Back to Journals » International Journal of Nanomedicine » Volume 15
Supramolecular Vesicles Based on Amphiphilic Pillar[n]arenes for Smart Nano-Drug Delivery
Authors Hua Y, Chen L, Hou C, Liu S, Pei Z, Lu Y
Received 26 March 2020
Accepted for publication 10 June 2020
Published 10 August 2020 Volume 2020:15 Pages 5873—5899
DOI https://doi.org/10.2147/IJN.S255637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Yijie Hua,1,* Lan Chen,1,* Chenxi Hou,2 Shengbo Liu,3 Zhichao Pei,2 Yuchao Lu1
1Analysis Center of College of Science & Technology, Hebei Agricultural University, Huanghua, Hebei 061100, People’s Republic of China; 2College of Chemistry & Pharmacy, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China; 3School of Chemistry, Biology, and Material Engineering, Suzhou University of Science and Technology, Suzhou, Jiangsu 215009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuchao Lu
Analysis Center of College of Science & Technology, Hebei Agricultural University, Huanghua, Hebei 061100, People’s Republic of China
Tel/ Fax +86 317 560 5226
Email [email protected]
Zhichao Pei
College of Chemistry & Pharmacy, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China
Tel +86 298 709 1196
Fax +86 298 709 2769
Email [email protected]
Abstract: Supramolecular vesicles are the most popular smart nano-drug delivery systems (SDDs) because of their unique cavities, which have high loading carrying capacity and controlled-release action in response to specific stimuli. These vesicles are constructed from amphiphilic molecules via host-guest complexation, typically with targeted stimuli-responsive units, which are particularly important in biotechnology and biomedicine applications. Amphiphilic pillar[n]arenes, which are novel and functional macrocyclic host molecules, have been widely used to construct supramolecular vesicles because of their intrinsic rigid and symmetrical structure, electron-rich cavities and excellent properties. In this review, we first explain the synthesis of three types of amphiphilic pillar[n]arenes: neutral, anionic and cationic pillar[n]arenes. Second, we examine supramolecular vesicles composed of amphiphilic pillar[n]arenes recently used for the construction of SDDs. In addition, we describe the prospects for multifunctional amphiphilic pillar[n]arenes, particularly their potential in novel applications.
Keywords: supramolecular vesicles, amphiphilic pillar[n]arenes, smart, drug delivery
Introduction
Drug-induced side effects on normal cells and acquired drug resistance of cancer cells are major obstacles to cancer chemotherapy. Smart nano-drug delivery systems (SDDs), with their characteristic intelligence, are promising tools for addressing these obstacles. SDDs improve the therapeutic efficiency of anticancer drugs by reducing the toxic effects induced by anticancer drugs in normal cells and reducing the side effects of chemotherapy.1–3 In recent years, SDDs have garnered attention in the scientific community and the pharmaceutical industry.4–6 Many nanocarriers are available for preparing SDDs, including nanoparticles,7–11 micelles,12–14 vesicles,15–17 nanogels,18 and quantum dots.19,20 Among these nanocarriers, vesicles have been the most extensively studied because of their unique cavity structure and their ability to encapsulate and control the release of water-soluble or water-insoluble drugs in response to specific stimuli.21,22 Currently, a variety of amphiphilic molecules have been used in the fabrication of vesicles; moreover, amphiphilic host molecules are particularly promising for vesicle construction because of their unique dynamic reversibility, which has led to the emergence of supramolecular vesicles.23–25 Supramolecular vesicles obtained through host-guest interactions have attracted much attention as SDDs because of their smart responsiveness to stimuli.
Pillar[n]arenes, novel macrocyclic host molecules, were first synthesized and named by Tomoki Ogoshi in 2008,26 and they have since attracted increasing interest, with many intelligent nanomaterials constructed based on them,27–34 and playing important roles in biotechnology and biomedicine. Notably, their unique capacity for facile and functional modification has endowed them with the ability to selectively bind different kinds of hydrophilic functional groups, including anions, neutral and cations groups, making them particularly useful for constructing amphiphilic pillar[n]arenes.35 Compared to pillar[n]arenes, amphiphilic pillar[n]arenes are intelligent and targeted according to functional hydrophilic group modifications, with potential applications in many fields, such as molecular recognition, biological analysis, and drug delivery.36–40
Because of the rapid development of the fascinating research field of amphiphilic pillar[n]arenes, in this review, we mainly categorize amphiphilic pillar[n]arenes according to the electric dipole properties of various hydrophilic groups and their means of synthesis: 1) neutral amphiphilic pillar[n]arenes. 2) anionic amphiphilic pillar[n]arenes. 3) cationic amphiphilic pillar[n]arenes (Scheme 1). When introducing examples, we discuss not only the synthesis of these amphiphilic pillar[n]arenes but also the construction of the supramolecular vesicles based on amphiphilic pillar[n]arenes, including their application in the targeted and stimuli-responsive drug delivery in SDDs.41–44 Moreover, the current challenges of and future prospects for multifunctional amphiphilic pillar[n]arenes are also illustrated.
Synthesis of Amphiphilic Pillar[n]arenes
Compared with pillar[n]arenes, amphiphilic pillar[n]arenes are more widely used to construct supramolecular vesicles because of their excellent properties.45–47 In this section, we mainly focus on the synthesis of neutral, anionic and cationic amphiphilic molecules based on pillar[n]arenes, depending on the type of functional group modification at the termini of the pillar[n]arene.
Synthesis of Neutral Amphiphilic Pillar[n]arenes
Many different kinds of neutral groups have been studied for modifying pillar[n]arenes. The neutral motifs include almost all common groups sugars, aliphatic chains, polyethylene glycol chains, etc. These groups endow amphiphilic pillar[n]arenes with desired properties, especially a targeting function and favorable biocompatibility. For example, in 2013, Yu et al obtained an amphiphilic pillar[5]arene by synthesizing asymmetric pillar[5]arene, with one end modified with a sugar moiety and the other end modified with a hydrophobic aliphatic chain. It self-assembled in a short time in aqueous solution to form nano-sugar vesicles, which were transformed into a nano-tubular structure after one week of being undisturbed (Figure 1).48
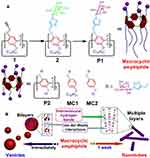 |
Figure 1 (A) Synthetic route and chemical structure of P1; (B) self-assembly of P1 in water. Reprinted with permission from Yu GC, Ma YJ, Han CY, et al. A sugar-functionalized amphiphilic pillar[5]arene: synthesis, self-assembly in water, and application in bacterial cell agglutination. J Am Chem Soc. 2013;135:10310–10313.48; Copyright 2013, American Chemical Society. |
In 2017, Wang’s group successfully developed a water-soluble pillar[5]arene (GalP5) based on a β-D-galactose functional group and used it for constructing supramolecular, a targeted and prodrug nanoparticle with stimuli responsiveness based on its host-guest interaction with the prodrug camptothecin (G). This pillar[5]arene has many potential applications in the field of targeted drug delivery.29 In addition, Pei and colleagues synthesized an amphiphilic pillar[5]arene (GALWP5) by modifying it with a galactose unit at each end to successfully develop a novel dual targeting supramolecular glycol-vesicle (GALWP5⊃D⊃TPP) based on the host-guest complexation of GALWP5 and a triphenyl phosphonium derivative (D-TPP).49 Recently, selenium-containing compounds have been selected as candidates for stimuli-responsive materials because of their sensitivity in the presence of oxidants or reductants. In 2017, Zhou et al prepared the first selenium-containing macrocyclic amphiphile by adopting selenium for pillar[5]arene modification.50 Subsequently, they reported a dual redox-responsive supramolecular amphiphile formed by their selenium-containing pillar[6]arene and a ferrocenium cationic guest, which could controlled by different reductant/oxidant pairs in water.51
In addition, ethoxylate is typically used as the neutral group for modifying pillar[n]arenes. Its high stability and resistance to strong electrolytes, acids and alkali is due to its lack of ionic state. It can effectively improve the biocompatibility of pillar[n]arenes and has a good solubilizing function. In 2012, Ogoshi et al synthesized a new water-soluble pillar[6]arene with triethylene oxide, which complexes with azobenzene derivatives to form a photoresponse system that underwent photo-reversible conversion at low critical solution temperatures.52 In 2013, Zhao and co-workers synthesized amphiphilic pillar[5]arene using water-soluble ethylene glycol and hydrophobic alkyl groups, which gave it significantly improved cellular biocompatibility.53 In 2014, Chi et al synthesized an ethoxy-modified water-soluble pillar[7]arene (WP7), from which the first pillararene-based supra-amphiphilic poly-pseudorotaxane was constructed, that WP7 could self-assemble in the presence of an azobenzene derivative to form vesicles in water.54 Moreover, Huang and colleagues reported a neutral amphiphilic pillar[5]arene modified by a polyethylene glycol chain, which formed a superamphiphile with a paraquat polymer that served as a redox reaction system (Figure 2).55 Furthermore, Huang’s group reported an ethoxy-modified water-soluble pillar[10]arene that was synergistically complexed with paraquat derivatives to generate a thermally responsive [3] pseudorotaxane.40 Subsequently, this group successfully prepared a multi-reactive amphiphilic supramolecular diblock copolymer based on the neutral pillar[10]arene and paraquat polymer in water. This copolymer could self-assemble at room temperature to form multiresponsive polymer micelles, which have application for the controlled release of small molecules.31 In 2016, it reported that, by linking an ethoxy chain to both ends of a pillar[n]arene, an amphiphilic pillar[5]arene could be synthesized successfully. Based on the recognition of the host-guest molecule with this amphiphilic pillar[5]arene and a viologen salt (M), P5-PEG⊃Biotin⊃PTPE self-assembled into supramolecular structure nanoparticles (SNPs) and acted as a self-imaging drug delivery system using an aggregation induced emission (AIE) effect. This study described a new method for the construction of self-imaging drug delivery systems that have great potential application value in the field of cancer treatment.27 The same year, Yu and colleagues synthesized a neutral polyethylene glycol-modified amphiphilic pillar[5]arene and linked with a biotin targeting ligand, which can constructed with a viologen salt polymer guest molecular to make supramolecular diblock copolymerized amphiphile.44
