Back to Journals » Clinical Ophthalmology » Volume 15
Suppression of Diurnal (9AM–4PM) IOP Fluctuations with Minimally Invasive Glaucoma Surgery: An Analysis of Data from the Prospective, Multicenter, Single-Arm GEMINI Study
Authors Pyfer MF, Gallardo M , Campbell A, Flowers BE, Dickerson Jr JE , Talla A , Dhamdhere K
Received 22 August 2021
Accepted for publication 13 September 2021
Published 24 September 2021 Volume 2021:15 Pages 3931—3938
DOI https://doi.org/10.2147/OPTH.S335486
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Mark F Pyfer,1 Mark Gallardo,2 Anita Campbell,3 Brian E Flowers,4 Jaime E Dickerson Jr,5,6 Alain Talla,5 Kavita Dhamdhere5,7
1Northern Ophthalmic Associates, Jenkintown, PA, USA; 2El Paso Eye Surgeons, PA, El Paso, TX, USA; 3Grene Vision Group, Wichita, KS, USA; 4Ophthalmology Associates, Fort Worth, TX, USA; 5Sight Sciences, Inc., Menlo Park, CA, USA; 6North Texas Eye Research Institute, University of North Texas Health Science Center, Fort Worth, TX, USA; 7Mahatma Gandhi Medical College and Research Center, Wardha, India
Correspondence: Kavita Dhamdhere
Sight Sciences, Inc., 4040 Campbell Avenue, Suite 100, Menlo Park, CA, USA
Tel +1 877 2661144
Email [email protected]
Purpose: This study analyzes diurnal IOP data (9AM, 12PM, 4PM) from a prospective 12-month trial of the OMNI Surgical System in open-angle glaucoma (OAG) patients with the aim of evaluating effect of MIGS surgery on the amplitude of the diurnal IOP profile pre- and postoperatively.
Setting: Fifteen ophthalmology practices and surgery centers located in 14 states in the United States.
Design: Prospective, multicenter, IRB approved study. Patients treated with canaloplasty (360°) and trabeculotomy (180°). Patients had cataract and mild–moderate OAG with intraocular pressure (IOP) ≤ 33 mmHg on zero to four hypotensive medications.
Methods: Post-hoc analysis of diurnal IOP data from the multicenter GEMINI study. Analysis includes comparison of IOP preoperatively and at month 12 for each of the diurnal time points, 9AM, 12PM, 4PM, change in magnitude of spread between the maximum IOP and minimum IOP for each patient and the proportions of patients preoperatively and at month 12 with IOPs at or below 25, 21, 18, and 15 mmHg, average variability (standard deviation of the 9AM, 12PM, and 4PM IOP) preoperatively and at month 12.
Results: A total of 128 patients included in this analysis. IOP at each diurnal timepoint was significantly lower postoperatively (p< 0.0001). The difference between highest and lowest IOP measurement for each patient averaged 2.8 mmHg preoperatively (SD 2.4, MAX 14, MIN 0) and 1.8 mmHg (SD 1.7, MAX 10, MIN 0) month 12 (P< 0.00001). The proportion with IOP ≤ to 25, 21, 18, and 15 mmHg increased; 75%– 97%, 27%– 88%, 1%– 79%, and < 1%– 56%, respectively. The average variability was greater at all time points preoperatively (P< 0.0001).
Conclusion: This study demonstrates that eyes with OAG can benefit from an overall decreased IOP and degree of IOP fluctuations for as long as 12 months after surgical treatment with canaloplasty and trabeculotomy.
