Back to Journals » Drug Design, Development and Therapy » Volume 14
Suitability of Amoxicillin–Clavulanic Acid for Administration via Prolonged Infusion
Authors Fawaz S , Dixon B , Barton S , Mohamed A, Nabhani-Gebara S
Received 10 September 2019
Accepted for publication 3 December 2019
Published 10 January 2020 Volume 2020:14 Pages 103—109
DOI https://doi.org/10.2147/DDDT.S230459
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Sarah Fawaz, Breanna Dixon, Stephen Barton, Amna Mohamed, Shereen Nabhani-Gebara
Faculty of Science, Engineering and Computing, Kingston University, London KT1 2EE, UK
Correspondence: Sarah Fawaz
Email [email protected]
Rationale: Previously, we have been able to outpace bacterial mutation by replacing increasingly ineffective antibiotics with new agents. However, with the discovery of new antibiotics diminishing, optimising the administration of existing broad-spectrum antibiotics such as co-amoxiclav has become a necessity.
Methods: A stability indicating HPLC method was developed and validated in compliance with International Council for Harmonisation (ICH) guidelines. Stability of co-amoxiclav at clinical concentration was evaluated at three temperatures (4°C, ambient (23– 25°C) and 37°C) in three diluents (water for injection (WFI), 0.9% w/v NaCl and Ringer’s solution). To establish whether there were significant differences at the level of both diluent and temperature, results were analysed using analysis of covariance (ANCOVA) to assess differences between the attained slopes of regression.
Results: Data obtained indicated co-amoxiclav stability superior to that previously proposed making it suitable for extended infusion therapy. The degradation of amoxicillin appeared to follow a linear trend, with the rate of degradation elevated at higher temperatures, demonstrated by the magnitude of the regression slopes in these conditions. Analysis of regression slopes via ANCOVA demonstrated that diluent and temperature both significantly affected co-amoxiclav stability. Amoxicillin retained 90% of its initial concentration for 7.8 to 10 hrs when stored at 4°C, 5.9 to 8.8 hrs at ambient and 3.5 to 4.5 hrs when incubated at 37°C.
Conclusion: Co-amoxiclav is suitable for administration via prolonged infusion. Findings from this study aid in ameliorating current dosing regimens to optimise antibiotic efficacy. Other valuable applications conferred from these findings include the ability to pre-prepare solutions for use in bolus administration, minimising preparation time and workload.
Keywords: prolonged infusion, co-amoxiclav and antibiotic resistance
Introduction
While the rate of antibiotic discovery has plummeted, the global burden of antimicrobial resistance (AMR) is on the rise and shows no signs of receding.1–3 Urgent action is required to address this public health threat and halt the advent of a post-antibiotic era.
Recently, the World Health Organisation (WHO) identified significant gaps in the present status of surveillance and information on AMR and confirmed that treatments for commonly acquired infections are becoming less effective.4 Reduced susceptibility to antibiotics, coupled with the lack of new agents has led to a renewed interest in optimising currently available antimicrobials. One growing area for reducing the development of AMR involves differential dosing regimens such as prolonged or continuous infusions of time-dependent antibiotics.5–9 However, this may not be possible for all antibiotics due to varying stability profiles.
The European Pharmacopeia considers pharmaceuticals stable providing they maintain 90% of their initial concentration.10 Uncertainty regarding β-lactam antibiotic stability after reconstitution and dilution presents a challenge in practice when assigning a shelf-life to injections that are pre-prepared and stored in ready-to-administer containers.10 These antibiotics display a time-dependent nature whereby maintaining concentrations above the minimum inhibitory concentration (MIC) promotes maximal bactericidal activity.11
One such drug is amoxicillin–clavulanic acid (co-amoxiclav), a combination β-lactam antibiotic/β-lactamase inhibitor that exhibits broad-spectrum activity against a wide variety of bacterial infections. Currently, parenteral administration of co-amoxiclav is via bolus intermittent infusion. A proposed dosing strategy for enhancing co-amoxiclav’s efficacy involves extending the time at which concentrations are maintained above the MIC via continuous/prolonged infusions.6 Prolonging infusion from 0.5 to 2 hrs has previously been associated with improvements in time above the MIC (T>MIC).12
Literature indicates that the main constraints of co-amoxiclav stability include infusion diluent and storage temperature. Co-amoxiclav has been found to be less stable at higher temperatures, with data suggesting that shelf-life ranges between 1 and 5.5 hrs at room temperature in water for injection (WFI) and up to 8 hrs at 4°C.13–16
To expand the breadth of current knowledge, this study utilises the bench-to-bedside approach, where challenges experienced in practice are addressed in the laboratory. Co-amoxiclav stability is a crucial parameter that needs to be determined to assess the feasibility of administration via continuous/prolonged infusions. To address this, a high-performance liquid chromatography (HPLC) stability indicating method (SIM) was developed and validated in compliance with International Council for Harmonisation (ICH) guidelines. Quantitative analysis of co-amoxiclav stability was then conducted in a range of temperatures and diluents to determine their effect on degradation.