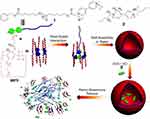 |
Figure 2 Chemical structures of WP5 and polymer 1, as well as schematic illustration of the redox-responsive self-assembly between WP5 and 1 in water.Notes: Reprinted with permission from Chi XD, Yu GC, Ji XF, et al. Redox-responsive amphiphilic macromolecular [2] pseudorotaxane constructed from a water-soluble pillar[5]arene and a paraquat-containing homopolymer. ACS Macro Lett. 2015;4:996–999.55; Copyright 2015, American Chemical Society. |
The effective reduction of cytotoxicity induction by antitumor peptides in normal cells remains a challenge. In 2019, Li et al synthesized a monofunctionalized amphiphilic pillar[5]arene (P-PEG) by modifying a pillar[5]arene with polyethylene glycol chain, and a pH/redox- responsive supramolecular structure (Pep-V⊂P-PEG) was designed by the host interacting with the Pep-V-modified guest peptide. This work proved that Pep-V⊂P-PEG has a supramolecular structure, is highly selective and induces few side effects, therefore, it can be used as a potential antitumor agent for development and application.56
Synthesis of Anionic Amphiphilic Pillar[n]arenes
To date, anionic motifs have been extensively studied and applied to pillar[n]arenes to synthesize negative amphiphilic pillar[n]arenes.57,58 Carboxyl groups are currently the most widely used moiety. In 2012, Yu et al successfully synthesized the first amphiphilic pillar[6]arene modified by a carboxyl group, and by changing the pH, its water solubility was reversibly controlled (Figure 3). The solubility of this water-soluble pillar[6]arene (WP6) enabled its morphology to be reversibly converted between a nanotube and vesicle.38 The same year, Yu et al used a carboxyl-modified pillararene to obtain an anionic amphiphilic pillararene, which was combined with paraquat by in a host-guest assembly to hinder direct contact between paraquat molecules and cells and inhibited the formation of paraquat free radicals successfully. To a certain extent, the anionic amphiphilic pillararene reduced the toxicity of paraquat in human cells, which is of great significance for the safe application of the pesticide paraquat.59 Li et al synthesized an anionic amphiphilic water-soluble pillar[7]arene (WP7) by introducing 14 anionic carboxylate groups at both edges of the pillar[7]arene, and the pH control complexation process with the paraquat G1 in water was studied. Using this novel molecular recognition motif, they produced a superamphiphile, WP7⊃G2, based on WP7 and the amphiphilic paraquat derivative G2, which self-assembled into supramolecular vesicles in water through host-guest interactions. Future work on controlled release and drug delivery will be based on this novel molecular recognition motif.60 In 2016, Huang’s group successfully increased the intensity of near-infrared emission by generating a host-guest complex of a carboxy-modified water-soluble pillar[5]arene and a cyanostyrene derivative in water.61 Recently, Zhao et al successfully prepared two amphiphilic poly pseudorotaxanes that self-assembled into supramolecular polymer vesicles according to the molecular recognition of carboxyl-modified water-soluble pillar[n]arenes and paraquat and studied the release of encapsulated drugs through pH-triggered self-assembling fluorescent (due to aggregation-induced emission of tetraphenylethylene) supramolecular polymers.62
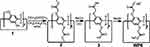 |
Figure 3 Synthetic route to WP6.Notes: Reprinted with permission from Yu GC, Xue M, Zhang ZB, et al. A water-soluble pillar[6]arene: synthesis, host−guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J Am Chem Soc. 2012;134:13248–13251.38; Copyright 2012, American Chemical Society. |
Recently, Wang’s group completed extensive work based on carboxylated functional pillar[n]arenes. They synthesized an amphiphilic pillar[6]arene using a carboxyl-modified pillar[6]arene and constructed the first pH responsive SDS molecule with an alkyl chain-modified ferrocene derivative (FcA).63 In 2014, Hu et al successfully constructed an amphiphilic pillar[6]arene (WP6) by modifying the carboxyl group at both ends of pillar[6]arene, it can form an amphiphilic host-guest inclusion complex with azobenzene derivative G1 or G2. Thus, two novel supramolecular nanocarriers were prepared.64 In another work, Wang and co-workers used a carboxyl-modified pillar[6]arene to obtain an anionic amphiphilic pillar[6]arene, and used it to construct a multi-stimulus-responsive vesicle with a pyridinium derivative guest.65 Recently, Wang’s group successfully constructed a WP6-based drug-drug conjugated supramolecular nanocarrier. The water solubility of amphiphilic pillar[6]arene (WP6) was achieved by linking carboxyl groups to both ends of WP6.43 There are many paradigms of amphiphilic pillar[5]arenes based on carboxyl modification. Hu, Wang and co-workers synthesized a water-soluble pillar[5]arene from carboxyl modified pillar[n]arenes in 2015, and successfully developed an amphiphilic inclusion complex based on it and the lysine derivative.42 Later, they synthesized amphiphilic pillar[5]arene in the same way and used it in a complex with a boron-dipyrromethene-derived compound in aqueous medium to form a superamphiphilic molecule.66 Recently, they successfully constructed a novel glucose-reactive supramolecular drug delivery system using the host-guest interaction of a carboxyl-modified water-soluble pillar[5]arene (WP5) and a pyridyl boronic acid derivative (G).67 Furthermore, in another work, Hu and colleagues synthesized an amphiphilic pillar[5]arene based on a carboxyl-modified water-soluble WP5 and bola-type supramolecular amphiphiles with dual pH and GSH responses were successfully constructed through complexation with a bola-type naphthalimide guest (G) in water.68
Pei’s group synthesized carboxylated amphiphilic pillar[6]arenes by modifying the carboxyl groups at the ends of a pillar[6]arene, and a novel supramolecular complex was constructed based on the host-guest complexation of this carboxyl-modified pillar[6]arene with a galactose derivative and doxorubicin (DOX)-loaded ZIF-8. This work opened up a new avenue for building multifunctional supramolecular hybrid materials for cancer therapeutic applications.69 Subsequently, in 2016, Pei and colleagues successfully synthesized an amphiphilic pillar[5]arene by attaching ferrocenecarboxylic acid to each end of pillar[5]arene, and through host-guest interactions with a galactose derivative, supramolecular vesicles were formed. Under oxidizing conditions, ferrocene exhibits electropositivity, which makes these amphiphilic pillararenes promising for siRNA co-delivery.70
In recent years, carboxy-pillar[n]arenes have received increasing attention because of their effective pH response. Ji et al synthesized an amphiphilic pillar[5]arene by using carboxyl-modified pillar[5]arenes, and a novel dual pH-responsive supramolecular prodrug micelle was constructed based on the interaction between this host and a guest with methyl viologen-functionalized doxorubicin (MV-DOX).28 In 2016, Shi and co-workers synthesized water-soluble pillar[6]arene (WP6) modified with a carboxyl group, and based on its complexation with a photoreactive azobenzene derivative serving as a guest, a stimulus-responsive nanoparticle carrier was prepared.71 This same year, Wheate et al demonstrated the wide-ranging biomedical applications of carboxylated pillar[n]arene nanocapsules by synthesizing carboxylated pillar[n]arenes.72 A water-soluble amphiphilic pillar[6]arene (CPA[6]) with a substituted carboxylate was synthesized by Du’s group, and a novel supramolecular vesicle was constructed based on the host-guest interaction of this carboxylated pillar[n]arene with a disulfide-bonded benzimidazole amphiphile.73 Fan and co-workers successfully synthesized a negative amphiphilic pillar[5]arene with carboxyl-modified water-soluble pillar[5]arene, which self-assembled upon complexation with a bola-type guest G, that absorbs near-infrared light (NIR) to form a multifunctional supramolecular vesicle. This work provides an innovative approach to building new nanocarriers.74 In 2017, Zhou and colleagues successfully synthesized a water-soluble pillar[6]arene (WP6) by linking carboxyl groups at both ends of pillararene, followed by the construction of a dual pH/light stimuli-responsive compound vesicle with a block copolymer (BCP) containing amphiphilic azobenzene.75 In 2018, Zhong and co-workers successfully synthesized a water-soluble amphiphilic WP5-modified by carboxyl groups, and constructed pH and redox dual-responsive polymer vesicles based on host-guest interactions of their modified WP5 with a paraquat-containing block copolymer (BCP).76 This same year, Zhou et al synthesized a water-soluble amphiphilic pillar[5]arene modified by a carboxyl group, and CO2-enhanced bola-type supramolecular amphiphiles were successfully constructed using CO2 responsive borosilicate GH2 driven by host-guest complexation with a positively charged ammonium salt. Finally, they successfully demonstrated the effective controlled release of self-assembled vesicles consisting of bola-type supramolecular amphiphiles containing calcein. This work provides an idea for building new smart CO2 responsive supramolecular materials (Figure 4).77 Moreover, Yang et al synthesized amphiphilic carboxylated pillar[6]arene, and used it to construct a multifunctional supramolecular material with a polypyrrole@UiO-66 nanocomposite for targeted chemiluminescence therapy.32 Yin et al successfully synthesized an amphiphilic pillar[5]arene (WP5) modified with a carboxyl group, and constructed host-guest supramolecular complexes based on WP5 and spiropyran derivatives (G); the structural changes of the visible light-inducing component were studied.39