Keywords: primary open-angle glaucoma, trabeculotomy, viscodilation, OMNI surgical system, IOP fluctuation, diurnal IOP
Introduction
Glaucoma is a progressive optic neuropathy that, if unchecked, can result in blindness. Treatment is aimed at preventing or retarding the rate of vision loss such that good visual function is maintained over a patient’s lifespan.1 Elevated intraocular pressure (IOP) is the most important risk factor for development and progression of glaucoma and the only one that can be modified.2 IOP has a normal circadian cycle with pressure generally increasing at night, peaking in the early morning hours, and then declining during the daytime, although peak IOP timing may vary.3–5 Diurnal fluctuation, particularly where peak IOP is relatively high, has been implicated as an important risk factor for glaucoma progression, independent of IOP alone.6–8 Therapies that temper or damp peak fluctuations could thus be of great value in reducing the rate of glaucoma progression.9
Reduction in the amplitude of diurnal IOP fluctuation has been a desired goal of therapeutics for glaucoma for many years. Extended duration of action over a 24-hour period (and longer) has been cited as an important benefit of some topical ocular hypotensive drugs such as the prostaglandin analogs.10 Implant and depot delivery of IOP-lowering drugs have sought to provide long-term IOP control with lower peak amplitude while mitigating the problems associated with daily dosing and patient adherence.11,12 Surgical intervention can also provide for long-term IOP control but, due to the risk and complication profile, was previously reserved for advanced glaucoma where significant and permanent damage had already occurred. The advent of minimally (or micro) invasive glaucoma surgery (MIGS) has, and continues, to change the thinking regarding the place of surgery in glaucoma treatment with surgical intervention becoming increasingly common for mild–moderate glaucoma treatment.13–15 Taking the patient behavior variable out of the treatment equation is an obvious benefit of a MIGS approach. Continuous IOP control that is not reliant on adherence to a dosing regimen should result in a more even and predictable IOP profile over time. The aim of the present study is to challenge this assumption, namely, does MIGS surgery result in an overall decreased amplitude in the diurnal IOP profile when compared to the diurnal IOP profile in the same patients presurgically?
Methods
This was a post hoc analysis of diurnal IOP data collected from patients treated with the OMNI Surgical System (Sight Sciences, Inc, Menlo Park, CA, USA) as participants in the multicenter, historically controlled GEMINI study. The study was a 12-month study with preoperative and terminal medication washouts. Washout durations were standard and based on medication class (eg, 4 weeks for beta-blockers or prostaglandin analogs). Six-month interim results of the a priori stated endpoints have been published16 with 12-month results pending. The GEMINI study was conducted under the oversight of an IRB (Aspire, Santee, CA, USA), all patients provided written informed consent prior to inclusion in the study, and the study was conducted according to the tenets of the Declaration of Helsinki. The GEMINI study was registered on clinicaltrials.gov (NCT03861169).
Patients
The patient population has been described elsewhere.16 Briefly, almost all were primary open-angle glaucoma (POAG) (93%), most were white (82%), 30% self-reported Hispanic ethnicity, and all were mild–moderate glaucoma with an average visual field mean deviation of −3.7 ± 3.6 dB. All were adults with a mean age of 68.7 ± 7.8. Diurnal IOP (DIOP) must have been a minimum of 21 mmHg following the preoperative medication washout.
Following the medication washout, all patients were treated with the OMNI Surgical System (Sight Sciences, Inc., Menlo Park, CA) in combination with phacoemulsification cataract surgery. Details of the treatment have been previously reported.16 Briefly, the OMNI device is used to perform a sequential ab interno canaloplasty followed by a trabeculotomy. In all cases, OMNI was used following phacoemulsification and was a standardized 360 degree canaloplasty and a 180 degree trabeculotomy.
IOP Measurements
Unmedicated washed out IOP was obtained at preoperative baseline and at either 12 or 13 months postoperative. Patients on no medication were assessed at 12 months, while those on medication underwent washout returning 1 month later. For the purposes of this paper both are referred to collectively as “12-month”. Intraocular pressure was measured at 9 AM ± 1.5 hours, 12 PM ± 1 hour, and 4 PM ± 2 hours using Goldmann applanation tonometry and the operator/reader protocol to prevent unintentional bias.17 Two measurements were taken from each eye with the mean value recorded as the IOP for that time point. If the measurements were different by greater than 2 mmHg a third measurement was taken and the median of the three measures was used. Tonometers were calibrated a minimum of every 3 months over the course of the study.