Materials and Methods
Materials
GSK pharmaceutical dosage form co-amoxiclav (1000mg/200mg) infusion vials were provided by St George’s Hospital, London, UK. Amoxicillin sodium, potassium clavulanate and caffeine reference standards were purchased from Sigma Aldrich, as were ammonium acetate and glacial acetic acid. Water for injection (WFI), 0.9% sodium chloride, and Ringer’s solution were purchased from The Pharmacy, Kingston, UK. Methanol (HPLC grade) and acetonitrile (HPLC grade) were purchased from VWR and distilled water was generated in the laboratory at Kingston University, London, UK.
Instrumentation
Quantitative analysis of amoxicillin–clavulanic acid was carried out using an Agilent 1260 HPLC system with single wavelength UV detection and Chemstation software.
HPLC-SIM Development & Validation
A SIM was developed and validated in accordance with ICH guidelines. Parameters investigated included column, mobile phase and internal standard selection. The method was optimised through the selection of suitable flowrate, wavelength, injection volume and column temperature.
To determine the developed method’s specificity, a forced degradation study was conducted. Co-amoxiclav solutions were exposed to oxidative, hydrolytic, photolytic and thermal stress. Stressed solutions were analysed to assess the method’s ability to separate the parent compounds from their degradation products.
Validation was conducted over the span of 3 days. The analytical range was determined by running reference standard amoxicillin–clavulanic acid at various concentrations (0, 10, 20, 30, 40, 50, 60, 70, and 80ppm).
Quality control (QC) solutions equating to approximately 25%, 50% and 75% of the working range, ie 15ppm, 45ppm and 75ppm, were prepared prior to each validation run to assess the accuracy. Precision was considered at three levels: repeatability, intermediate precision and reproducibility through analysis of prepared QC solutions. Three sets of each sample were prepared daily and each sample was run in triplicate.
Method selectivity was demonstrated by analysing “sample blanks” to observe any interferences at the determined amoxicillin and clavulanic acid retention times. Robustness was examined by marginally varying the following parameters: flow rate (±0.5mL/min), column temperature (±5°C), wavelength (±10nm), injection volume (±5μL) and mobile phase composition.
Quantitative HPLC Assay
In clinical settings, a 1.2g amoxicillin–clavulanic acid vial is reconstituted with 20mL WFI and is diluted further with 50mL of diluent (1200mg/70mL = 17 143ppm). Nine pharmaceutical formulation vials were reconstituted with 20mL WFI, further diluted with either 50mL of 0.9% sodium chloride (n=3), Ringer’s solution (n=3) or WFI (n=3) and stored at 4°C, ambient and 37°C in vials.
Standard practice involves dilution of co-amoxiclav with sodium chloride solution. Other compatible diluents including Ringer’s solution and WFI were trialled to examine the influence of diluent on co-amoxiclav stability. To evaluate preparation and storage feasibility, the stability of co-amoxiclav solutions was assessed at 4°C. Ambient and 37°C temperature conditions were considered in order to mimic average and high temperatures experienced in hospital wards due to seasonal variations.
To achieve a nominal concentration of 34.3ppm, a dilution factor of 500x was used. Sampling was undertaken every 2 hrs and the infusion solution was considered stable while the percentage recovery of amoxicillin remained above 90%.
Data and Statistical Analysis
Raw data obtained were corrected for drift and internal standard. Results are reported as the residual ratio of amoxicillin concentration from three replicates. To determine whether amoxicillin concentration decreased significantly over time, the slope of the linear regression line for each condition was tested against the null hypothesis (H0 = no deviation from zero) using a one-tailed t-test at the 99% level of significance. To establish whether there were significant differences at the level of both diluent and temperature, results were analysed using analysis of covariance (ANCOVA) to assess differences between the attained slopes of regression.
Results
HPLC-SIM Development and Validation
Amoxicillin, clavulanic acid and caffeine separation were attained using conditions presented in Table 1. The method demonstrated specificity with parent compounds separated from their degradation products with sufficient resolution. A representative chromatogram is displayed in Figure 1.
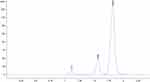 |
Figure 1 Chromatogram displaying clavulanic acid (tR: 1.117 mins), amoxicillin (tR: 1.565 mins) and caffeine (tR: 1.820 mins) separation. |
 |
Table 1 Displaying HPLC Parameters Investigated |
The calibration curve (Figure 2) obtained demonstrated good linearity over the concentration range of 0–80ppm. The representative linear equation was y = 0.9951x + 0.00822 with correlation coefficient (R2) of 0.9999. Good intra-sample precision was achieved with %RSD ranging between 0.07% and 1.66%. Intra-day precision ranged between 0.65% and 5.63%.