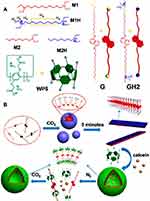 |
Figure 4 (A) Chemical structures and cartoon representations of WP5, and guest molecules G and GH2; (B) Cartoon representation of the self-assembly of GH2, (WP5)2⊃GH2, and the controlled behavior.Notes: Reprinted with permission from Zhou YJ, Li ER, Zhao R, Jie KC. CO2-enhanced bola-type supramolecular amphiphile constructed from pillar[5]arene-based host−guest recognition. Org Lett. 2018;20:4888–4892.77; Copyright 2018, American Chemical Society. |
Phosphoric acid groups can also be used to modify and synthesize amphiphilic pillar[n]arenes. In 2016, Hu, Wang et al synthesized negative amphiphilic pillar[n]arenes WP[n]s based on phosphorylated pillar[n]arenes, and the introduction of a phosphate group enhanced their water solubility and biocompatibility.78 Du’s group synthesized a phosphorylated amphiphilic pillar[5]arene (PPA[5]), which was constructed as a novel supramolecular nanowire based on its host-guest interactions with choline-functionalized mesoporous silica nanoparticles (MSNs). PPA[5] surrounds choline or pyridinium stems to form supramolecular nanotubes for the encapsulation and release of drugs in MSN wells. PPA[5] exhibits high binding affinity for quaternary ammonium salt, which it binds through host-guest interactions primarily based on ion pairing between the phosphate and quaternary ammonium (guest) moieties to minimize the premature release of the drug (Figure 5).30
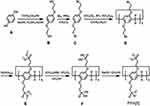 |
Figure 5 Synthetic route to phosphonated pillar[5]arene (PPA[5]).Notes: Reprinted with permission from Huang X, Wu SS, Ke XK, Li XY, Du XZ. Phosphonated pillar[5]arene-valved mesoporous silica drug delivery systems. ACS Appl Mater Interfaces. 2017;9:19638–19645.30; Copyright 2017, American Chemical Society. |
In 2019, Chang et al reported that a charge-reversing amphiphilic pillar[5]arene P5NH-DCA, was induced by the hydrolysis of acid-labile amide groups in the acidic microenvironment of tumors. Specifically, the head group charge of P5NH-DCA changed from negative to positive, enabling it to destroy cancer cells with high efficiency. In normal cells, the negatively charged P5NH-DCA remains stable, which greatly reduces the toxicity it induces in normal cells and improves its anticancer bioavailability (Figure 6).79 Compared with the wide application of carboxyl groups in the synthesis of amphiphilic pillar[n]arenes, sulfonic acid groups have not been extensively studied. However, Jin et al synthesized a monosulfonicpillar[5]arene in 2018. The host-guest interactions between it and six alcohol molecules were also studied in detail.80 Recently, Yang’s group synthesized a water-soluble disulfonic pillar[5]arene and a pH-stable fluorescence system was constructed using it and a water-soluble tetraphenylethylene guest (Figure 7).81 Thus, sulfonic acid group has great potential for their development and application as amphiphilic pillar[n]arene modifications in the future.
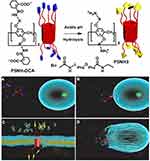 |
Figure 6 (A) Charge reversal process of P5NH-DCA; (B) Binding to cancer cell membrane; (C) Disrupting cancer cell membrane; (D) Killing cancer cells.Notes: Reprinted with permission from Chang YC, Chen JY, Yang JP, et al. Targeting the cell membrane by charge-reversal amphiphilic pillar[5]arene for the selective killing of cancer cells. ACS Appl Mater Interfaces. 2019;11:38497–38502.79; Copyright 2019, American Chemical Society. |
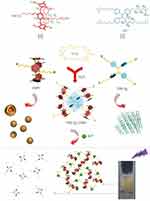 |
Figure 7 Chemical structures and cartoons of DSP5 and TPE-Q4 and the schematic presentation of their self-assembly into a fluorescent supramolecular system for the selective detection of Fe3+ ions.Notes: Reprinted with permission from Wang X, Lou XY, Jin XY, Liang F, Yang YW. A binary supramolecular assembly with intense fluorescence emission, high pH stability, and cation selectivity: supramolecular assembly-induced emission materials. Research. 2019;2019:1454562.81; Copyright 2019, Science. |
Synthesis of Cationic Amphiphilic Pillar[n]arenes
Compared to the numerous anionic and neutral motifs used to modify pillar[n]arenes, the number of cationic motifs reported for using as motifs in pillar[n]arene modifications are relatively limited. Ogoshi et al modified both ends of pillararene with tetra-alkylphosphine to obtain an cationic amphiphilic pillararene, which could be simultaneously dissolved in an aqueous solution and an organic solvent, and used as a highly efficient substrate selectivity phase transfer catalyst (Figure 8).82 Li et al successfully synthesized a cationic water-soluble pillar[6]arene with twelve pyridinium moieties, which showed strong binding affinity for two naphthalene sulfonate derivatives in an aqueous medium, therefore it has the potential for effective complexation with specific important anionic guests.83
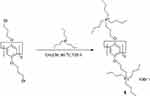 |
Figure 8 Synthetic route of tetra-alkylphosphine capped amphiphilic pillar[5]arene 1.Notes: Reprinted with permission from Ogoshi T, Ueshima N, Yamagishi TA. An amphiphilic pillar[5]arene as efficient and substrate-selective phase-transfer catalyst. Org Lett. 2013;15:3742–3745.82; Copyright 2019, American Chemical Society. |
In 2011, Huang’s group successfully prepared a cationic water-soluble pillar[5]arenes by introducing trimethyl-ammonium groups on the upper and lower edges of the pillar[5]arenes, which formed a stable 1:1 host guest complex with sodium 1-octanesulfonate in water. This novel cationic pillar[5]arene provides more opportunities for related research in the field of host-guest chemistry.84
In another work, Huang’s group successfully synthesized a new water-soluble pillar[5]arene with ten imidazolium groups on the edges, which could be used as a stabilizer to make gold nanoparticles smaller than 6 nm in water.85 Yao et al constructed an amphiphilic pillar[5]arene based on an asymmetric pillar[5]arene by modifying the amino group at one end and the hydrophobic fatty chain at the other end, it enabling the self-assembled formation of nanovesicles in aqueous solution through hydrophobic interactions. They also loaded these vesicles with calcein molecules and found that, upon pH stimulation, the regulated release of calcein molecules could be achieved.36 In 2015, Jie et al successfully synthesized a cationic amphiphilic pillar[5]arene using a tertiary amine modified pillar[5]arene and developed a new type of CO2-responsive molecular recognition motif in combination with the anionic surfactant sodium dodecyl sulfate (SDS). DLS, SEM and TEM experiments confirmed that the inclusion complex had has supramolecular amphiphilic properties and could be used to construct spherical double-layer supramolecular vesicles, and the assembly and disassembly of these vesicles was successfully used for the release of gas and surfactant-triggered calcein (Figure 9).37 Hu et al obtained a cationic amphiphilic pillar[5]arene by modifying it with functionalized monoalkylamines, which underwent host-guest interactions with guanidine carbon phenylpropanol (GCP) in aqueous solution to form amphiphilic peptides and spontaneously self-assembled supramolecular vesicles. In addition, experiments have shown that these peptides have effective gene transfection properties.86 In a recent study, Hu et al successfully synthesized a cationic amphiphilic WP5, the ends of which were modified with quaternary ammonium salt, a pillar[5]arene prodrug, WP5-DOX; and an Arg-Gly-Asp (RGD) modified sulfonate guest, RGD-SG; these components formed a stimuli-responsive tumor-targeted drug delivery system actualized by host-guest recognition.87
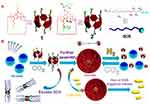 |
Figure 9 (A) Chemical structures and cartoon representations of 1, 2, and SDS and (B) Cartoon representation of gas-controlled self-assembly and dual-triggered release of calcein.Notes: Reprinted with permission from Jie KC, Zhou YJ, Yao Y, Shi BB, Huang FH. CO2-responsive pillar[5]arene-based molecular recognition in water: establishment and application in gas-controlled self-assembly and release. J Am Chem Soc. 2015;137:10472–10475.37; Copyright 2015, American Chemical Society. |
In addition, Pei’s group successfully constructed a cation amphiphilic pillar[5]arene (FCAP) with a terminal ferrocene, which self-assembled in aqueous solution to form a novel cationic vesicle with GSH-triggering and siRNA co-delivery capacity.88 Subsequently, in 2016, a Trp-modified pillar[5]arene (TP5) was reported, to be a novel water-soluble amphiphilic macrocyclic molecule. Its water solubility was produced by a free amino group, and a supramolecular vesicle (TP5G) was constructed based on the host-guest interaction of TP5 and a galactose derivative (G).89 Moreover, by attaching cysteamine hydrochloride to both ends of the pillar[5]arene, Lu and co-workers successfully synthesized a cationic amphiphilic pillar[5]arene and via host-guest interactions between it and a galactose derivative, a multifunctional supramolecular vesicle was assembled.90
Amphiphilic Pillar[n]arene-Based Supramolecular Vesicles for Smart Nano-Drug Delivery
Supramolecular vesicles are the most widely used nanocarriers in smart drug delivery systems due to their facile construction, high-cargo-loading capacity, and effective biocompatibility, which enhances the therapeutic efficiency of anticancer drugs and overcomes obstacles to chemotherapy. Currently, aiming at the differences between the microenvironment of cancer cells and normal cells, many functional supramolecular vesicles have been constructed based on amphiphilic pillar[n]arenes. In this section, we focus on the progress of amphiphilic pillar[n]arene-ased supramolecular vesicle applications in SDDs.
