Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Supporting Shared Decision-Making and Home Dialysis in End-Stage Kidney Disease
Authors Campbell-Montalvo R , Jia H , Shukla AM
Received 2 June 2022
Accepted for publication 30 August 2022
Published 8 September 2022 Volume 2022:15 Pages 229—237
DOI https://doi.org/10.2147/IJNRD.S375347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pravin Singhal
Rebecca Campbell-Montalvo,1,2 Huanguang Jia,2 Ashutosh M Shukla2,3
1Department of Curriculum and Instruction, Neag School of Education, University of Connecticut, Storrs, CT, USA; 2Department of Medicine, North Florida/South Georgia Veteran Healthcare System, Gainesville, FL, USA; 3Division of Nephrology, Hypertension and Transplantation, Department of Medicine, University of Florida, Gainesville, FL, USA
Correspondence: Ashutosh M Shukla, North Florida South Georgia Veteran Healthcare System, Division of Nephrology, Hypertension and Transplantation, University of Florida, 1600 Archer Road, Gainesville, FL, 32610, USA, Tel +1 352-273-8821, Fax +1 352-392-5465, Email [email protected]
Abstract: It has been widely demonstrated that patient education and empowerment, especially involving shared treatment decisions, improve patient outcomes in chronic medical conditions, including chronic kidney disease requiring kidney replacement therapies. Accordingly, regulatory agencies in the US and worldwide recommend shared decision-making for finalizing one’s choice of kidney replacement therapy. It is also recognized that the US needs to substantially increase home dialysis utilization to leverage its positive impacts on patient and healthcare cost-related outcomes. This perspective highlights how the routine clinical use of the recommended practice of shared decision-making can exist in synergy with the system’s goal for increased home dialysis use. It introduces a pragmatic provider checklist, The Nephrologist’s Shared Decision-Making Checklist, grounded in the relevant theories of shared decision-making, and, unlike some research assessments and extant tools, is easy to understand and implement in clinical practice. This qualitative Checklist can help providers ensure that they have co-constructed an SDM experience with the patient and involved caretakers, helping them benefit from the improved outcomes associated with SDM.
Keywords: shared decision-making, informed decision-making, kidney failure, end-stage renal disease, home dialysis, patient engagement, patient empowerment
Introduction
High morbidity and mortality, poor health-related quality of life, and disproportionately high healthcare costs make kidney failure, commonly referred to in the literature as end stage kidney disease (ESKD), a significant concern for the US healthcare system.1 Pragmatic challenges of managing advanced chronic kidney disease (CKD) combined with decades of regulatory policies have unearthed several societal concerns in the management of ESKD. While kidney transplantation provides the most desirable form of kidney replacement therapy, limitations in the availability of organs combined with ineligibility for transplantation due to high comorbidity index necessitate initiation of dialysis in overwhelming majority (~97%) of incident ESKD patients. Currently, home dialysis consisting of peritoneal dialysis and home hemodialysis is used in about 10% of the ESKD patients, and over 90% of the incident and prevalent ESKD patients use in-center hemodialysis as kidney replacement therapy.2 Considering that home dialysis therapies provide equivalent survivals and potential for improved patient-centered and health services outcomes,1,3–5 these paradoxically low rates of utilizations have prompted regulatory agencies, such as the Center for Medicare and Medicaid Services (CMS) to repeatedly advocate policies that increase home dialysis use. A recent presidential executive order established a high target of over 80% for home dialysis and kidney transplantation use in incident ESKD patients by 2025.2
While feasibility constraints play some role, lack of patient awareness and engagement constitute a significant and addressable barrier in this arena. Many at-risk advanced CKD patients, even those under nephrology care have poor CKD awareness and do not have opportunities to receive information about therapeutic considerations from a nephrologist before they developed ESKD.6,7 Prior studies in several chronic disease models show that improving patient awareness significantly improves patient outcomes. Studies in the CKD population have also shown that patient education, especially involving shared decision-making (SDM) between patients and providers, improves self-management8–12 and increases the rates of kidney transplantations and home dialysis utilization.13–16 Therefore, investigators and professional renal organizations have repeatedly recommended using SDM to increase patient-centered utilization of home dialysis therapies for all patients with advanced, stage 4 and 5 CKD.17,18
