Back to Journals » Drug Design, Development and Therapy » Volume 10
Sulfasalazine inhibits inflammation and fibrogenesis in pancreas via NF-κB signaling pathway in rats with oxidative stress-induced pancreatic injury
Authors Wang Y, Tian F, Yan M, Fan J, Wang L, Kuang R, Li Y
Received 3 March 2016
Accepted for publication 4 April 2016
Published 24 May 2016 Volume 2016:10 Pages 1743—1751
DOI https://doi.org/10.2147/DDDT.S107679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
This paper has been retracted.
Ya-Ru Wang, 1,* Fei-Long Tian, 2,* Ming-Xian Yan, 1 Jin-Hua Fan, 1 Li-Yun Wang, 1 Rong-Guang Kuang, 1 Yan-Qing Li 3
1Department of Gastroenterology, Shandong Qianfoshan Hospital, Shandong University, 2Shandong University School of Medicine, 3Department of Gastroenterology, Qilu Hospital, Shandong University, Ji’nan, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Background: Pathogenesis and effective therapeutics of chronic pancreatic inflammation and fibrosis remain uncertain.
Purpose: To investigate the effects of sulfasalazine (SF) on pancreatic inflammation and fibrogenesis.
Methods: Chronic pancreatic injury in rats was induced by diethyldithiocarbamate (DDC) and interfered by SF through intraperitoneal injection. The rats were divided into five groups: group N, normal control group, rats were treated with dilated water only; group DS1, rats received SF (10 mg/kg) 2 hours before DDC treatment; group DS2, rats were treated with DDC and then SF (100 mg/kg, twice a week); group DS3, rats were treated with DDC, then SF (100 mg/kg, thrice a week); and group DDC, rats were treated with DDC only. Pancreatic inflammation and fibrosis were determined by hematoxylin and eosin staining and Sirius red staining. The genes and proteins related to NF-κB pathway and fibrogenesis including NF-κB/p65, TNF-α, ICAM-1, α-SMA, and Con 1 were detected by immunohistochemical staining, reverse transcription polymerase chain reaction, and Western blotting.
Results: Rats in the DDC and DS1 groups showed the highest histological scores after DDC treatment, but the scores of DS2 and DS3 groups decreased significantly when compared with the DDC group. Sirius red staining showed collagen formation clearly in DDC and DS1 rats rather than in DS2 and DS3 rats. NF-κB/p65, ICAM-1, and α-SMA were strongly expressed in DDC and DS1 rats, while DS2 and DS3 rats showed mild to moderate expression by immunohistochemistry. Reverse transcription polymerase chain reaction showed increased levels of NF-κB/p65, ICAM-1, TNF-α, α-SMA, and Con 1 mRNA in DDC and DS1 rats in comparison to normal controls. The mRNA levels of these molecules in DS2 and DS3 rats were significantly lower than those in DS1 and DDC rats. Western blotting demonstrated that the NF-κB/p65, ICAM-1, and α-SMA expressions in pancreatic tissues of the rats of the DDC group were more clear than those of the normal control, DS2, and DS3 rats.
Conclusion: SF inhibits pancreatic inflammation and fibrogenesis via NF-κB signaling pathway.
Keywords: sulfasalazine, pancreatic injury, inflammation, fibrogenesis, NF-κB
Corrigendum for this paper has been published
Introduction
Acute or chronic inflammatory cells infiltration in pancreas tissues is a typical pathological characteristic in acute or chronic pancreatitis.1,2 Fibrogenesis is temporary in acute pancreatitis but persistent in chronic pancreatitis. The causes that induce these pathological alterations in pancreas are bile duct stones, alcohol abuse, severe hyperlipidemia, and others. No matter what the etiological factors are, one of the important underlying pathogenesis is oxidative stress, which would incite inflammatory cells activation, promote proinflammatory cytokines release, and therefore lead to pancreatic damage.3–6 NF-κB signaling pathway is important in the development of inflammation process, and recurrent or chronic inflammation could induce transfer growth factor beta activation and then lead to pancreatic stellate cells (PSCs) activation and pancreatic fibrosis. Previous studies demonstrate the close relationship between oxidative damage and NF-κB activation; overactivated NF-κB signaling incites upregulation of a series of inflammatory molecules, PSCs activation, and then contributes to development of pancreatic lesions.7,8 Therefore, NF-κB signaling could be a therapeutic target to ameliorate inflammation and fibrogenesis within pancreas tissues.