Amphiphilic Pillar[n]arene-Based Stimuli-Responsive Supra-Molecular Vesicles for Smart Nano-Drug Delivery
The stimuli-responsive supramolecular vesicles not only encase drugs but also possess intelligence, which can be used to improve the therapeutic efficiency of anticancer drugs. To date, stimuli-responsive supramolecular vesicles with various levels of responsiveness to external stimuli, such as pH, redox enviroments, light, GSH, and other chemical/physical stimuli, have application that have been significantly expanded the applications in smart drug delivery.57
Amphiphilic Pillar[n]arene-Based Single Stimuli-Responsive Supra-Molecular Vesicles for Smart Nano-Drug Delivery
The first pH-responsive smart drug delivery system (DDSs) was constructed by Wang and co-workers. It had an alkyl chain-modified ferrocene derivative (FcA) that could strongly bind WP6 in water to form a stable inclusion complex (WP6*FcA) via hydrophobic effects.63 The complex was assembled into supramolecular vesicles that were capable of encapsulating hydrophilic mitoxantrone (MTZ) to achieve MTZ-loaded vesicles for rapid MTZ release in low-pH environments. Wang et al proved that DDSs could dramatically decrease the toxicity induced by MTZ in normal cells without compromising the therapeutic effects of MTZ on cancer cells (Figure 10). In another work, they used a new glucose-reactive supramolecular drug delivery system based on WP5 and pyridylboronic acid derivatives (G) for insulin delivery and controlled release under physiological conditions.67 Insulin-loading and in vitro drug release experiments showed that insulin could be successfully encapsulated in these vesicles. This was the first time that small supramolecular vesicles were used to encapsulate large proteins. This work provides a good example of the potential application of smart supramolecular vesicle systems in the treatment of diabetes.
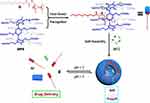 |
Figure 10 Schematic illustration of the formation of supramolecular vesicles and their pH-responsive drug release.Notes: Reprinted with permission from Duan QP, Cao Y, Li Y, et al. pH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J Am Chem Soc. 2013;135:10542–10549.63; Copyright 2013, American Chemical Society. |
In 2015, Huang and co-workers used redox reaction systems consisting of neutral WP[5] (WP5) and paraquat polymers to make redox-sensitive polymer vesicles.55 TEM showed that the aggregates exhibited a spherical morphology, and dynamic light scattering (DLS) showed that the sizes of the aggregates were similar to those observed under TEM. After the addition of Na2S2O4 as a reducing agent, the vesicles underwent a dramatic morphological changes. In addition, the anticancer drug doxorubicin hydrochloride (DOX·HCl) was used to test the controlled release function of these vesicles. The results indicated that these DOX-containing vesicles had a higher release rate in the presence of Na2S2O4 and greater payload release than they did in the absence of stimulation, suggesting they can be used as redox stimuli-responsive drug delivery systems. Due to the sensitivity of selenium-containing compounds in the presence of oxidants or reducing agents, they have been selected as good candidates for stimulus-responsive materials. In 2017, Zhou et al used amphiphilic selenium-containing pillar[5]arenes that self-assembled in water to form redox-reactive vesicles; moreover, these vesicles could be used for the controlled release of DOX.50
Multi-Stimuli-Responsive Supramolecular Vesicles
Hu et al constructed two novel supramolecular nanocarriers prepared from an amphiphilic host-guest inclusion complex consisting of water-soluble pillar[6]arene (WP6) and azobenzene derivative G1 or G2 (Figure 11).64 G1 was structurally similar to G2, but had an additional phenoxy group in the hydrophobic region. WP6 with G1 formed supramolecular micelles that gradually transformed into a layered structure with liquid crystal properties, while WP6 and G2 formed stable supramolecular vesicles in water, that exhibited dual light and pH responsiveness. It is notable that the WP6⊃G2 supramolecular vesicles effectively encapsulated the anticancer drug mitoxantrone (MTZ), and remained stable in the simulated normal physiological environment; however, in acidic environment similar to that of tumor cells or upon external UV irradiation, the encapsulated drug was promptly released.
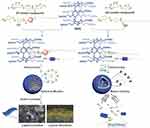 |
Figure 11 Schematic illustration of the construction of supramolecular micelles (WP6⊃G1) or vesicles (WP6⊃G2) and the application of supramolecular vesicles in drug delivery.Notes: Reprinted with permission from Hu XY, Jia KK, Cao Y, et al. Dual photo- and pH-responsive supramolecular nanocarriers based on water-soluble pillar[6]arene and different azobenzene derivatives for intracellular anticancer drug delivery. Chem Eur J. 2015;21:1208–1220.64; © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. |
Subsequently, Wang and co-workers constructed a multi-stimuli-responsive vesicle from WP6 and a pyridinium derivative guest.65 Drug-loading and controlled-release testing indicated that the anticancer drug DOX was efficiently loaded into the nanocarrier and the vesicles showed good Ca2+ and pH responses. In addition, cell uptake experiments indicated that the encapsulation of anticancer drugs into nanocarriers did not reduce the therapeutic effect of the drugs on tumor cells and significantly reduced the toxic side effects of the drug in normal cells. The study provides a strategy for building smart nanocarriers that have tremendous potential for smart drug delivery. In 2015, Wang et al constructed amphiphilic inclusion complexes formed by water-soluble pillar[5]arene and lysine derivatives that formed novel dual GSH and pH-responsive supramolecular vesicles.42 These vesicles exhibited excellent GSH and pH responsiveness in a simulated acidic tumor environment with a high GSH concentration and released the encapsulated MTZ effectively. Finally, MTT assays used to assess cytotoxicity showed that these MTZ-loaded vesicles were effective in inhibiting the proliferation of cancer cells and had potent antitumor activity. Currently, research on the active targeting of this supramolecular vesicle platform in response to the tumor microenvironment is ongoing, and is expected to provide better platforms for the efficient treatment of cancer and tumors.
In 2017, Wang et al successfully constructed a dual pH- and GSH (glutathione)-responsive bola supramolecular amphiphile based on soluble pillar[5]arene (WP5) and bolaform naphthalimide guest (G) in water.68 The resulting bola-type amphiphile self-assembled into supramolecular binary vesicles in water because of host-guest interactions, and at low pH and/or high GSH concentration environment will decompose. In addition, drug-loading and release assays showed that the hydrophobic anticancer drug doxorubicin (DOX) was successfully encapsulated in the vesicle and that the loaded drug was rapidly released in an acidic microenvironment with high GSH concentration. It is worth noting that cytotoxicity experiments showed that these DOX-loaded supramolecular vesicles had a significantly increased anticancer effect on tumor cells compared to that of free DOX, and the reduced side effects on normal cells with simultaneous pH/GSH dual responsiveness was remarkable. In vitro cellular uptake and subcellular localization assays indicated that the vesicles entered cancer cells primarily through endocytosis and caused excellent drug accumulation in cancer cells. This study provides an example of the successful development of effective bola-type multi-stimuli-responsive supramolecular nanocarriers, which have great potential applications in the field of drug delivery, particularly with respect to controlled release. Later, Wang et al prepared biocompatible nanocarriers for anticancer treatment based on phosphorylated WP[n]s and a pyridinium bromide guest.78 The micelles were formed by the self-assembly of WP5 and the guest, whereas the hollow vesicles were formed by WP6 and the guest. Both the micelles and vesicles exhibited dual pH-/Zn2+-responsiveness. DOX was loaded inside the micelle, and MTZ was encapsulated by the vesicle. This study provided a new method for constructing nanocarriers with different forms of smart drug delivery. In 2018, Wang’s group reported complex vesicles formed by self-assembly of a host and guest based on pillar[5]arene and phenyl boronic acid derivatives, which contained both insulin and glucose oxidase (GOx).91 This supramolecular vesicle selectively recognized glucose and underwent structural damage. In the process of GOx-promoted glucose-specific oxidation to gluconic acid, high glucose concentrations, in situ production of H2O2 and the acidic microenvironment triggered continuous and controlled insulin release (Figure 12). In vivo experiments demonstrated that this smart supramolecular nanocarrier exhibited a rapid response to hyperglycemic conditions and could effectively modulate glucose levels in a mouse model of type I diabetes over a prolonged period. This intelligent insulin delivery system with multi-stimuli responsiveness provides a good opportunity for the delivery of closed-loop insulin, and the development of a nanoplatform for continuous blood glucose monitoring will be facilitated by the use of these or similar intelligent artificial nanocarriers.
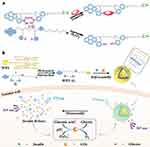 |
Figure 12 Schematic illustration of the glucose-responsive supramolecular insulin delivery system. (A) Chemical structure and the mechanism of multiresponsive diphenylboronic acid guest G. (B) Supramolecular self-assembly of the host–guest complex WP5⊃G into vesicles and their successful encapsulation of insulin and GOx as well as the efficient insulin release under hyperglycemic state.Notes: Reprinted with permission from Zuo MZ, Qian WR, Xu ZQ, et al. Multiresponsive supramolecular theranostic nanoplatform based on pillar[5]arene and Diphenylboronic acid derivatives for integrated glucose sensing and insulin delivery. Small. 2018;14:e1801942.91; © 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. |
Similarly, Zhou and colleagues reported the generation of dual pH/light-stimulating responsive compound vesicles based on host-guest interactions between water-soluble pillar[6]arene (WP6)and an amphiphilic azobenzene-containing block copolymer (BCP).75 Reversible morphological transformation between complex vesicles and solid aggregates through repeated exposure to pH and light stimulation enabled controlled release of the water-soluble anticancer drug doxorubicin hydrochloride (DOX•HCl). Under external stimuli, DOX•HCl showed a faster release rate with stimulation than it did without stimulation. In addition, MTT (viability) and cell uptake assay results revealed that the composite vesicles showed excellent cytocompatibility with human breast cancer cells (MCF-7), and the drug-loaded composite vesicles exhibited lower cytotoxicity than the free drug. This system may promote new prospects in the field of drug delivery. To regulate the rate of drug release, in 2018, Zhong and co-workers successfully constructed dual pH- and redox-responses polymer vesicles generated by the host-guest interactions of WP5 and a paraquat-containing block copolymer BCP.76 These polymeric vesicles loaded with DOX showed modulated drug release rates based on exposure to a single stimulus or a combination of both stimuli. In addition, under the conditions of this study, the DOX-loaded polymeric vesicles showed anticancer activity similar to that of free DOX in vitro, indicating that these vesicles may play important roles in the treatment of cancer as controlled-release drug carriers.