Unfortunately, the existing SDM measures, such as SDM-Q9-DOC, OPTION5, and DSAT10, while being helpful research and methodological tools and conceptual pieces, have not been studied well in the ESKD context. Thus, physician awareness of these measures is poor, their applicability in pragmatically guiding ESKD patients’ lifestyle goals vis-a-vis their medical situation and access to dialysis modalities is limited, and they are uncommonly implemented in routine CKD and ESKD care. A recent United States Renal Data System (USRDS) analysis showed that less than 1% of incident ESKD patients between 2010 and 2014 received targeted kidney disease education in their pre-ESKD period.19,20 Furthermore, these concerns are syndemic in that health disparities and social inequality influence the risks, therapy, and outcomes of many chronic illnesses including ESKD.21–24 For example, compared to their white counterparts, racial/ethnic minority groups, including Latinx, Asian/Pacific Islander, Native American, and Black individuals, not only have a higher lifetime probability of being diagnosed with CKD and ESKD but also have lower access to pre-ESKD kidney disease education,20 which further correlates with lower quality-of-care at incident ESKD and kidney replacement therapy. Thus, disparities in SDM contribute to disparities in ESKD care in terms of lower rates of functioning vascular access,19,20 kidney transplantations, and home dialysis therapies.17,25
We propose a parsimonious and clinically relevant SDM model, The Nephrologist’s SDM Checklist, which providers can use easily to ensure that they have co-constructed an SDM experience with their patients and caretakers/partners. We hope that increasing its ease and implementation will allow nephrologists to provide an SDM experience more universally, irrespective of the sociodemographic characteristics and thus, addressing some of the existing inequalities of the differential SDM access. The Checklist follows the gold standard of ESKD treatment strategy: Educate early for later intervention. Additionally, a strength of the tool is that it is purposively qualitative. This is vital given that SDM interactions for ESKD management, unlike many acute care scenarios, occur over an extended period during which patients’ clinical, psychological, and lifestyle needs are everchanging. This is useful for a range of patients, including those who have difficulty making decisions about themselves. Additionally, kidney disease management decisions, whether relating to dialysis or other treatments, require the patient to digest a lot of information and balance it within their own understanding of their health case as well as their lifestyle preferences and other factors. Providing multiple checkpoints relating to these concerns over a longer period supports our argument that the Checklist can be an important tool in guiding a practitioner in their SDM execution and allow them to be reasonably assured that SDM has occurred. Overall, the perspective aims to appraise clinicians to the concepts of SDM and introduce an easy-to-implement blueprint of SDM for routine clinical use to enhance the quality of advanced CKD care, with an eventual goal of improving patient-centered outcomes, including informed home dialysis utilization.
Shared Decision-Making in General Health
In the past decade, there has been extensive work articulating the general principles, practices, and models of SDM.10,12,26–28 In a landmark paper Barry et al initially surmised that SDM resides at the pinnacle of modern-day patient-centered care. Following, Elwyn et al defined SDM as
an approach where clinicians and patients share the best available evidence when making decisions, and where patients are supported to consider options to achieve informed preferences.29
Given the scarce relative guidance on the clinical application of SDM, Elwyn et al originally proposed a three-phase SDM general practitioner model consisting of Choice Talk —similar to the model used in the two case vignettes, providing patients options from which to make a choice. It is followed with Option Talk, clarifying additional details about the choices relevant for the patient, and then Decision Talk, revolving around physician support of the patient’s processes where they weigh options vis-à-vis their preferences to decide for themselves. Critical in this model are interventions that support decisions in which information is presented and summarized in a way that is up-to-date, inclusive of risks and benefits, and summarizes the main points in an accessible manner. Recently, Elwyn et al updated their original three-talk model, replacing the concept of Choice Talk with Team Talk, aimed at forging a partnership or team-based approach between the practitioner and patient to meet the patient’s needs,28 and adding how the talking model can be adjusted as patient goals change over time.27 These principles and subsequent evolutions have been further incorporated into the Consumer Assessment of Healthcare Provider and System (CAHPS) Ambulatory Care Improvement Guide (2020).30