It is reported that sulfasalazine (SF) is an inhibitor of NF-κB signaling pathway, which can inhibit NF-κB translocation and activation and downregulate inflammatory cytokines release and expression of some adhesion molecules, such as TNF-α and ICAM-1.9–11 We speculate that SF may inhibit PSCs activation and prevent pancreatic fibrogenesis on the basis of previous studies which reported its antifibrogenesis effect on experimental liver fibrosis.10,12 Diethyldithiocarbamate (DDC) is a superoxide dismutase inhibitor, which can induce pancreatitis in rats.13 In this study, we induced pancreatic damage by DDC and intervened by SF in rats, observed the pancreatic histological alterations and molecules expressions, and investigated whether SF prevents or ameliorates oxidative stress-induced pancreatic injuries.
Materials and methods
Animals and reagents
This animal study was approved by the Ethics Committee of Shandong University and the experiments were performed in accordance with the Laboratory Animal Care and Use Regulations of Shandong University. All Wistar rats, weighing 160–185 g, were obtained from The Laboratory Animal Center of Shandong University and housed in a temperature and humidity controlled room for 1 week. The rats were then divided into five groups (15 rats per group) on the basis of comparable mean body weight as follows: group N, normal control group, rats were treated with dilated water only (intraperitoneal [ip], twice a week) for 10 weeks; group DS1, rats received SF treatment (ip, 10 mg/kg) 2 hours before DDC treatment (ip, 750 mg/kg, twice a week) for 10 weeks; group DS2, rats were treated with DDC (ip, 750 mg/kg, twice a week) first and then SF (ip, 100 mg/kg, twice a week) for 10 weeks; group DS3, rats were treated with DDC (ip, 750 mg/kg, twice a week) for 10 weeks, then SF (ip, 100 mg/kg, thrice a week) for 2 weeks; and group DDC, rats were treated with DDC only (ip, 750 mg/kg, twice a week) for 10 weeks. DDC and SF were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
H&E staining
Each formalin-fixed and paraffin-embedded pancreas specimen was cut into 5 μm thick sections. Hematoxylin and eosin (H&E) staining was performed for routine histologic observations.
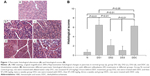
Histological inflammation in pancreas was evaluated quantitatively based on three aspects: acinar cell atrophy, vacuolization, and inflammatory cell infiltration, and was presented as histological score.
Sirius red staining
Slides were deparaffinized and immersed for 25 minutes in saturated aqueous picric acid containing 0.5% Sirius red. All the Sirius red-stained sections were observed and photographed under both common light and polarization microscopes. Under polarization microscope, collagen appears bright orange-red and/or bright green. The images were digitized using ImageJ software (Version 1.50g, National Institutes of Health, USA).
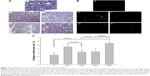
Pancreas collagen deposition was presented by a fibrosis index (%) that indicates the ratio of the mean collagen stained area to the mean whole area of the section.