In 2016, Shi and colleagues prepared a dual-stimuli-responsive nanocarrier based on the complexation of WP6 and a photoreactive azobenzene derivative,71 which self-assembled into vesicles. The nanocarriers exhibited responsiveness to light and pH, and the controlled release of DOX was achieved by the system. In the same year, Du’s group reported a novel supramolecular vesicle generated based on CPA[6] co-assembled with the disulfide-bonded benzimidazole amphiphiles,73 this supramolecular vesicle responded to five triggering factors, GSH, pH, CO2, Zn2+ and HDA, resulting in disulfide bond cleavage, protonation of carboxylate groups, metal chelation and competitive bonding to achieve controlled drug release (Figure 13). This is the first example of a smart PA-based supramolecular vesicle with antibacterial properties that met the various requirements for controlling drug release. Smart stimuli-responsive supramolecular vesicles have broad application prospects in the treatment of tumors and related diseases.
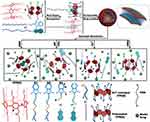 |
Figure 13 Chemical structures of G1, G2, and G3, as well as illustrations of the controlled drug release in response to the five stimuli.Notes: Reprinted with permission from Jiang L, Huang X, Chen D, et al. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar-[6]arenes that are responsive to five stimuli. Angew Chem Int Ed. 2017;56:2655–2659.73; © 2017 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. |
Amphiphilic Pillar[n]arene-Based Targeted Supramolecular Vesicles for Smart Nano-Drug Delivery
Anticancer drugs are scattered in the body and cannot reach tumor tissues effectively, which influences the intensity and duration of their pharmacological effects and leads to side effects on normal cells. Therefore, supramolecular vesicles with targeting functions have received considerable attention, specifically because they can precisely target tumors, enhance antitumor efficacy and effectively reduce side effects on normal cells.
Amphiphilic Pillar[n]arene-Based Targeted Supramolecular Vesicles for Smart Nano-Drug Delivery
Yu and colleagues constructed a supramolecular diblock copolymer amphiphile based on a biotin-targeted ligand-modified water-soluble pillar[5]arene and a viologen salt polymer (M). Promptly, host-guest complex supramolecular vesicles were formed and successfully used as nanocarriers for the targeted delivery of the anticancer drug DOX.44
In 2018, Pei and colleagues successfully developed a galactose dual-targeted supramolecular ethylene glycol vesicle (GALWP5⊃D-TPP) based on a neutral pillar[5]arene and a triphenylphosphine derivative (D-TPP) host-guest complex (Figure 14).49 GALWP5⊃D-TPP loaded with DOX not only had the ability to target HepG2 liver cancer cells overexpressing ASGP-R but also had good mitochondrial targeting ability, which facilitated enhanced mitochondrial accumulation of drugs upon entering the cancer cells. This study provides a good example of the rational design and construction of dual-targeting supramolecular vesicles for drug delivery.
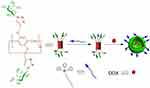 |
Figure 14 Cartoon representation of constructing dual-target supramolecular vesicles and their properties in efficient drug delivery.Notes: Reprinted with permission from Shang K, Wang Y, Lu YC, Pei ZC, Pei YX. Dual-targeted supramolecular vesicles based on the complex of galactose capped pillar[5]arene and triphenylphosphonium derivative for drug delivery. Isr J Chem. 2018;58:1205–1209.49; © 2018 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. |
Amphiphilic Pillar[n]arene-Based Supramolecular Vesicles for Smart Nano-Drug Delivery for Targeted Stimulus Responsiveness
In 2016, Pei and co-workers successfully constructed a supramolecular vesicle (FACP5G) based on pillar[5]arene with ferrocene carboxylic acid-modified termini and a galactose derivative host-guest complex.70 Drug-loading experiments showed that DOX could be successfully encapsulated in these vesicles, which showed GSH and pH dual-responsiveness and cancer cell targeting based on the ferrocene carboxylic acid and galactose moieties, respectively. This work provides a good example of the rational design and construction of multifunctional nanocarriers, which has great potential for application in the field of targeted drug delivery. Moreover, Lu et al constructed a multifunctional supramolecular vesicle composed of a multifunctional cystamine dihydrochloride-blocked pillar[5]arene (CAAP5) and a galactose derivative (G) based on host-guest interaction, and it demonstrated effective drug loading.90 Furthermore, DOX-loaded CAAP5G exhibited not only excellent dual-stimulation responsiveness in cancer cells but also rapid release of DOX, and targeting of ASGP-R overexpressing HepG2 cells. Most notably, at high concentrations GSH, vesicles release cysteamine, which increases the anticancer efficiency of DOX and reduces the resistance of cancer cells. This study provides new ideas for the rational design and construction of targeted and stimuli-responsive nanocarriers for drug delivery, which has applications in adjuvant chemotherapy (Figure 15). Tryptophan (Trp) is an essential amino acid in the human diet, because it can interaction with DNA through the anthracycline in the molecule. Using this, In 2016, Pei and colleagues reported a supramolecular vesicle (TP5G) based on the host-guest interaction assembly of Trp-modified pillar[5]arene (TP5) and a galactose derivative (G).89 Drug-loading experiments showed that the anticancer drug DOX was successfully encapsulated in these supramolecular vesicles, and the galactose residue on the guest is used to the asialoglycoprotein overexpressing HepG2 cells through carbohydrate-protein interaction (Figure 16). Therefore, this DOX-loaded supramolecular vesicle had the ability to target liver cancer against ASGP-R overexpressing HepG2 cells, but more importantly because of significant mutual interaction between TP5 and the DNA in the cells. This study developed a targeted supramolecular vesicle drug delivery system based on DNA interactions.
 |
Figure 15 Schematic illustration of the synthesis of CAAP5, the host-guest complexation with galactose derivatives (G), formation of vesicles (CAAP5G), and their GSH/pH dual-responsive drug release.Notes: Reprinted with permission from Lu YC, Hou CX, Ren JL, et al. A multifunctional supramolecular vesicle based on complex of cystamine dihydrochloride capped pillar[5]arene and galactose derivative for targeted drug delivery. Int J Nanomed. 2019;14:3525–3532.90; Copyright 2019, Dovepress. |
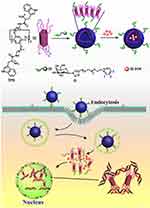 |
Figure 16 Cartoon of the self-assembly and drug-loading process of a vesicle based on Trp-modified pillar[5]arene and a galactose derivative and its possible cellular pathways.Notes: Reprinted with permission from Yang K, Chang YC, Wen J, et al. Supramolecular vesicles based on complex of Trp-modified pillar[5]arene and galactose derivative for synergistic and targeted drug delivery. Chem Mater. 2016;28:1990–1993.89; Copyright 2016, American Chemical Society. |
In 2018, Hu and colleagues reported stimuli-responsive targeted drug delivery systems formed by host-guest recognition between the novel pillar[5]arene prodrug WP5-DOX and sulfonate guest RGD-SG, a modified by Arg-Gly-Asp (RGD).87 The morphology and size of this nanocarrier, which targets supramolecular tumors, could be controlled by using different ratios of hosts to guests. The amphiphilic WP5-DOX⊃RGD-SG complex at a molar ratio of 5:1 self-assembled into vesicles. Smaller micelles were obtained by changing the molar ratio to 1:3. Both prodrug nanocarriers selectively delivered doxorubicin to the RGD-overexpressing in cancer cells and responsively released the drug in an acidic environment, thereby enhancing antitumor efficacy. This work demonstrates the potential of this nanocarrier in stimulating responsive targeted drug delivery.
Amphiphilic Pillar[n]arene-Based Multifunctional Supramolecular Vesicles for Smart Nano-Drug Delivery
The multifunctional supramolecular vesicles improve not only anticancer efficacy but also reduce the side effects induced by a drug. Notably, they can also overcome the drug resistance of tumor cells. Therefore, these vesicles have received a tremendous amount of attention and have exhibited enormous potential in the development of cancer treatment.