Extending Elwyn et al’s model, Légaré and Thompson-Leduc have clarified the myths of the pragmatic application of SDM in clinical practice.31 These authors contended that SDM is not necessarily easy, cannot be replaced by a tool, and is not a fad that leaves patients alone to make decisions, but is widely desired, that the patients are generally well suited to take part in it, and can be performed inexpensively without devoting unsustainable amounts of time. The authors argued that even in instances where the patients ask the physician, “what they would do” can be handled well within the scope of clinical guidelines of SDM practice. Finally, they cautioned against the complacency; even when the physicians believe that they are practicing SDM, they need to look closely to ensure that clinical decision-making is patient-centered and free of patient and provider bias. They advised that the SDM should be achieved through a bilateral agreement that accounts for patients’ care goals within the limitations of evidence-based medicine.31 These rationales have been further validated by the CAHPS Ambulatory Care Improvement Guide (2020) SDM guidelines, which contend that practitioner assessment of patient involvement in SDM can help improve SDM.30
The Current State of SDM in ESKD
To background the concept and myths of SDM in ESKD, we highlight two commonly occurring vignettes from clinical practice (Box 1). These accounts present the micro-level of patient interaction, illustrating how patients in need of education may not receive it –even when receiving nephrology care for an extended period. Additionally, while these examples further show the importance of patient education on ESKD treatment options for a meaningful impact on their lives, the vignettes, by their design and need, are focused on the role of SDM concerning the underused home dialysis therapies and are not intended to draw a contrast with the conventional hemodialysis. Finally, the discussions following show that while recent advances in SDM contextualize some of the methodological concerns and rationale, more work remains on assessing when SDM has occurred for advanced CKD patients – an essential before more widespread SDM can be envisioned.
 |
Box 1 Case Vignettes Highlighting the Pragmatic Occurrence of SDM in Advanced CKD and ESKD |
While regulatory agencies and professional societies have repeatedly called for widespread adoption of SDM to increase rates of home dialysis and kidney transplantation,13 few studies have examined the impact of SDM on ESKD. At the same time, there is a general lack of understanding of how SDM can be effectively implemented in clinical practice and the barriers to SDM use/adoption.8
Among the studies examining the importance of SDM in advanced CKD, Thamer et al used a self-developed 7-item tool to evaluate the medical records of nearly 70,000 patients and predict the risk of death among elderly patients starting kidney replacement therapy. Survival did not differ significantly among elderly patients with high levels of comorbidities –whether they underwent dialysis therapy or conservative management. However, the authors showed that the risk score developed, can help inform practice of SDM to support informed patient care choices.32 Others have examined the role of SDM on patient satisfaction and outcomes across dialysis modalities, reaching similar conclusions. In a survey-based study of over 780 dialysis patients in Germany, Robinski et al found that satisfaction with treatment modalities and the perception of the SDM were higher among patients on peritoneal dialysis than hemodialysis. While SDM affected patient satisfaction, the factors affecting patients’ treatment choices likely further contributed to their perception of SDM. Hemodialysis patients reported being restricted because of medical issues, decisions by their practitioner, or their desire to rely on the support of hemodialysis staff. Peritoneal dialysis patients highlighted their desire for independence for their choice. The authors concluded that screening patient preferences early on, identifying practitioner biases in consultation, employing accessible vocabulary, and encouraging patients, especially those who are passive, to participate in selecting therapy, likely helped patients understand the quality of life and treatment benefits of dialysis.33
Drawing further on patient-centeredness, Benito and Luis suggest that protocol and practice of SDM would likely vary across different populations, given heterogeneous needs across groups. They considered that an elderly patient might desire greater symptom control, a younger individual more of an active lifestyle, or a caregiver increased scheduling flexibility.34 While this is important, providers also need to critically examine variations in SDM for societal bias and to reduce disparities. For example, Barrett et al17 assessed responses from participants enrolled in a randomized trial aimed at evaluating patient–nephrologist interactions about kidney replacement therapies. They considered whether patients had spoken with their doctor about how various options could affect their quality of life, life expectancy, finances, family, and need for assistance. The study found that women, Black participants, people with low income, and those with lower levels of education had more thoroughly discussed dialysis compared to transplants with their nephrologists. Thus, patients in these subgroups were not provided the same access to full SDM, i.e., all options for kidney replacement therapy, including transplant. As these groups may have decreased access to knowledge about transplants, increasing the tendency for nephrologists to share this option could increase the access to transplants for these groups.