Statistical Analysis
Paired t-tests (one-sided, significance level 0.05) were used to compare IOP preoperatively and at month 12 both for mean diurnal IOP and for each of the diurnal time points, 9AM, 12PM, 4 PM. Repeated measures analysis of variance (ANOVA) was used to evaluate differences in IOP based on time of measurement, preoperatively and at month 12. Levene’s and Bartlett’s tests were used to check homogeneity of variances of IOP at each of the measurement timepoints. The degrees of freedom of the F-test for time effect in the above ANOVA were adjusted appropriately to account for sphericity. With a significant result from the ANOVA-specific contrasts comparing measurements made at different times of day, either preoperatively or postoperatively, were made using paired t-tests with a Bonferroni correction to control the family-wise error rate at 0.05. The question of “Is the magnitude of spread between the maximum IOP and minimum IOP for each patient significantly reduced at month 12 compared to preoperatively” was evaluated using a paired t-test. All statistical analyses were conducted in R (R Core Team, 2021). Chi-square tests were used to compare the proportions of patients preoperatively and at month 12 with IOPs at or below 25, 21, 18, and 15 mmHg.
Results
There were 149 patients enrolled in the GEMINI study and, of these, 128 completed the month 12 visit and were available for this analysis. DIOP was reduced from a preoperative mean of 23.7 ± 3.1 mmHg to 15.6 ± 4.2 at the 12-month endpoint (P<0.0001). IOP at each of the diurnal timepoints was also significantly lower postoperatively than for the corresponding timepoints at the preoperative baseline. Figure 1 shows that 95% (122/128) of patients experienced an IOP reduction, 91% (117/128) an IOP reduction of at least 3 mmHg (an IOP reduction of uncontested clinical significance), and 86% (110/128) an IOP reduction of at least 20%.
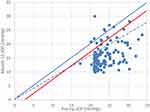 |
Figure 1 Scatterplot of pre-op DIOP versus post-op DIOP for each patient. Points below the diagonal represent a decrease in IOP. Red line indicates a 3 mmHg reduction; Dashed line a 20% reduction. |
The magnitude of the difference between the highest and lowest IOP measurement for each patient at either the preoperative visit or the month 12 postoperative visit provides an index of the IOP fluctuations experienced by each patient before and after treatment (Figure 2). Preoperatively this range averaged 2.8 mmHg (SD 2.4, MAX 14, MIN 0) which was decreased to 1.8 mmHg (SD 1.7, MAX 10, MIN 0) at month 12, a difference that was highly significant (P<0.00001). Excluding those patients that required IOP-lowering medication during the 12-month follow-up and underwent a terminal washout prior to diurnal IOP measurements at month 12 yielded results similar to the group as a whole albeit with an even greater difference between preoperative and month 12 diurnal range in IOP (Figure 3). Regression analysis showed that change in diurnal range of IOP was not associated with baseline preoperative IOP (r2=0.028).
Patient-level IOP data are presented in Figure 3 where all preoperative and all month 12 IOPs are plotted. It is clear from a superficial visual examination of the charts that the amplitude of the IOP measured at each time point for each patient is almost always substantially lower at month 12 when compared to the corresponding preoperative measurement. The exceptions are relatively few in number and are clustered toward the right side of the graphs. There were 24 patients that resumed topical medical therapy during the 12-month follow-up period. These patients underwent a 4-week washout of their medication before measuring and recording the month 12 DIOP used for analysis. These 24 patients are represented by the last 24 data point pairs (on the right side of the graphs in Figure 3). Table 1 presents the proportion of IOP measurements > or ≤ 25 mmHg, 21 mmHg, 18 mmHg, and 15 mmHg at both preoperative and month 12 for each of the three diurnal timepoints. Significantly greater proportions of patients are at or below each of these IOP thresholds at 12 months compared to pre-operatively at all three diurnal timepoints (all P<0.0001, chi-square test, Table 1). Taken together, the proportion of IOP measurements less than or equal to 25, 21, 18, and 15 mmHg increased from 75% to 97%, 27% to 88%, 1% to 79%, and <1% to 56%, respectively. The absolute variability (standard deviation of the 9 AM, noon, and 4 PM IOP) preoperatively and at month 12 is shown in Figure 4. Note that the preoperative variability is greatest for 61% (78/128), equal for 6% (8/128), and less for 33% (42/128) statistically significant (P<0.0001, 3-sample test for equality of proportions, chi-square 86.2, df=2).
