo cell differentiation. There was an average increase in ALP level of 68% and 24% in cells on the HESP5 samples, as compared with ALP level of cells on the p-Ti samples, at days 4 and 7 of culture, respectively (P<0.05).
Discussion
Surface nanocrystallization is a new concept that has been proposed for the formation of nanotopography on the surface layer of materials.20 It has been demonstrated that materials with nanotopography have unusual and extraordinary mechanical properties and biocompatibilities that are fundamentally different from, and often far superior to, those of their conventional coarse-grained counterparts.15,20
It has been found in this study that distinct nanofeatures were successfully created on the surface of titanium by HESP. The formation of nanosized grains is attributed to the great amount of deformation being introduced onto the surface of the Ti samples within a very short period. In addition, the original coarse grains were gradually divided by dislocation walls and dislocation tangles.15 According to the SEM and AFM results of HESP3 and HESP5 samples, and in accordance with previous studies,15,21,22 the specific surface nanocrystallization process induced by HESP may occur as follows. At first, some of the dislocations intersect and the stress field at the initial point of deformation is formed. Then, mechanical twinning is induced when the stress increases to the resolved shear limit. With increase of the twin system and enhancement of the twin intersections, grains are refined. Finally, nanosized grains with equiaxed shapes and random crystallographic orientation form under much greater strain, with a high strain rate and multidirectional repeated loads.21,22
In the traditional shot-peening technique commonly used in industry, the surface layer microstructure is not refined to nanosizes due to the low microstrain rate, the magnitude of low peening pressure, and the low coverage rate.23 However, the peening parameters have been optimized through our preliminary experiments; as such, high peening pressure, 100% coverage rate, and bigger pills of shot peening were used so that a large amount of twins with different orientations appeared on the surface layer, and the twin insertion could induce nanocrystallization.
In the current study, titanium with nanoscale topography was cocultured with MG-63 cells to evaluate the effects of nanotopography on cell behavior. The results indicated that after 1 day of culture, the cells spread well and presented with a more widely spread morphology on the HESP5 surface than on the p-Ti surface; moreover, a greater number of cells was also observed on the HESP5 samples (P<0.05). In addition, the ALP activity of MG-63 cells increased over time and was significantly higher in cells on the HESP5 samples than in cells on the p-Ti samples at days 4 and 7 (P<0.05). In this way, it was determined that HESP processing of titanium – which was initially only intended as a means for improving titanium’s mechanical properties – also produces an effect that holds extraordinary significance for the potential applications of titanium as an implant material, namely, the favorable cytocompatibility of osteoblast cells.
To our knowledge, this promising effect of the nanotopography by HESP on osteoblast responses can be illustrated as follows. The nanoscale topography is a useful way to change the surface roughness and wettability.13,20 After HESP treatment, the surface roughness and wettability were improved significantly. The increased surface roughness was found to facilitate the adhesion of biomacromolecules and cells.24 Together with surface roughness, surface wettability is an important factor influencing osteoblast cell responses to implant surfaces. It has been confirmed that an increased initial hydrophilicity could improve early bone healing response at the cell–biomaterial interface by increasing absorption of adhesive proteins, such as fibronection, collagen, and laminin. Enhancing the number of absorbed adhesive proteins could induce increased osteoblast adhesion by recognition of integrins located on the cellular membrane.14
Titanium surfaces with microtopography and additional submicrotopography have been shown to promote early development of the mineralized matrix, which is occasionally observed on the surfaces presenting with microtopography and absent on machined surfaces.25,26 The cellular behavior is also influenced by surfaces with nanotopography. Recent studies3,27,28 have shown that surfaces with micro- and nanoscale topography are more conducive to the proliferation of bone cells and to the formation of bone of good quality and at good quantity. The complex interactions in the cell–matrix substrate and in cell signaling events have been confirmed at the nanoscale.29,30 Different signaling pathways regulate adhesion, migration, differentiation, and gene expression in osteoblasts.31 Thus, it has been shown previously that different nanotopographical surfaces influence protein adsorption, cell adhesion, cell proliferation, and synthesis differently, as well as the secretion of extracellular matrix molecules in vitro.27,28 The results presented in this study are consistent with those of recent and related studies,27,28 which show that topography at the nanoscale level definitely has a positive effect on osteoblast adhesion, viability, and differentiation, thus influencing the bone cell response as it comes in contact with a titanium substrate.
In light of the aforementioned roles of the different parameters that affect cell behavior, the observed favorable bone cell behavior on the shot-peened surface can be concluded to be the net result of the surface nanostructure’s effectiveness in promoting bone cell responses. Further in-depth biological studies (which we are planning to conduct) will be performed to investigate the mechanisms that control the enhancement of cell responses caused by the nanotopographical surface obtained via HESP treatment.
Thus, this investigation illustrated that there is great potential for this novel process of surface modification. Specially, the attractiveness of this method in enhancing the bone cell responses is an added benefit when combined with the mechanical properties of the titanium implant that this method produces, which is not offered by other techniques.
Conclusion
It has been shown that the nanotopography of titanium can be successfully prepared by the HESP technique. Our in vitro study results verify that nanostructured titanium is beneficial for osteoblast responses such as cell adhesion, cell viability, and cell differentiation. We believe that the application of nanocrystallization by HESP on implant surfaces is feasible in clinical practice. Titanium with nanotopography created by HESP is confirmed as a good candidate for improving the biocompatibility of the dental implant.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81371182 and 81100782), the Zhejiang Provincial Natural Science Foundation of China (Y2110400), and the Wenzhou Municipal Science and Technology Bureau Foundation of China (Y20140112).