Evaluating the role of SDM on patient outcomes, healthcare quality, and healthcare utilization in about 64,000 patients, Hughes et al35 found that individuals who were a race other than white, unmarried, un/underinsured, and with lower levels of education or socioeconomic status were less likely to receive quality SDM. They further showed that this reduced SDM quality was associated with more negative patient-reported health outcomes, lower quality of life indicators, and increased healthcare utilization. Similarly, examining the role of SDM on the quality of delivered care, Ruchi et al examined the rates of vascular access creation among the US incident hemodialysis patients.19 Despite substantial racial and ethnic disparities in the delivery of SDM, the presence of pre-ESKD kidney disease education services among incident hemodialysis patients had twice the rates of incident vascular access. These authors argue that increasing physician education may help the utilization and quality of SDM.
Together, this literature shows that while the concept of SDM has significantly improved in recent times, its implementation and practice in routine clinical care requires additional attention. In a systemic review showing that patients with opportunities for SDM are more satisfied with their dialysis modality, Yu et al articulated multiple barriers practitioners encounter in implementing SDM, including lack of pertinent tools and training in SDM, high clinical workload, and an assortment of language, education, literacy, and technology-related barriers inhibiting patient engagement in SDM. The authors list 17 recommendations contextualized within Elwyn et al’s original three-talk model,12 providing a backdrop to our introduction of a more practitioner-friendly model that fills a critical gap in existing materials and can ease the implementation of SDM.
The Nephrologist’s SDM Checklist
SDM takes concerted effort, and providers need to specifically evaluate their practices and how they are serving patients in this regard.31 While models exist on what SDM may look like in clinical practice,29 it is important for providers to clearly understand when SDM can be said to have occurred. Such assessment is crucial in increasing its routine implementation across the socio-demographic spectrum.30
The goal of the Nephrologist’s SDM Checklist (Table 1) is to help providers support patients in making crucial decisions regarding treatment, addressing their future needs and care, and maintaining the lifestyle they desire.36,37 The Checklist helps providers determine whether they have succeeded in supporting patients’ treatment choices, assist patients’ access to evidence, and support patients in weighing options in light of informed preferences.29 A routine and universal execution of the Checklist for all patients with advanced CKD with high likelihood of progression to ESKD is suggested given the established needs and recommendations for SDM and patient autonomy, including the understanding that patients want SDM and can participate.31 We expect providers to use the Nephrologist’s SDM Checklist within clinical guidelines,31 and in concert with such considerations as risk scores.32 Finally, the use of the Checklist can be potentiated by combining it with the employment of additional resources such as decision aids, decision coaching, or evaluation questionnaire or surveys.37
 |
Table 1 The Nephrologist’s Checklist for Shared Decision-Making in ESKD |
The Checklist below is scaffolded on the authors’ collective experience in renal and non-renal arenas and informed by the literature. The Checklist has four pragmatic questions that providers ask themselves to determine whether SDM has been achieved in clinical practice. For SDM to occur, providers do not have to engage in the entire spectrum of activity in one sitting but can (and often preferably so) achieve these through a series of steps and visits during routine care. Additionally, the providers also may not engage in every aspect of patient education by themselves but can engage the multidisciplinary team consisting of a combination of dieticians, social workers, or dialysis nurses, or trained educators, etc, to achieve the SDM. Once the answer to a question in the Checklist is “Yes”, the provider can move to the next question. We recommend that the four questions are reviewed serially and periodically, for example, as patient context changes.
We suggest the practitioner begin with question 1, by first asking and allowing the patient to articulate the goals of their life in the context of their diagnosis of advanced CKD and its progression with respect to their overall health. This necessitates that the provider ensures that the patient is informed of their CKD diagnosis and their overall healthcare burden. Once articulated, these goals should be recorded and updated as needed with any significant medical or social changes in patients’ lives. Addressing the goals of care provides an opportunity to clarify the diagnosis for the patient, an issue highlighted in the above-described Vignette 2, allowing the patient to form realistic expectations of the ESKD diagnosis in their life. Decision aids in the form of questionnaires that allow patients to consider their needs and preferences for travel, schedule, responsibilities, and transportation can be administered during this stage to initiate patient familiarity with ESKD management options.38 A detailed review of the currently available decision aids was recently published, but attention is needed to ensure that the aid used in the clinical care is current with clear evidence-based guidance, culturally and linguistically compatible, and is easy to implement in clinical practice.12
The burden of advanced CKD rises with increasing age. Accordingly, many elderly (and non-elderly) patients, such as people with affected cognition, may have a caregiver or partner to help them deal with kidney disease and consider treatment options. Thus, practitioners may find that SDM conversations are had with a caregiver in addition to the patient. Once the goals of life and goals of care are determined, which are primarily the domain of the patient’s wishes, with explicit patient permission, the provider may conduct discussions with the patient and their caregiver(s) together—as a unit (question 2). The Option Talk needs to be all-inclusive, covering dialysis, transplantation, and conservative care, irrespective of the individual patient’s eligibility for a given form of therapy. This avoids later confusion and provides a trustful base on which the provider can conduct the later choice discussions applicable to individual patients’ medical and social conditions. Constant communication and restatement of lifestyle preferences and diagnosis may be needed—such practices likely could have helped the patient in Vignette 1 take more control of his disease at an earlier stage.