Immunohistochemistry staining
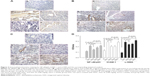
Immunohistochemistry staining for NF-κB/p65, ICAM-1, and α-SMA was performed as follows: the sections were deparaffinized, immersed in 3% H2O2 (v/v) to quench endogenous peroxidase activity, and microwaved in 10 mM sodium citrate (pH 6.0) for 15 minutes for antigen retrieval. Then, the avidin and biotin were applied to eliminate endogenous biotin-related background staining. The sections were then incubated with primary antibodies (1:150) (Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 4°C overnight and incubated, respectively, with biotinylated goat anti-mouse antibodies and horseradish peroxidase-conjugated streptavidin (Santa Cruz Biotechnology Inc.) for 15 minutes at room temperature. The slides were washed and the chromogen was developed for 5 minutes with liquid 3,3′-diaminobenzidine before observation. Distilled water with 0.4% Tween-20 was used as a rinsing solution. Positive staining areas were measured by ImageJ software and expressed as integrated optical density.
All histological samples were evaluated blindly by the same pathologist. To evaluate the histological changes, three sections were randomly selected from each rat, and five nonoverlapping fields per section were captured for observation.
RT-PCR assay for mRNA levels
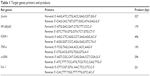
mRNA levels were determined by reverse transcription polymerase chain reaction (RT-PCR). Pancreatic samples were rapidly immersed in RNAlater (Sigma-Aldrich Co.) for RNA protection and stored at −20°C before assay. Total RNA was extracted from pancreatic tissues using a TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and was reverse-transcribed using oligo (dT) as a primer. The sequences used as an internal standard control, housekeeping gene β-actin, and target genes are listed in Table 1.
  | Table 1 Target genes primers and products |
PCR amplification cycles were carried out under the following conditions: initial activation of 95°C for 5 minutes followed by 35 cycles of 45 seconds of denaturation at 95°C, 45 seconds of annealing at (58°C for β-actin, ICAM-1, and α-SMA; 60°C for NF-κB/p65; 50°C for TNF-α), 45 seconds of extension at 72°C followed by one final extension at 72°C for 7 minutes. Completed reactions were held at 4°C.
PCR products were separated by gel electrophoresis (1.5% agarose stained with ethidium bromide). Specific bands were visualized with an image system (FluorChem 9900; Alpha Innotech, San Leandro, CA, USA). The detection of each gene was repeated for three times. The optical density (OD) values of the bands were analyzed using ImageJ software and standardized to the β-actin signal.
Western blotting for NF-κB/p65, ICAM-1, and α-SMA expression
Briefly, frozen tissues were lysed in lysis buffer. The lysates were centrifuged at 4,000 × g, and supernatants were immediately stored at −70°C until use. Protein concentration was determined using the Lowery method, and 50 μg of protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Nonspecific binding was blocked by preincubation of the nitrocellulose membrane in Tris-buffered saline containing 5% nonfat milk for 1 hour. The nitrocellulose membrane was incubated overnight at 4°C with anti-NF-κB/p65, ICAM-1, and α-SMA antibodies (Santa Cruz Biotechnology Inc.). Bound primary antibody was detected using a peroxidase-conjugated secondary antibody (Boshide, Wuhan, People’s Republic of China) and enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA). The detection of each protein was repeated for three times. The OD values of the bands were quantified using ImageJ software and standardized to the signal of the control.
Statistical analysis
Data were expressed as mean ± standard deviation. The statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. P<0.05 was considered significant.
Results
General information
Rats demonstrated discomfort after DDC injection but recovered soon. The rats’ urine turned deep yellow after SF treatment, but became normal in a day. All rats survived well during the experimental period.
H&E staining
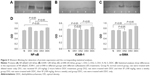
After DDC treatment, acinar cells atrophy and vacuolization, enlarged space between lobules, and inflammatory cells infiltration were demonstrated. Rats in DDC and DS1 groups showed the highest histological scores; DS2 and DS3 groups had all aforementioned presentations, but the histological scores decreased significantly when compared with the DDC group (Figure 1).
Sirius red staining for fibrogenesis
Pancreatic fibrogenesis was detected by Sirius red staining, and observed under common light and polarized microscopes. Collagen formation was clearly observed in DDC and DS1 rats, but in DS2 and DS3 rats, collagen presentation was less in comparison to that in DDC rats (Figure 2).