 |
Table 1 Number and Proportion of Patients with IOP Above and Below 15, 18, 21, and 25 mmHg at Preoperative and Month 12 Postoperative Visits at 9AM, Noon, and 4PM |
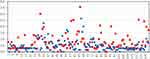 |
Figure 4 Variability in the three diurnal IOP measurements (9 AM, noon, 4 PM) for each patient at preoperative (red) and month 12 (blue). |
Discussion
Our analysis of GEMINI data shows that in addition to significant overall mean IOP reduction, the amplitude of mean IOP was reduced at each of the diurnal time points where IOP was measured. Moreover, 95% of patients had diminished peak IOP post-surgically when compared to the preoperative measurements. The importance of IOP reduction is inarguable and has been demonstrated in landmark studies such as the AGIS and the EMGT.2,18 The importance of IOP fluctuation in glaucoma progression is also generally accepted. Asrani et al showed that range of home-measured IOP over 5 days was the strongest predictor of progression over 8 years. Cumulative risk of progression was 88% for the patients with the greatest range in IOP (upper quartile) and 57% for those in the lower quartile.6 Kim et al classified eyes using the Glaucoma Rate Index (GRI) as fast progressors (GRI ≤ −37) or slow progressors (GRI between −37 and −6). Logistic regression analysis identified higher peak IOP as a significant predictor for fast progression.7 In the present study, we show that the month 12 variability and range in the IOP measurements was significantly decreased from the preoperative level, effectively, a “flattening” of the diurnal curve, at least during daylight (ie, office) hours. Whether or not this can be extrapolated to the nighttime hours, where IOP was not measured, cannot be determined from this dataset.
Glaucoma is a pernicious disease that can cause substantial and significant damage before it is detected.19,20 This fact, and the development of surgical treatments with favorable safety profiles, has led to an increasing willingness to intervene as early as possible in the course of the disease.21 The OHTS showed that over a 5-year period, the likelihood of developing glaucoma, defined as a reproducible visual field defect or clinically significant optic disc defect, was over twice as great in untreated versus treated eyes.22 Similarly, the EMGT demonstrated that early intervention (SLT and betaxolol drops) in patients with early glaucoma resulted in a smaller proportion of patients showing progression compared to untreated controls (59% and 76%, median follow-up of 8 years) and for those showing progression, mean time to progression was greater for treated than for the untreated controls (67 months versus 49 months).18 Migdal et al showed that, in newly diagnosed glaucoma, early surgical treatment with trabeculectomy resulted in no significant visual field deterioration over the 5-year follow-up period.23 While lowering IOP is universally acknowledged to be key in limiting progressive glaucomatous damage, modulation of peak 24-hour IOP has been increasingly recognized as important.24 IOP fluctuation and peak IOP have been shown to be significant risk factors for progression of glaucoma.7,9 Our data are limited to showing modulation of IOP fluctuation during just one-third of the full 24-hour diurnal cycle and we do not know if this can be generalized to the span of hours (including nighttime) where IOP was not measured. However, it is not unreasonable to speculate that interventions reducing both IOP and the magnitude of diurnal fluctuations, even if limited to a portion of the full diurnal cycle, should have a salutary effect on disease progression.
Evidence that IOP increases during the night, likely due to both circadian factors as well as supine body position,25 suggests that any effort directed at IOP reduction should also attempt to provide 24-hour efficacy. A beta-blocker, for example, would provide little if any nighttime protection because aqueous humor production is substantially diminished at night.26 On the other hand, it seems reasonable that the influence of surgical modification of the conventional outflow pathway, by reducing resistance to outflow, may be operative throughout the 24-hour cycle. While certain drugs, such as the prostaglandins, can have a 24-hour duration of IOP-lowering effect,27 they remain reliant on patient adherence and behavior to consistently achieve this benefit. In contrast, depending on whether IOP-lowering medications have been eliminated or simply reduced, patient adherence has a lesser (or no) impact on IOP-control following surgical intervention.