Once Decision Talk and other relevant practices have been completed, the practitioner needs to evaluate whether the patient has reached a decision for kidney replacement therapy in question 3. Analyzing long-term outcomes of ESKD among patients undergoing kidney disease education, Devins et al found that denial of the disease state is associated with poor long-term outcomes, including lower rates of incident vascular access and increased mortality.39 Thus, while structuring the frequency and delivery of education, it may be vital for the provider to emphasize the need for and target dates for decision-making with options for adjusting them to the individual patient’s comfort. The provider can then assess whether the decision has been reached with a reasonable confidence level, also in question 3. In the era of internet ubiquity and misinformation, many nephrologists, including the authors, have countless examples of well-informed ESKD patients wishing for home dialysis being swayed away by inaccurate information, occasionally even that from other medical practitioners. Evaluating the impact of education on the quality of incident ESKD care, Hanko et al found that while education substantially increases the rates of home dialysis utilization, the rates of vascular access are lower among the patients who remain indecisive after education compared to those choosing hemodialysis.40 A pragmatic measure of assessing patients’ confidence is by asking how confident the patient/caregiver feels about their ability to explain the pros and cons of their choice to their inner circle or primary care provider.
Next, the provider must consider whether the patient’s choice is congruent with the patient’s stated goals of care and comorbidity burden and is without significant misconceptions or fear about dialysis therapies (question 4). If the answer to question 4 is “No”, or when the chosen modality and the stated goals of care appear incongruent to the provider, the provider may need to revisit the first three items with particular emphasis on relevant clinical evidence. Providers must be cautious regarding their own biases, as highlighted in Vignette 2, which shows a common misconception that patients with a perceived history of non-adherence may not be good candidates for home dialysis. Finally, while it may not be possible to avoid all fear and apprehension, the provider needs to minimize the element of fear as an overriding motivator of the decision. A detailed discussion about the pros and cons of dialysis vs conservative care might have avoided the life-threatening illness in Vignette 1. Once the answer to all four items is “Yes”, the practitioner can be assured that SDM has most likely occurred, but they still need to watch for future social and clinical events likely to affect the SDM choices in real-time.
Implications
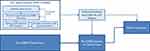
The design of the Nephrologist’s Checklist has important implications. It addresses some pragmatic concerns experienced by clinicians and articulated by Engels et al and informs on how SDM can be implemented effectively and routinely in clinical practice, and supports the increase of SDM use by providers.8 The Checklist’s utility in potentially increasing physician fidelity in implementing SDM could improve patient SDM receipt.35 The Checklist may further help address the documented differential access of groups to SDM by introducing a way for practitioners to evaluate their provision of SDM to patients systematically.17 Indeed, it is our aim that appropriate implementation of the Checklist across groups by providers will increase SDM access for those often at a disadvantage when it comes to SDM, including minorities, women, and lower-income patients.17,35 We echo Benito and Luis in cautioning that the details of SDM and implementation of the Checklist will vary based on individual case needs.34 However, establishing optimal ESKD treatment lead time through early SDM supports improvement in the quality of pre-ESKD nephrology care and may even improve patient engagement and empowerment. This ultimately can promote significant improvements in healthcare outcomes such as improved vascular access, home dialysis, and pre-emptive kidney transplantation rates, and it promotes patient comfort and ability to maintain life goals in the face of their disease (Figure 1).13,15,17,33
Conclusion
In sum, research shows there is a need for a universal practice of SDM for all people with advanced CKD and ESKD, since when practiced inconsistently, people on the lower end of various social axes, or structurally vulnerable, are at increased risk of developing ESKD and report receiving less frequent and lower quality SDM. Despite this evident need, there are limited objective data regarding the methods and measures of SDM broadly applicable for patients with advanced CKD and informed KRT selection. While the Checklist provides a pragmatic way forward, we expect additional vital questions and concerns to arise over the coming phases, especially regarding the differing need and efficacy of the SDM for patients with different sociodemographic and educational background. The future workforce will need to be cognizant of these concepts, and physician education may need to highlight the role of physicians in better understanding and providing culturally relevant care in a manner accessible to their patients.