Hu and colleagues successfully constructed a drug-drug conjugated supramolecular nanocarrier based on WP6. In this study, two anticancer drugs, chlorambucil and camptothecin were linked by disulfide bonds, and new conjugated prodrugs were generated through the recognition of a host and guest using WP6.43 This complex could self-assemble into vesicles in an aqueous medium. The disulfide bond had GSH responsive and could be destroyed in tumor cells, causing the simultaneous release of the two drugs and achieving synergistic treatment against cancer cells (Figure 17). Cytotoxicity measurements showed that the vesicle not only killed tumor cells, but also significantly reduced the toxic side effects on normal cells. This work provides a new approach to the preparation of nanocarriers and synergistic chemotherapy. To take advantage of combination therapy, Wang’s group synthesized a compound derived from boron-dipyrromethene (G), which acted as both a guest and a photosensitizer in aqueous medium. It was complexed with WP5 to form superamphiphilic molecules and self-assembled into supramolecular vesicles.66 This vesicle had high DOX encapsulation efficiency and exhibited rapid drug release under acidic conditions. Cytotoxicity experiments showed that these vesicles also had good biocompatibility. Cell internalization tests showed that they localized to lysosomes. Furthermore, DOX-loaded vesicles showed significant therapeutic efficacy through a combination of photodynamic and chemical activity against A549 cancer cells, suggesting that DOX can be used in dual chemical photodynamic treatments against cancer. In 2018, Fan and co-workers constructed supramolecular vesicles for joint chemical photothermal therapy.92 They synthesized a perylene diimide dye derivative that acted as both a photothermal agent and a guest; it was complexed with WP5 to form supramolecular vesicles and had a high loading capacity for the hydrophobic anticancer drug DOX. Cytotoxicity experiments showed that this vesicle had a synergistic effect on MCF-7 cancer cells and a better therapeutic effect than PTT treatment or chemotherapy alone. Moreover, this nanocarrier could easily enter tumor cells by endocytosis, thereby obtaining adequate drug accumulation in the diseased cells. In another work, Fan and co-workers successfully constructed multifunctional supramolecular vesicles through self-assembly based on WP5 and NIR-absorbing bola-type guest diketopyrrolopyrrole (DPP) by simultaneously combining photothermal- and photodynamic-triggered, hypoxia-activated chemotherapy.74 These nanocarriers exhibited excellent encapsulation of the hypoxia-activatable prodrug tirapazamine (TPZ) and rapidly released drugs at tumor sites in an acidic microenvironment. These vesicles produced high heat and ROS under 660 nm laser irradiation for effective anticancer treatment, which indicated that they also had excellent photothermal stability. Since oxygen is continuously consumed during the PDT process, a hypoxic microenvironment could be availably formed, and in this case, the antitumor activity of the prodrug TPZ was further activated to achieve synergistic treatment. In summary, this work provides an innovative approach to building SDDS that shows great advantages for cancer treatment (Figure 18). 2014, Pei’s group constructed a novel cationic vesicle through self-assembled ferrocene-terminated amphiphilic pillar[5]arene (FCAP) in aqueous solution that enabled GSH triggering and siRNA co-delivery, showing low cytotoxicity and significant redox-responsive behavior leading to rapid drug release and gene transfection in cancer cells at high GSH concentrations.88 The cationic vesicle not only effectively improved the bioavailability of anticancer drugs and reduced their adverse side effects on normal cells, but also overcame the problem of cancer cell drug resistance. This is the first report of redox-reactive vesicles for drug/siRNA co-delivery assembled from pillar[n]arenes, which provides a good example of a rational design of an effective stimulus-responsive drug/siRNA co-delivery system.
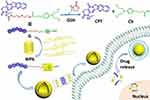 |
Figure 17 Schematic design of the drug–drug conjugate SVs for co-delivery of different anticancer drugs. Reprinted with permission from Shao W, Liu X, Sun GP, Hu XY, Zhu JJ, Wang LY. Construction of drug-drug conjugate supramolecular nanocarriers based on water-soluble pillar[6]arene for combination chemotherapy. Chem Commun. 2018;54:9462–9465.43; Copyright 2018, Royal Society of Chemistry. |
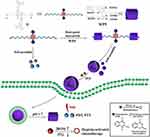 |
Figure 18 Construction of multifunctional supramolecular vesicles for combination cancer therapy.Notes: Reprinted with permission from Wang Q, Tian L, Xu JZ, et al. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem Commun. 2018;54:10328–10331.74; Copyright 2018, Royal Society of Chemistry. |
Conclusion
In conclusion, recent advances in stimuli-responsive supramolecular vesicles generated based on amphiphilic pillar[n]arenes for smart drug delivery were reviewed. Currently, the most widely synthesized amphiphilic pillar[n]arenes are anionic amphiphilic pillar[n]arenes modified by carboxyl groups. Compared to carboxyl groups, sulfonic groups have not been excessively studied in the synthesis of amphiphilic pillar[n]arenes. Yang’s group recently synthesized amphiphilic pillar[n]arenes based on mono-sulfonic acid groups and disulfonic acid groups and used them to construct fluorescent supramolecular systems. In addition, neutral and cationic pillar[n]arenes have been continuously discovered and used because of their excellent biocompatibility, targeting function and co-delivery capacities. Therefore, the synthesis of novel multifunctional amphiphilic pillar[n]arenes is a development trend that is characterized by significant challenges.
Recently, through the development of related research, supramolecular vesicles based on amphiphilic pillar[n]arene have been developed in increasing numbers and used for smart drug delivery. It has become a tremendous research area for potential development. Among recent developments, supramolecular vesicles have been constructed using amphiphilic pillar[n]arenes that can overcome the drug resistance of cancer cells. For example, Hu and co-workers utilized water-soluble pillar[6]arenes in an aqueous medium to construct two anticancer drug-conjugated supramolecular vesicles for chemo-photothermal therapy and chemo-photodynamic therapy for cancer. This work provides a novel method for synergistic chemotherapy. Moreover, Pei’s group developed a different multifunctional cationic capsule capable of GSH triggering and siRNA co-delivery via the ferrocene-modified termini of pillar[5]arenes which provided good examples of effective stimulation of responsive drug release and gene co-transport system design (Figure 19). The abovementioned amphiphilic pillar[n]arene-based multifunctional supramolecular vesicles not only have the functions of single targeting or stimulus-responsive supramolecular vesicles to improve anticancer efficiency and reduce cytotoxicity and side effects induced by the drug in normal cells but also, more importantly, the ability to overcome the drug resistance of tumor cells, thereby improving the effectiveness of chemotherapy. Therefore, in future research, we should continue to pay attention to and research multifunctional supramolecular nanomaterials based on amphiphilic pillar[n]arenes, which will hopefully open up new and promising prospects for synergistic combination therapy for use against cancer.
 |
Scheme 1 Chemical structures of amphiphilic pillar[n]arenes. |
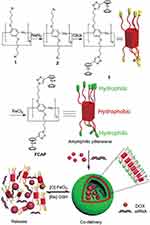 |
Figure 19 Synthetic route of ferrocenium capped amphiphilic PA[5], Illustration of the formation of cationic vesicles, and their redox-responsive drug/siRNA release.Notes: Reprinted with permission from Chang YC, Yang K, Wei P, et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed. 2014;53:13126–13130.88; © 2014 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. |
Funding
This research work was supported by the Specialized Research Fund for the Doctoral Program of Hebei Agricultural University (ZD201712), Undergraduate innovation and entrepreneurship training program (201810086062), Natural Science Foundation of Hebei Province (B2019204243) and Science & Technology Fund of Hebei Agricultural University (LG201811).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gießen-Jung C, von Baumgarten L. Chemotherapie-induzierte periphere Neuropathie. Dtsch Med Wochenschr. 2018;113:970–978. doi:10.1055/s-0043-120839
2. Kalaydina RV, Bajwa K, Qorri B, Decarlo A, Szewczuk MR. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int J Nanomed. 2018;13:4727–4745. doi:10.2147/IJN.S168053
3. Minko T, Dharap SS, Pakunlu RI, Wang Y. Molecular targeting of drug delivery systems to cancer. Curr Drug Targets. 2004;5:389–406. doi:10.2174/1389450043345443
4. Bildstein L, Dubernet C, Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev. 2011;63:3–23. doi:10.1016/j.addr.2010.12.005