Disclosure
The authors report no conflicts of interest in this work.
References
Diz P, Scully C, Sanz M. Dental implants in the medically compromised patient. J Dent. 2013;41(3):195–206. | ||
Steigenga JT, Al-Shammari KF, Nociti FH, et al. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003;12(4):306–317. | ||
Xia L, Feng B, Wang P, et al. In vitro and in vivo studies of surface-structured implants for bone formation. Int J Nanomedicine. 2012;7:4873–4881. | ||
Cheng Y, Wu J, Gao B, et al. Fabrication and in vitro release behavior of a novel antibacterial coating containing halogenated furanone-loaded poly(L-lactic acid) nanoparticles on microarc-oxidized titanium. Int J Nanomedicine. 2012;7:5641–5652. | ||
Chen WC, Ko CL. Roughened titanium surfaces with silane and further RGD peptide modification in vitro. Mater Sci Eng C Mater Biol Appl. 2013;33(5):2713–2722. | ||
Wang Z, Sun Y, Wang D, Liu H, Boughton RI. In situ fabrication of silver nanoparticle-filled hydrogen titanate nanotube layer on metallic titanium surface for bacteriostatic and biocompatible implantation. Int J Nanomedicine. 2013;8:2903–2916. | ||
Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis YF. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22(11):1241–1251. | ||
Lamers E, Walboomers XF, Domanski M, et al. The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials. 2010;31(12):3307–3316. | ||
Novaes AB Jr, de Souza SL, de Barros RR, et al. Influence of implant surfaces on osseointegration. Braz Dent J. 2010;21(6):471–481. | ||
Kim KH, Ramaswamy N. Electrochemical surface modification of titanium in dentistry. Dent Mater. 2009;28(1):20–36. | ||
Forster Y, Rentsch C, Schneiders W, et al. Surface modification of implants in long bone. Biomatter. 2012;2(3):149–157. | ||
Zhang W, Li Z, Liu Y, et al. Biofunctionalization of a titanium surface with a nano-sawtooth structure regulates the behavior of rat bone marrow mesenchymal stem cells. Int J Nanomedicine. 2012;7:4459–4472. | ||
Jindal S, Bansal R, Singh BP, et al. Enhanced osteoblast proliferation and corrosion resistance of c p-Ti through surface nanostructuring by ultrasonic shot peening and stress relieving. J Oral Implantol. 2014;40(suppl 1):347–355. | ||
Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010;28(4):198–206. | ||
Lu K, Lu J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mater Sci Eng. 2004;375–377:38–45. | ||
Ballo A, Agheli H, Lausmaa J, Thomsen P, Petronis S. Nanostructured model implants for in vivo studies: influence of well-defined nanotopography on de novo bone formation on titanium implants. Int J Nanomedicine. 2011;6:3415–3428. | ||
Yao ZQ, Ivanisenko Y, Diemant T, et al. Synthesis and properties of hydroxyapatite-containing porous titania coating on ultrafine-grained titanium by micro-arc oxidation. Acta Biomater. 2010;6(7):2816–2825. | ||
Serrano MC, Pagani R, Vallet-Regí M, et al. In vitro biocompatibility assessment of poly(epsilon-caprolactone) films using L929 mouse fibroblasts. Biomaterials. 2004;25(25):5603–5611. | ||
Sibilla P, Sereni A, Aguiari G, et al. Effects of a hydroxyapatite-based biomaterial on gene expression in osteoblast-like cells. J Dent Res. 2006;85(4):354–358. | ||
Lu K, Lu J. Surface nanocrystallization (SNC) of metallic materials: presentation of the concept behind a new approach. J Mater Sci Tech. 1999;15:193–197. | ||
Pi Y, Agoda-Tandjawa G, Potiron S, Demangel C, Retraint D, Benhayoune H. Surface nanocrystallization of Ti-6Al-4V alloy: microstructural and mechanical characterization. J Nanosci Nanotechnol. 2012;12(6):4892–4897. | ||
Aliofkhazraei M, Rouhaghdam AS. Formation of nanocrystalline layers by surface severe plastic deformation and pulsed plasma electrolytic carburizing. J Nanosci Nanotechnol. 2010;10(7):4777–4781. | ||
Ma G, Luo Y, Chen C. Surface nanocrystallization of commercial pure titanium by shot peening. Trans Nonferrous Met Soc China. 2004;14(special 2):204–209. | ||
Bang SM, Moon HJ, Kwon YD, Yoo JY, Pae A, Kwon IK. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin Oral Implants Res. 2014;25(7):831–837. | ||
Schwartz Fo HO, Novaes AB Jr, de Castro LM, Rosa AL, de Oliveira PT. In vitro osteogenesis on a microstructured titanium surface with additional submicron-scale topography. Clin Oral Implants Res. 2007;18(3):333–344. | ||
Raimondo T, Puckett S, Webster TJ. Greater osteoblast and endothelial cell adhesion on nanostructured polyethylene and titanium. Int J Nanomedicine. 2010;5:647–652. | ||
Oshida Y, Tuna EB, Aktoren O, Gencay K. Dental implant systems. Inter J Mol Sci. 2010;11(4):1580–1678. | ||
Puckett S, Pareta R, Webster TJ. Nano rough micron patterned titanium for directing osteoblast morphology and adhesion. Int J Nanomedicine. 2008;3(2):229–241. | ||
de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A. 2007;80(3):554–564. | ||
de Oliveira PT, Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials. 2004;25(3):403–413. | ||
Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001;80(6):1540–1544. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.