Immunohistochemistry staining
Inflammation and fibrosis-associated molecules were detected by immunohistochemistry staining. As a result, NF-κB/p65, ICAM-1, and α-SMA were strongly expressed in DDC and DS1 rats, while DS2 and DS3 rats showed mild-to-moderate expression. ICAM-1 was expressed in endothelial cells and in a small amount of acinar cells, while α-SMA was expressed around vessels and between acinar cells (Figure 3).
RT-PCR assay
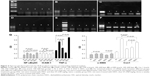
The results of PCR showed increased mRNA levels of NF-κB/p65, ICAM-1, TNF-α, α-SMA, and Con 1 in DDC and DS1 rats in comparison to normal controls. The mRNA levels of these molecules in DS2 and DS3 rats were significantly lower than those of DS1 and DDC rats (Figure 4).
Western blotting
Western blotting demonstrated that the NF-κB/p65, ICAM-1, and α-SMA expressions in pancreatic tissues of DDC rats were significantly higher than those of normal control rats. The differences in the expression of these molecules between DS2 and DDC and DS3 and DDC rats were all significant (Figure 5).
Discussion
Pancreatitis is a complex disorder the exact mechanism of which remains controversial. Recurrent acute pancreatitis or chronic pancreatitis of various origins damage pancreatic parenchymal cells, including acinar and islet cells, and result in exocrine or/and endocrine insufficiency. So far, effective and widely accepted therapeutic methods for pancreatic injuries are not well established because of the vague mechanisms underlying this pathophysiological process. Although the exact pathogenesis of pancreatitis remains uncertain, several mechanisms related to oxidative and inflammatory stress are implicated. Injuries to the pancreatic cells cause a complex cascade of events that includes increased production of reactive oxygen species (ROS), which leads to oxidation of lipids and proteins and disruption of the cell membrane. Thus, oxidative stress is considered to play a key role in the development of chronic pancreatic injury.14,15 We found high-fat diet induced vascular disturbances and oxidative stress in pancreas and led to pancreatic injury in our previous study.16 In our present study, rats were treated with DDC, a reagent which could incite oxidative stress and lead to cell damage.17 As a result, inflammation, acinar atrophy, and fibrogenesis were observed inside the pancreatic tissues after DDC administration, suggesting development of oxidative stress-induced pancreatic injuries. In consideration of the role of oxidative stress in development of pancreatic injury, antioxidant is believed to be an effective therapy.
Actually, in the past decade, experimental and clinical investigations have demonstrated that antioxidants have protective effects on acute8,18–20 and chronic5,21–23 pancreatitis. SF is used comprehensively for treatment of inflammatory bowel disease and arthritis, but recent studies have demonstrated its antioxidant properties based on experimental researches. It is reported that SF, especially its metabolite 5-aminosalicylic acid, scavenges ROS, inhibits oxidative stress, and therefore has therapeutic effects on inflammation.11,24,25 It is also reported that a new drug, which was developed from SF, ameliorates amyotrophic lateral sclerosis because of its antioxidant property.26 Additionally, in an experimental study of CCl4-induced liver fibrosis, SF showed antifibrotic effects because of its antioxidant ability and its ability to inhibit NF-κB nuclear translocation.10 Accordingly, we treated the rats with SF before and after DDC stimulation in our study in order to investigate whether SF could ameliorate pancreatic injuries. The results show that SF treatment decreases inflammatory cells infiltration, inhibits PSCs activation and fibrogenesis in pancreas tissues, suggesting the protective and therapeutic effects of SF on oxidative stress-induced pancreatic damage.