Showing diminished IOP peaks and fluctuations should not be confused with demonstrating an effect on progression of disease. Direct demonstration of retarding or halting progression requires a specific protocol design with rigorous visual field testing, repeat testing when defects are detected, and generally a multi-year follow-up period.28,29 Nevertheless, there is strong evidence linking IOP peaks and fluctuations to progression of disease.6,7 Significantly reducing IOP peaks and fluctuation, in addition to significant and clinically meaningful overall IOP reduction, as shown in the GEMINI study, suggests that the patients in this study treated with a MIGS procedure such as OMNI should be less likely to progress as rapidly as before the surgical intervention. Kim et al showed that eyes categorized as “fast decaying” in terms of visual field had mean IOP of 14.1 mmHg, a peak IOP of 22.4 mmHg, and IOP fluctuation of 3.3 mmHg. Conversely, “stable” or “improving” eyes had essentially the same mean IOP as fast decayers (14.1 vs 14.3 vs 13.8 mmHg) but a peak IOP that was 2.6 to 3.4 mmHg lower and IOP fluctuation that was approximately 1 mmHg lower (3.3; 2.5 mmHg).7 The results from the present study show peak IOP reduced by over 8 mmHg and a reduction in fluctuation of 1 mmHg (from 2.8 to 1.8) suggesting that the risk of visual field progression may have been ameliorated, particularly in comparison to their pre-study, unmedicated status.
There are limitations to the present analysis. First, analysis of fluctuation was not an a priori goal of the GEMINI study. However, we have assiduously employed appropriate statistical analyses including testing for normality and using distribution-free methods where appropriate, controlling for multiplicity using Bonferroni correction, and limiting our data interrogation to focus on intrapatient IOP variability. Second, as stated above, this study does not address progression. The GEMINI study was not designed to assess progression. Automated perimetry was performed at preoperative baseline and at month 12 primarily for eligibility and safety reasons; however, there were no adverse events for worsening of visual field mean deviation. Third, there was not an untreated control group. Including a “no treatment” or placebo control is problematic for ethical reasons in a 12-month long study such as GEMINI where patients have a progressive and potentially blinding disease. However, the analysis does compare preoperative with postoperative IOP data for the same patients; effectively each patient serving as their own control. Finally, our data suggest that MIGS could be effective in controlling IOP fluctuations, but our data are restricted to eyes treated with canaloplasty and trabeculotomy using the OMNI Surgical System. We do not know if our findings can be generalized to other MIGS procedures and implants.
The present study demonstrates that eyes with primary open-angle glaucoma can benefit from an overall decreased IOP and degree of IOP fluctuations for as long as 12 months after surgical treatment with canaloplasty and trabeculotomy. Further study with a greater sample size and longer follow-up are needed and ongoing. Study using other MIGS procedures to determine the generalizability of these results are also needed.
Data Sharing Statement
Subject-level data beyond what is included in this article are not publicly available. Requests for data will be considered on a case by case basis and should be directed to the corresponding author (KD).
Disclosure
Dr Mark F Pyfer reports personal fees from Sight Sciences, during the conduct of the study; advisory board from Aerie and Johnson & Johnson, advisory board speakers bureau from Omeros and Eyevance, speakers bureau from Sight Sciences, outside the submitted work. Dr Mark Gallardo reports grants, consultant and speaker from Sight sciences, during the conduct of the study and outside the submitted work; grants, personal fees from Sight sciences. Dr Anita Campbell reports paid speaker and site PI for Sight Sciences, during the conduct of the study. Dr Brian E Flowers reports grants, personal fees from Sight Sciences, during the conduct of the study; personal fees from iStar, grants from Alcon, grants from Santen, grants from Nicox, personal fees from Ivantis, grants from Arie, personal fees from Glaukos, grants, personal fees from Sight Sciences, outside the submitted work. Dr Jaime E Dickerson Jr reports the GEMINI study was funded by Sight Sciences. He is an employee of Sight Sciences. Dr Kavita Dhamdhere reports full-time employee of Sight Sciences. The authors report no other conflicts of interest in this work.