5. Kratz F, Müller IA, Ryppa C, Warnecke A. Prodrug strategies in anticancer chemotherapy. ChemMedChem. 2008;3:20–53. doi:10.1002/cmdc.200700159
6. Kim K, Lee M, Park H, et al. Cell-permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J Am Chem Soc. 2006;128:3490–3491. doi:10.1021/ja057712f
7. Hong MH, Zhu SJ, Jiang YY, Tang GT, Pei YY. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J Controlled Release. 2009;133:96–102. doi:10.1016/j.jconrel.2008.09.005
8. Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi:10.1021/ja057254a
9. Basiruddin SK, Maity AR, Saha A, Jana NR. Gold-nanorod-based hybrid cellular probe with multifunctional properties. J Phys Chem C. 2011;115:19612–19620. doi:10.1021/jp206641k
10. Kuo TR, Hovhannisyan VA, Chao YC, et al. Multiple release kinetics of targeted drug from gold nanorod embedded polyelectrolyte conjugates induced by near-infrared laser irradiation. J Am Chem Soc. 2010;132:14163–14171. doi:10.1021/ja105360z
11. Xue YD, Bao L, Xiao XR, et al. Noncovalent functionalization of carbon nanotubes with lectin for label-free dynamic monitoring of cell-surface glycan expression. Anal Biochem. 2011;410:92–97. doi:10.1016/j.ab.2010.11.019
12. Nasongkla N, Bey E, Ren JM, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–2430. doi:10.1021/nl061412u
13. Li GY, Guo L, Ma SM. Self-assembly and drug delivery behaviors of thermo-sensitive poly(t-butyl acrylate)-b-poly (N-isopropylacrylamide) micelles. J Appl Polym Sci. 2009;113:1364–1368. doi:10.1002/app.30033
14. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2006;24:1–16. doi:10.1007/s11095-006-9132-0
15. Napoli A, Valentini M, Tirelli N, MÜller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater. 2004;3:183–189. doi:10.1038/nmat1081
16. Feng WW, Jin M, Yang K, Pei YX, Pei ZC. Supramolecular delivery systems based on pillararenes. Chem Commun. 2018;54:13626–13640. doi:10.1039/C8CC08252A
17. Huang Y, Dong X, Liang J. Review of the application of nanovesicles and the human interstitial fluid in gastrointestinal premalignant lesion detection, diagnosis, prognosis and therapy. Int J Nanomed. 2019;14:9469–9482. doi:10.2147/IJN.S208559
18. Dickerson EB, Blackburn WH, Smith MH, et al. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer. 2010;10:10. doi:10.1186/1471-2407-10-10
19. Guo CX, Yang HB, Sheng ZM, et al. Layered graphene/quantum dots for photovoltaic devices. Angew Chem Int Ed. 2010;49:3014–3017. doi:10.1002/anie.200906291
20. Chen K, Li ZB, Wang H, Cai WB, Chen XY. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35:2235–2244. doi:10.1007/s00259-008-0860-8
21. Yang XQ, Grailer JJ, Rowland IJ, et al. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano. 2010;4:6805–6817. doi:10.1021/nn101670k
22. Shirbin SJ, Ladewig K, Fu Q, et al. Cisplatin-induced formation of biocompatible and biodegradable polypeptide-based vesicles for targeted anticancer drug delivery. Biomacromol. 2015;16:2463–2474. doi:10.1021/acs.biomac.5b00692
23. Xiao TX, Zhong WW, Xu LX, et al. Supramolecular vesicles based on pillar[n]arenes: design, construction, and applications. Org Biomol Chem. 2019;17:1336–1350. doi:10.1039/C8OB03095B
24. Wang K, Guo DS, Wang X, Liu Y. Multistimuli responsive supramolecular vesicles based on the recognition of p-sulfonatocalixarene and its controllable release of doxorubicin. ACS Nano. 2011;5:2880–2894. doi:10.1021/nn1034873
25. Wang S, Yao CH, Ni MF, et al. Thermo- and oxidation-responsive supramolecular vesicles constructed from self-assembled pillar[6]arene-ferrocene based amphiphilic supramolecular diblock copolymers. Polym Chem. 2017;8:682–688. doi:10.1039/C6PY01961G
26. Ogoshi T, Kanai S, Fujinami S, Yamagishi TA, Nakamoto Y. Para-bridged symmetrical pillar[5]arenes: their lewis acid catalyzed synthesis and host–guest property. J Am Chem Soc. 2008;130:5022–5023. doi:10.1021/ja711260m
27. Yu GC, Zhao R, Wu D, et al. Pillar[5]arene-based amphiphilic supramolecular brush copolymer: fabrication, controllable self-assembly and application in self-imaging targeted drug delivery. Polym Chem. 2016;7:6178–6188. doi:10.1039/C6PY01402J
28. Wang Y, Du JW, Wang YX, Jin Q, Ji J. Pillar[5]arene based supramolecular prodrug micelles with pH induced aggregate behavior for intracellular drug delivery. Chem Commun. 2015;51:2999–3002. doi:10.1039/C4CC09274K
29. Liu X, Shao W, Zheng YJ, et al. GSH-responsive supramolecular nanoparticles constructed by β-D-galactose-modified pillar[5]arene and camptotecin prodrug for targeted anticancer drug delivery. Chem Commun. 2017;53:8596–8599. doi:10.1039/C7CC04932C
30. Huang X, Wu SS, Ke XK, Li XY, Du XZ. Phosphonated pillar[5]arene-valved mesoporous silica drug delivery systems. ACS Appl Mater Interfaces. 2017;9:19638–19645. doi:10.1021/acsami.7b04015
31. Chi XD, Ji XF, Shao L, Huang FH. A multiresponsive amphiphilic supramolecular diblock copolymer based on pillar[10]arene/Paraquat complexation for rate-tunable controlled release. Macromol Rapid Commun. 2017;38:1600626. doi:10.1002/marc.201600626
32. Wu MX, Yan HJ, Gao J, et al. Multifunctional supramolecular materials constructed from polypyrrole@UiO-66 nanohybrids and pillararene nanovalves for targeted chemophotothermal therapy. ACS Appl Mater Interfaces. 2018;10:34655–34663. doi:10.1021/acsami.8b13758
33. Xia W, Ni MF, Yao CH, et al. Responsive gel-like supramolecular network based on pillar[6]arene−ferrocenium recognition motifs in polymeric matrix. Macromol. 2015;48:4403–4409. doi:10.1021/acs.macromol.5b00889
34. Guo SW, Liang TXZ, Song YS, et al. Supramolecular polymersomes constucted from water-soluble pillar[5]arene and cationic poly(glutamamide)s and their applications for targeted anticancer drug delivery. Polym Chem. 2017;8:5718–5725. doi:10.1039/C7PY01259D
35. Jie KC, Zhou YJ, Yao Y, Huang FH. Macrocyclic amphiphiles. Chem Soc Rev. 2015;44:3568–3587. doi:10.1039/C4CS00390J
36. Yao Y, Xue M, Chen JZ, Zhang MM, Huang FH. An amphiphilic pillar[5]arene: synthesis, controllable self-assembly in water, and application in calcein release and TNT adsorption. J Am Chem Soc. 2012;134:15712–15715. doi:10.1021/ja3076617
37. Jie KC, Zhou YJ, Yao Y, Shi BB, Huang FH. CO2-responsive pillar[5]arene-based molecular recognition in water: establishment and application in gas-controlled self-assembly and release. J Am Chem Soc. 2015;137:10472–10475. doi:10.1021/jacs.5b05960
38. Yu GC, Xue M, Zhang ZB, et al. A water-soluble pillar[6]arene: synthesis, host−guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J Am Chem Soc. 2012;134:13248–13251. doi:10.1021/ja306399f
39. Li PY, Yao QF, Lü BZ, Ma GP, Yin MZ. Visible light–induced supra-amphiphilic switch leads to transition from supramolecular nanosphere to nanovesicle activated by pillar[5]arene-based host–guest interaction. Macromol Rapid Commun. 2018;39:1800133. doi:10.1002/marc.201800133
40. Chi XD, Yu GC, Shao L, Chen JZ, Huang FH. A dual-thermoresponsive gemini-type supra-amphiphilic macromolecular [3] pseudorotaxane based on pillar[10]arene/paraquat cooperative complexation. J Am Chem Soc. 2016;138:3168–3174. doi:10.1021/jacs.5b13173
41. Cui YH, Deng R, Li Z, et al. Pillar[5]arene pseudo[1]rotaxane-based redox-responsive supramolecular vesicles for controlled drug release. Mater Chem Front. 2019;3:1427–1432. doi:10.1039/C9QM00237E
42. Wu X, Li Y, Lin C, Hu XY, Wang LY. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem Commun. 2015;51:6832–6835. doi:10.1039/C5CC01393C
43. Shao W, Liu X, Sun GP, Hu XY, Zhu JJ, Wang LY. Construction of drug-drug conjugate supramolecular nanocarriers based on water-soluble pillar[6]arene for combination chemotherapy. Chem Commun. 2018;54:9462–9465. doi:10.1039/C8CC05180A
44. Yu GC, Yu W, Shao L, et al. Fabrication of a targeted drug delivery system from a pillar[5]arene-based supramolecular diblock copolymeric amphiphile for effective cancer therapy. Adv Funct Mater. 2016;26:8999–9008. doi:10.1002/adfm.201601770
45. Xiao TX, Qi LJ, Zhong WW, et al. Stimuli-responsive nanocarriers constructed from pillar[n]arene-based supra-amphiphiles. Mater Chem Front. 2019;3:1973–1993. doi:10.1039/C9QM00428A
46. Zhang HC, Liu ZN, Zhao YL. Pillararene-based self-assembled amphiphiles. Chem Soc Rev. 2018;47:5491–5528. doi:10.1039/C8CS00037A
47. Yu GC, Jie KC, Huang FH. Supramolecular amphiphiles based on host−guest molecular recognition motifs. Chem Rev. 2015;115:7240–7303. doi:10.1021/cr5005315
48. Yu GC, Ma YJ, Han CY, et al. A sugar-functionalized amphiphilic pillar[5]arene: synthesis, self-assembly in water, and application in bacterial cell agglutination. J Am Chem Soc. 2013;135:10310–10313. doi:10.1021/ja405237q
49. Shang K, Wang Y, Lu YC, Pei ZC, Pei YX. Dual-targeted supramolecular vesicles based on the complex of galactose capped pillar[5]arene and triphenylphosphonium derivative for drug delivery. Isr J Chem. 2018;58:1205–1209. doi:10.1002/ijch.201800080
50. Zhou YJ, Jie KC, Huang FH. A redox-responsive selenium-containing pillar[5]arene-based macrocyclic amphiphile: synthesis, controllable self-assembly in water, and application in controlled release. Chem Commun. 2017;53:8364–8367. doi:10.1039/C7CC04779G