Funding
This manuscript was supported by a grant from the Department of Veterans Affairs, Health Service Research and Development Awards (I01HX002639), and Office of Rural Health FY21 Awards. Shukla AM reports additional ongoing grant support from the Department of Veterans Affairs, Clinical Science Research and Development Merit Grant (I01CX001661).
Disclosure
The authors have no financial or non-financial competing interests to disclose in this work.
References
1. Saran R, Robinson B, Abbott KC, et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3):A7–A8. doi:10.1053/j.ajkd.2019.01.001
2. House TW. Executive order on advancing American kidney health. Executive orders; 2019. Available from: https://www.whitehouse.gov/presidential-actions/executive-order-advancing-American-kidney-health/.
3. Mehrotra R, Chiu Y, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171(2):110–118. doi:10.1001/archinternmed.2010.352
4. Li PK-T, Chow KM. Peritoneal dialysis–first policy made successful: perspectives and actions. Am J Kidney Dis. 2013;62(5):993–1005. doi:10.1053/j.ajkd.2013.03.038
5. Lee MB, Bargman JM. Survival by dialysis modality—who cares? Clin J Am Soc Nephrol. 2016;11:1083–1087. doi:10.2215/CJN.13261215
6. Greer RC, Cooper LA, Crews DC, Powe NR, Boulware LE. Quality of patient-physician discussions about CKD in primary care: a cross-sectional study. Am J Kidney Dis. 2011;57(4):583–591. doi:10.1053/j.ajkd.2010.08.027
7. Murphy KA, Greer RC, Roter DL, et al. Awareness and discussions about chronic kidney disease among African-Americans with chronic kidney disease and hypertension: a mixed methods study. J Gen Intern Med. 2020;35(1):298–306. doi:10.1007/s11606-019-05540-3
8. Engels N, de Graav G, van der Nat P, van den Dorpel M, Bos WJ, Stiggelbout AM. Shared decision-making in advanced kidney disease: a scoping review protocol. BMJ Open. 2020;10(2):e034142. doi:10.1136/bmjopen-2019-034142
9. Finderup J, Jensen JD, Lomborg K. Shared decision-making in dialysis choice has potential to improve self-management in people with kidney disease: a qualitative follow-up study. J Adv Nurs. 2021;77(4):1878–1887. doi:10.1111/jan.14726
10. Murray MA, Bissonnette J, Kryworuchko J, Gifford W, Calverley S. Whose choice is it? Shared decision making in nephrology care. Semin Dial. 2013;26(2):169–174. doi:10.1111/sdi.12056
11. Navaneethan SD, Jolly SE, Schold JD, et al. Pragmatic randomized, controlled trial of patient navigators and enhanced personal health records in CKD. Clin J Am Soc Nephrol. 2017;2017:45.
12. Yu X, Nakayama M, Wu M-S, et al. Shared decision-making for a dialysis modality. Kidney Int Rep. 2021;7(1):15–27.
13. Lee CT, Cheng CY, Yu TM, et al. Shared decision making increases living kidney transplantation and peritoneal dialysis. Transplant Proc. 2019;51(5):1321–1324. doi:10.1016/j.transproceed.2019.02.025
14. Shukla AM, Easom A, Singh M, et al. Effects of a comprehensive predialysis education program on the home dialysis therapies: a retrospective cohort study. Perit Dial Int. 2017;37(5):542–547. doi:10.3747/pdi.2016.00270
15. Shukla AM, Hinkamp C, Segal E, et al. What do the US advanced kidney disease patients want? Comprehensive pre-ESRD Patient Education (CPE) and choice of dialysis modality. PLoS One. 2019;14(4):e0215091. doi:10.1371/journal.pone.0215091
16. Easom AM, Shukla AM, Rotaru D, et al. Home run-results of a chronic kidney disease Telemedicine Patient Education Study. Clin Kidney J. 2020;13(5):867–872. doi:10.1093/ckj/sfz096
17. Barrett TM, Davenport CA, Ephraim PL, et al. Disparities in discussions about kidney replacement therapy in CKD care. Kidney360. 2021;3:158–163. doi:10.34067/KID.0004752021
18. Moss AH. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol. 2010;5(12):2380–2383. doi:10.2215/CJN.07170810
19. Ruchi R, Bozorgmehri S, Chamarthi G, et al. Provision of kidney disease education service is associated with improved vascular access outcomes among the US incident hemodialysis patients. Kidney360. 2021;3:91–98. doi:10.34067/KID.0004502021
20. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int Dec. 2020;4:56.