It is known that inflammatory transcription factor NF-κB signal pathway is important during development of inflammation. Activation of NF-κB has been shown to elicit acute pancreatitis as an early event, together with trypsinogen activation.27,28 Previous investigations have shown that NF-κB and its modulated molecules, such as TNF-α and ICAM-1, have close relations with oxidative damage and oxidants’ protective effects.3 Lv et al found that “lycopene” protects pancreatic acinar cells against necrosis and apoptosis through NF-κB/JNK pathway.18 In another experimental study, Gulcubuk et al found “resveratrol” can reduce oxidative damage, prevent IκB degradation, and decrease the levels of NF-κB, TNF-α, and IL-6.20 “Gallic acid”, which is a strong antioxidant, upregulates the expression of Nrf2 and attenuates experimental colitis.29 In our previous study, we also demonstrated the role of NF-κB signal pathway in high-fat diet-induced pancreatic injury.7 SF is reported to be a potent inhibitor of NF-κB activation, which partly explains its pharmacological effects as an immunomodulatory agent in chronic inflammation.9 In an animal study of CCl4-induced liver fibrosis, SF was found to have antifibrotic effects because of its antioxidant property and its ability to inhibit NF-κB nuclear translocation and TGF-β expression.10 According to our current study, we found elevated NF-κB/p65 expression after DDC stimulation, and its corresponding regulatory molecules including TNF-α and ICAM-1 were upregulated simultaneously in pancreatic tissues in comparison to controls. The genes of these inflammatory molecules changed in the same way. These findings indicate the role of NF-κB signal pathway in the ameliorative effects of SF on pancreatic injuries.
Conclusion
Our study suggests that SF might be a potential candidate for the treatment of pancreatic inflammatory disease. But, the adverse effects of SF should also be considered. It is reported that SF itself could induce oxidative stress and act with ROS, and these reactions might be a possible mechanism of male infertility, hepatotoxity, and nephrotoxity.24,30 SF-associated pancreatitis is also reported during treatment of inflammatory bowel disease. But there is a possibility that therapeutic rather than adverse effects might be dominant when treated with SF during the pathophysiological process of chronic pancreatic injuries. Whether SF is suitable for clinical treatment of patients with acute or chronic pancreatitis needs further experimental and clinical investigations and should be well assessed in the future.
Acknowledgments
This work was supported partly by grants from the Department of Science & Technology of Shandong Province (Project No 2012GSF1187) and the Health and Family Planning Commission of Shandong Province (Project No 2009HZ069), People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–1415. | ||
Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon. 2015;61(1):5–37. | ||
Yu JH, Kim H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J Gastroenterol. 2014;20(46):17324–17329. | ||
Armstrong JA, Cash N, Soares PM, Souza MH, Sutton R, Criddle DN. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res. 2013;47(11):917–933. | ||
Bhardwaj P, Yadav RK. Chronic pancreatitis: role of oxidative stress and antioxidants. Free Radic Res. 2013;47(11):941–949. | ||
Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11(1):135–165. | ||
Yan MX, Ren HB, Kou Y, Meng M, Li YQ. Involvement of nuclear factor kappa B in high-fat diet-related pancreatic fibrosis in rats. Gut Liver. 2012;6(3):381–387. | ||
Lima PR, de Melo TS, Carvalho KM, et al. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-κB activity in mice. Life Sci. 2013;92(24–26):1195–1201. | ||
Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. | ||