References
1. Spaeth GL, Lopes JF, Junk AK, Grigorian AP, Henderer J. Systems for staging the amount of optic nerve damage in glaucoma: a critical review and new material. Surv Ophthalmol. 2006;51(4):293–315. doi:10.1016/j.survophthal.2006.04.008
2. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi:10.1016/S0002-9394(00)00538-9
3. Zeimer RC, Wilensky JT, Gieser DK, Viana MA. Association between intraocular pressure peaks and progression of visual field loss. Ophthalmology. 1991;98(1):64–69. doi:10.1016/S0161-6420(91)32340-6
4. De Venecia G, Davis MD. Diurnal variation of intraocular pressure in the normal eye. Arch Ophthalmol. 1963;69:752–757. doi:10.1001/archopht.1963.00960040758013
5. Drance SM. The significance of the diurnal phasic variation of intraocular pressure in normal and glaucomatous eyes. Trans Can Opthalmolog Soc. 1960;23:131–140.
6. Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9(2):134–142. doi:10.1097/00061198-200004000-00002
7. Kim JH, Rabiolo A, Morales E, et al. Risk factors for fast visual field progression in glaucoma. Am J Ophthalmol. 2019;207:268–278. doi:10.1016/j.ajo.2019.06.019
8. Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118(9):1766–1773. doi:10.1016/j.ophtha.2011.01.047
9. Caprioli J. Intraocular pressure fluctuation: an independent risk factor for glaucoma? Arch Ophthalmol. 2007;125(8):1124–1125. doi:10.1001/archopht.125.8.1124
10. Dubiner HB, Sircy MD, Landry T, et al. Comparison of the diurnal ocular hypotensive efficacy of travoprost and latanoprost over a 44-hour period in patients with elevated intraocular pressure. Clin Ther. 2004;26(1):84–91. doi:10.1016/S0149-2918(04)90008-2
11. Robin AL, Clark AF, Covert DW, et al. Anterior juxtascleral delivery of anecortave acetate in eyes with primary open-angle glaucoma: a pilot investigation. Am J Ophthalmol. 2009;147(1):45–50.e2. doi:10.1016/j.ajo.2008.07.039
12. Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–1641. doi:10.1016/j.ophtha.2020.06.018
13. European Glaucoma Society. European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Br J Ophthalmol. 2017;101(6):130–195.
14. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern®. Ophthalmology. 2021;128(1):P71–P150.
15. Yang SA, Mitchell W, Hall N, et al. Trends and usage patterns of minimally invasive glaucoma surgery in the United States: IRIS® registry analysis 2013–2018. Ophthalmol Glaucoma. 2021. doi:10.1016/j.ogla.2021.03.012
16. Gallardo MJ, Sarkisian SR, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification in open-angle glaucoma: interim results from the GEMINI study. Clin Ophthalmol. 2021;15:481–489. doi:10.2147/OPTH.S296740
17. Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117(5):573–583. doi:10.1001/archopht.117.5.573
18. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121(1):48–56. doi:10.1001/archopht.121.1.48
19. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–464. doi:10.1016/0002-9394(89)90488-1
20. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741–748.
21. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi:10.1097/ICU.0b013e32834ff1e7
22. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):
23. Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101(10):
24. Wax MB, Camras CB, Fiscella RG, Girkin C, Singh K, Weinreb RN. Emerging perspectives in glaucoma: optimizing 24-hour control of intraocular pressure. Am J Ophthalmol. 2002;133(6):S1–10. doi:10.1016/S0002-9394(02)01459-9
25. Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912–2917.
26. Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci. 1991;32(13):3145–3166.
27. Toris CB, Zhan G, Fan S, et al. Effects of travoprost on aqueous humor dynamics in patients with elevated intraocular pressure. J Glaucoma. 2007;16(2):189–195. doi:10.1097/IJG.0b013e31802fc6d3
28. De Moraes CG, Liebmann JM, Levin LA. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res. 2017;56:107–147. doi:10.1016/j.preteyeres.2016.10.001
29. Weinreb RN, Kaufman PL. The glaucoma research community and FDA look to the future: a report from the NEI/FDA CDER Glaucoma Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2009;50(4):1497–1505. doi:10.1167/iovs.08-2843
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