21. Campbell-Montalvo R, Castañeda H. School employees as health care brokers for multiply-marginalized migrant families. Med Anthropol. 2019;38(8):733–746. doi:10.1080/01459740.2019.1570190
22. Campbell-Montalvo R, Sidorova O, Valdovinos M, Cong X, Lucas R. Healthcare access brokerage by school employees for Immigrant Mexican and Indigenous Guatemalan farmworking families in a Connecticut elementary school. AERA Open. 2022;8(1), 1 -15. doi:10.1177/23328584211068071
23. Quesada J, Hart LK, Bourgois P. Structural vulnerability and health: latino migrant laborers in the United States. Med Anthropol. 2011;30(4):339–362. doi:10.1080/01459740.2011.576725
24. Singer M. Introduction to syndemics: a critical systems approach to public and community health. Vol ISBN: 978-0-470-47203-3; 2009. Available from: wiley.com.
25. Mehrotra R, Soohoo M, Rivara MB, et al. Racial and ethnic disparities in use of and outcomes with home dialysis in the United States. J Am Soc Nephrol. 2016;27(7):2123–2134. doi:10.1681/ASN.2015050472
26. Légaré F, Kearing S, Clay K, et al. Are you SURE?: assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308–314.
27. Elwyn G, Vermunt NPCA. Goal-based shared decision-making: developing an integrated model. J Patient Exp. 2019;7(5):688–696. doi:10.1177/2374373519878604
28. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
29. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi:10.1007/s11606-012-2077-6
30. The CAHPS Ambulatory Care Improvement Guide. Practical strategies for improving patient experience; 2021. Available from: https://www.ahrq.gov/cahps/quality-improvement/improvement-guide/improvement-guide.html.
31. Légaré F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns. 2014;96(3):281–286. doi:10.1016/j.pec.2014.06.014
32. Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis. 2015;66(6):1024–1032. doi:10.1053/j.ajkd.2015.05.014
33. Robinski M, Mau W, Wienke A, Girndt M. Shared decision-making in chronic kidney disease: a retrospection of recently initiated dialysis patients in Germany. Patient Educ Couns. 2016;99(4):562–570. doi:10.1016/j.pec.2015.10.014
34. Heras Benito M, Fernández-Reyes Luis MJ. Shared decision-making in advanced chronic kidney disease in the elderly. Med Clin (Barc). 2019;152(5):188–194. doi:10.1016/j.medcli.2018.07.011
35. Hughes TM, Merath K, Chen Q, et al. Association of shared decision-making on patient-reported health outcomes and healthcare utilization. Am J Surg. 2018;216(1):7–12. doi:10.1016/j.amjsurg.2018.01.011
36. Morton RL, Tong A, Howard K, Snelling P, Webster AC. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ. 2010;340:c112. doi:10.1136/bmj.c112
37. Murray MA, Brunier G, Chung JO, et al. A systematic review of factors influencing decision-making in adults living with chronic kidney disease. Patient Educ Couns. 2009;76(2):149–158. doi:10.1016/j.pec.2008.12.010
38. Morton R. Do dialysis decision aids improve treatment decision-making? Perit Dial Int. 2016;36(4):359–361. doi:10.3747/pdi.2016.00017
39. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088–1098. doi:10.1053/j.ajkd.2005.08.017
40. Hanko J, Romann A, Taylor P, Copland M, Beaulieu M. Optimizing AVF creation prior to dialysis start: the role of predialysis renal replacement therapy choices. Nephrol Dial Transpl. 2012;27(11):4205–4210. doi:10.1093/ndt/gfs378
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.