51. Zhou YJ, Jie KC, Huang FH. A dual redox-responsive supramolecular amphiphile fabricated by selenium-containing pillar[6]arene-based molecular recognition. Chem Commun. 2018;54:12856–12859. doi:10.1039/C8CC06406G
52. Ogoshi T, Kida K, Yamagishi TA. Photoreversible switching of the lower critical solution temperature in a photoresponsive host−guest system of pillar[6]arene with triethylene oxide substituents and an azobenzene derivative. J Am Chem Soc. 2012;134:20146–20150. doi:10.1021/ja3091033
53. Zhang HC, Ma X, Nguyen KT, Zhao YL. Biocompatible pillararene-assembly-based carriers for dual bioimaging. ACS Nano. 2013;7:7853–7863. doi:10.1002/chem.201801315
54. Chi XD, Ji XF, Xia DY, Huang FH. A dual-responsive supra-amphiphilic polypseudorotaxane constructed from a water-soluble pillar[7]arene and an azobenzene-containing random copolymer. J Am Chem Soc. 2015;137:1440–1443. doi:10.1021/ja512978n
55. Chi XD, Yu GC, Ji XF, et al. Redox-responsive amphiphilic macromolecular [2] pseudorotaxane constructed from a water-soluble pillar[5]arene and a paraquat-containing homopolymer. ACS Macro Lett. 2015;4:996–999. doi:10.1021/acsmacrolett.5b00525
56. Li MY, Wang SJ, Xu J, Xu SH, Liu HL. pH/Redox-controlled interaction between lipid membranes and peptide derivative with a “Helmet”. J Phys Chem B. 2019;123:6784–6791. doi:10.1021/acs.jpcb.9b05367
57. Yang K, Pei YX, Wen J, Pei ZC. Recent advances in pillar[n]arenes: synthesis and application based on host-guest interactions. Chem Commun. 2016;52:9316–9326. doi:10.1039/C6CC03641D
58. Kakuta T, Yamagishi TA, Ogoshi T. Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes. Acc Chem Res. 2018;51:1656–1666. doi:10.1021/acs.accounts.8b00157
59. Yu GC, Zhou XY, Zhang ZB, et al. Pillar[6]arene/paraquat molecular recognition in water: high binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J Am Chem Soc. 2012;134:19489–19497. doi:10.1021/ja3099905
60. Li ZT, Yang J, Yu GC, et al. Water-soluble pillar[7]arene: synthesis, pH-controlled complexation with paraquat, and application in constructing supramolecular vesicles. Org Lett. 2014;16:2066–2069. doi:10.1021/ol500686r
61. Shi BB, Jie KC, Zhou YJ, et al. Nanoparticles with near-infrared emission enhanced by pillararene-based molecular recognition in water. J Am Chem Soc. 2016;138:80–83. doi:10.1021/jacs.5b11676
62. Zhao R, Zhou YJ, Jie KC, et al. Fluorescent supramolecular polymersomes based on pillararene/paraquat molecular recognition for ph-controlled drug release. Chinese J Polym Sci. 2020;38:1–8. doi:10.1007/s10118-019-2305-1
63. Duan QP, Cao Y, Li Y, et al. pH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J Am Chem Soc. 2013;135:10542–10549. doi:10.1021/ja405014r
64. Hu XY, Jia KK, Cao Y, et al. Dual photo- and pH-responsive supramolecular nanocarriers based on water-soluble pillar[6]arene and different azobenzene derivatives for intracellular anticancer drug delivery. Chem Eur J. 2015;21:1208–1220. doi:10.1002/chem.201405095
65. Cao Y, Hu XY, Li Y, et al. Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release. J Am Chem Soc. 2014;136:10762–10769. doi:10.1021/ja505344t
66. Meng LB, Zhang WY, Li DQ, et al. pH-responsive supramolecular vesicles assembled by water-soluble pillar[5]arene and BODIPY photosensitizer for chemo-photodynamic dual therapy. Chem Commun. 2015;51:14381–14384. doi:10.1039/C5CC05785J
67. Gao L, Wang TT, Jia KK, et al. Glucose-responsive supramolecular vesicles based on water-soluble pillar[5]arene and pyridylboronic acid derivative for controlled insulin delivery. Chem Eur J. 2017;23:6605–6614. doi:10.1002/chem.201700345
68. Liu X, Jia KK, Wang YC, et al. A dual-responsive bola-type supra-amphiphile constructed from water-soluble pillar[5]arene and naphthalimide-containing amphiphile for intracellular drug delivery. ACS Appl Mater Interfaces. 2017;9:4843–4850. doi:10.1021/acsami.7b00643
69. Yang K, Yang K, Chao S, et al. A supramolecular hybrid material constructed from pillar[6]arene-based host-guest complexation and ZIF-8 for targeted drug delivery. Chem Commun. 2018;54:9817–9820. doi:10.1039/C8CC05665J
70. Chang YC, Hou CX, Ren JL, et al. Multifunctional supramolecular vesicles based on complex of ferrocenecarboxylic acid capped pillar[5]arene and galactose derivative for targeted drug delivery. Chem Commun. 2016;52:9578–9581. doi:10.1039/C6CC03637F
71. Xia DY, Shangguan LQ, Xue M, Shi BB. Dual-responsive self-assembly of a bola-type supra-amphiphile constructed from a new pillar[6]arene-based recognition motif in water and its application in controlled release. New J Chem. 2016;40:9890–9894. doi:10.1039/C6NJ02269C
72. Wheate NJ, Dickson KA, Kim RR, et al. Host-guest complexes of carboxylated pillar[n]arenes with drugs. J Pharm Sci. 2016;105:3615–3625. doi:10.1016/j.xphs.2016.09.008
73. Jiang L, Huang X, Chen D, et al. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar-[6]arenes that are responsive to five stimuli. Angew Chem Int Ed. 2017;56:2655–2659. doi:10.1002/anie.201611973
74. Wang Q, Tian L, Xu JZ, et al. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem Commun. 2018;54:10328–10331. doi:10.1039/C8CC05560B
75. Zhou JY, Xu HA, Tong ZZ, Yang YH, Jiang GH. Photo/pH-controlled host–guest interaction between an azobenzene-containing block copolymer and water-soluble pillar[6]arene as a strategy to construct the “compound vesicles” for controlled drug delivery. Mater Sci Eng. 2018;89:237–244. doi:10.1016/j.msec.2018.04.010
76. Zhong JX, Tang QJ, Ju YS, et al. Redox and pH responsive polymeric vesicles constructed from a water-soluble pillar[5]arene and a paraquat-containing block copolymer for rate-tunable controlled release. J Biomater Sci, Polym Ed. 2019;30:202–214. doi:10.1080/09205063.2018.1561814
77. Zhou YJ, Li ER, Zhao R, Jie KC. CO2-enhanced bola-type supramolecular amphiphile constructed from pillar[5]arene-based host−guest recognition. Org Lett. 2018;20:4888–4892. doi:10.1021/acs.orglett.8b02033
78. Hu XY, Liu X, Zhang WY, et al. Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem Mater. 2016;28:3778–3788. doi:10.1021/acs.chemmater.6b00691
79. Chang YC, Chen JY, Yang JP, et al. Targeting the cell membrane by charge-reversal amphiphilic pillar[5]arene for the selective killing of cancer cells. ACS Appl Mater Interfaces. 2019;11:38497–38502. doi:10.1021/acsami.9b13492
80. Jin XY, Song N, Wang X, et al. Monosulfonicpillar[5]arene: synthesis, characterization, and complexation with tetraphenylethene for aggregation-induced emission. Sci Rep. 2018;8:4035. doi:10.1038/s41598-018-22446-y
81. Wang X, Lou XY, Jin XY, Liang F, Yang YW. A binary supramolecular assembly with intense fluorescence emission, high pH stability, and cation selectivity: supramolecular assembly-induced emission materials. Research. 2019;2019:1454562.
82. Ogoshi T, Ueshima N, Yamagishi TA. An amphiphilic pillar[5]arene as efficient and substrate-selective phase-transfer catalyst. Org Lett. 2013;15:3742–3745. doi:10.1021/ol4016546
83. Chen W, Zhang YY, Li J, et al. Synthesis of a cationic water-soluble pillar[6]arene and its effective complexation towards naphthalenesulfonate guests. Chem Commun. 2013;49:7956–7958. doi:10.1039/c3cc44328k
84. Ma YJ, Ji XF, Xiang F, et al. A cationic water-soluble pillar[5]arene: synthesis and host–guest complexation with sodium 1-octanesulfonate. Chem Commun. 2011;47:12340–12342. doi:10.1039/c1cc15660h
85. Yao Y, Xue M, Chi XD, et al. A new water-soluble pillar[5]arene: synthesis and application in the preparation of gold nanoparticles. Chem Commun. 2012;48:6505–6507. doi:10.1039/c2cc31962d
86. Hu XY, Ehlers M, Wang TT, et al. Formation of twisted B-sheet tapes from a self-complementary peptide based on novel pillararene-GCP host-guest interaction with gene transfection properties. Chem Eur J. 2018;24:9754–9759.
87. Hu XY, Gao L, Mosel S, et al. From supramolecular vesicles to micelles: controllable construction of tumor-targeting nanocarriers based on host–guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small. 2018;14:e1803952. doi:10.1002/smll.201803952
88. Chang YC, Yang K, Wei P, et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed. 2014;53:13126–13130. doi:10.1002/anie.201407272
89. Yang K, Chang YC, Wen J, et al. Supramolecular vesicles based on complex of Trp-modified pillar[5]arene and galactose derivative for synergistic and targeted drug delivery. Chem Mater. 2016;28:1990–1993. doi:10.1021/acs.chemmater.6b00696
90. Lu YC, Hou CX, Ren JL, et al. A multifunctional supramolecular vesicle based on complex of cystamine dihydrochloride capped pillar[5]arene and galactose derivative for targeted drug delivery. Int J Nanomed. 2019;14:3525–3532. doi:10.2147/IJN.S191256
91. Zuo MZ, Qian WR, Xu ZQ, et al. Multiresponsive supramolecular theranostic nanoplatform based on pillar[5]arene and Diphenylboronic acid derivatives for integrated glucose sensing and insulin delivery. Small. 2018;14:e1801942. doi:10.1002/smll.201801942
92. Wang Q, Zhang P, Xu JZ, et al. NIR-absorbing dye functionalized supramolecular vesicles for chemo-photothermal synergistic therapy. ACS Appl Bio Mater. 2018;1:70–78. doi:10.1021/acsabm.8b00014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