Chávez E, Castro-Sánchez L, Shibayama M, Tsutsumi V, Moreno MG, Muriel P. Sulfasalazine prevents the increase in TGF-β, COX-2, nuclear NFκB translocation and fibrosis in CCl4-induced liver cirrhosis in the rat. Hum Exp Toxicol. 2012;31(9):913–920. | ||
Dirlik M, Karahan A, Canbaz H, et al. Effects of sulfasalazine on lipid peroxidation and histologic liver damage in a rat model of obstructive jaundice and obstructive jaundice with lipopolysaccharide-induced sepsis. Curr Ther Res Clin Exp. 2009;70(4):299–315. | ||
Oakley F, Meso M, Iredale JP, et al. Inhibition of inhibitor of kappa B kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128(1):108–120. | ||
Joo KR, Shin HP, Cha JM, Nam S, Huh Y. Effect of Korean red ginseng on superoxide dismutase inhibitor-induced pancreatitis in rats: a histopathologic and immunohistochemical study. Pancreas. 2009;38(6):661–666. | ||
Siriwardena AK. Reappraisal of xenobiotic-induced, oxidative stress-mediated cellular injury in chronic pancreatitis: a systematic review. World J Gastroenterol. 2014;20(11):3033–3043. | ||
Grigsby B, Rodriguez-Rilo H, Khan K. Antioxidants and chronic pancreatitis: theory of oxidative stress and trials of antioxidant therapy. Dig Dis Sci. 2012;57(4):835–841. | ||
Yan MX, Li YQ, Meng M, Ren HB, Kou Y. Long-term high-fat diet induces pancreatic injuries via pancreatic microcirculatory disturbances and oxidative stress in rats with hyperlipidemia. Biochem Biophys Res Commun. 2006;347(1):192–199. | ||
Rahden-Staroń I, Grosicka-Maciąg E, Kurpios-Piec D, Czeczot H, Grzela T, Szumiło M. The effects of sodium diethyldithiocarbamate in fibroblasts V79 cells in relation to cytotoxicity, antioxidative enzymes, glutathione, and apoptosis. Arch Toxicol. 2012;86(12):1841–1850. | ||
Lv JC, Wang G, Pan SH, Bai XW, Sun B. Lycopene protects pancreatic acinar cells against severe acute pancreatitis by abating the oxidative stress through JNK pathway. Free Radic Res. 2015;49(2):151–163. | ||
Tang QQ, Su SY, Fang MY. Zinc supplement modulates oxidative stress and antioxidant values in rats with severe acute pancreatitis. Biol Trace Elem Res. 2014;159(1–3):320–324. | ||
Gulcubuk A, Haktanir D, Cakiris A, et al. The effects of resveratrol on tissue injury, oxidative damage, and pro-inflammatory cytokines in an experimental model of acute pancreatitis. J Physiol Biochem. 2014;70(2):397–406. | ||
Kodydkova J, Vavrova L, Stankova B, Macasek J, Krechler T, Zak A. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas. 2013;42(4):614–621. | ||
Singh N, Bhardwaj P, Pandey RM, Saraya A. Oxidative stress and antioxidant capacity in patients with chronic pancreatitis with and without diabetes mellitus. Indian J Gastroenterol. 2012;31(5):226–231. | ||
Tandon RK, Garg PK. Oxidative stress in chronic pancreatitis: pathophysiological relevance and management. Antioxid Redox Signal. 2011;15(10):2757–2766. | ||
Linares V, Alonso V, Domingo JL. Oxidative stress as a mechanism underlying sulfasalazine-induced toxicity. Expert Opin Drug Saf. 2011;10(2):253–263. | ||
Joshi R, Kumar S, Unnikrishnan M, Mukherjee T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radic Res. 2005;39(11):1163–1172. | ||
Clement AM. Two in one against motor neuron degeneration: tackling oxidative stress and inflammation with a sulfasalazine derivative. J Neurochem. 2012;122(5):869–871. | ||
Aleksic T, Baumann B, Wagner M, Adler G, Wirth T, Weber CK. Cellular immune reaction in the pancreas is induced by constitutively active I kappaB kinase-2. Gut. 2007;56:227–236. | ||
Baumann B, Wagner M, Aleksic T, et al. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest. 2007;117:1502–1513. | ||
Pandurangan AK, Mohebali N, Norhaizan ME, Looi CY. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des Devel Ther. 2015;9:3923–3934. | ||
Alonso V, Linares V, Bellés M, et al. Sulfasalazine induced oxidative stress: a possible mechanism of male infertility. Reprod Toxicol. 2009;27(1):35–40. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.