Good inter-day precision was also obtained with %RSD ranging between 1.16% and 1.80% (ICH acceptance criteria: %RSD ≤ 5%). The percentage error ranged between 0.07% and 8.78% and percentage recovery between 91.22% and 105.23% (ICH acceptance criteria: %Recovery: 80–120%), demonstrating good accuracy.
The method continued to perform optimally when parameters were varied, exhibiting slight changes in retention time, peak areas and heights. LOD and LOQ were calculated to be 1.57ppm and 4.76ppm, respectively.
 |
Figure 2 Amoxicillin–clavulanic acid nine-point calibration (internal standard corrected) displaying linearity over the range of 0–80ppm. |
Quantitative HPLC Assay
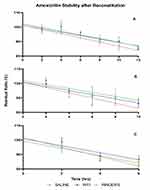
In general, amoxicillin–clavulanic acid solutions retained more of the initial drug concentration at lower temperatures compared with solutions stored at higher temperatures. The influence of diluent on amoxicillin concentrations at 4°C, ambient and 37°C over time is shown in Figure 3. The slopes of the regression lines for each condition showed significant deviation from zero at the 99% level of confidence, indicating amoxicillin exhibits degradation with time (Table 2).
 |
Table 2 Displaying the Linear Regression Equations for Each Condition Used to Calculate the Predicted Time at Which Residual Ratio of Amoxicillin Falls Below 90% |
Amoxicillin retained 90% of its initial concentration for 7.8 to 10 hrs when stored at 4°C, 5.9 to 8.8 hrs at ambient and 3.5 to 4.5 hrs when incubated at 37°C. Stability data for all conditions are displayed in Table 2. Significant differences between regression slopes of temperature conditions for each diluent were observed, as were differences between diluents for each temperature condition (Table 3). Clavulanic acid appeared to maintain concentrations within 90% of the initial concentration for the entirety of the sampling duration in all conditions analysed.
 |
Table 3 Results of ANCOVA Analyses Performed at the Level of Diluent and Temperature. |
Discussion
The emergence of resistance threatens our capacity to treat common infectious diseases as antibiotics progressively become less effective.1–3 Optimising dosing regimens of antibiotics has shown potential for controlling the spread of resistance.5–9
The utilisation of the bench-to-bedside approach enables outcomes to be translated directly from the laboratory to the clinical setting, integrating these advancements into practice. Sustaining serum concentrations above the MIC by employing methods which exploit the time-dependent nature of co-amoxiclav such as prolonged infusion could improve its clinical effectiveness.17 Optimising co-amoxiclav efficacy serves not only to improve treatment strategies but also to minimise the further development of antimicrobial resistance.
To our knowledge, this is the first study of its kind to utilise a multifactor analysis for determination of co-amoxiclav stability. By concomitantly examining drug stability at clinical concentrations in multiple diluents across a range of temperatures, a more comprehensive evaluation of multi-parameter stability was attained. The consequent understanding of the molecular stability of co-amoxiclav allows for a greater evaluation of its suitability for administration via prolonged infusion, aiding in the development of novel treatment strategies. Co-amoxiclav has not previously been considered for prolonged infusion; however, findings from this study demonstrate its feasibility.
Results obtained confirm that reconstitution diluent and storage temperature significantly influence co-amoxiclav stability. The degradation of amoxicillin appeared to follow a linear trend, with the rate of degradation elevated at higher temperatures as demonstrated by the magnitude of the regression slopes in these conditions. Storage at lower temperatures correlated with increased shelf-life, which is concurrent with previous studies on other β-lactam antibiotics6,18,19 (Table 2).
Dilution with Ringer’s solution demonstrated the least stability, exhibiting significant differences compared to saline and WFI when stored at both 4°C and ambient conditions (Table 3). Greatest stability of amoxicillin was achieved in WFI and saline solutions at 4°C, where shelf-lives of 9.6 and 10.0 hrs, respectively, were determined (Table 2).
Data obtained indicated co-amoxiclav stability superior to that previously proposed13–16 making it suitable for extended infusion therapy. Prolonged co-amoxiclav infusion would improve the effectiveness of therapy without altering the dose or dosing schedule, giving no increase in toxicity. Insight into this greater stability paves the way for further investigation of differential dosing regimens and optimisation of current treatment strategies, which have the potential to improve and enhance clinical efficacy.
Another valuable application conferred from these findings includes the ability to pre-prepare solutions for use in bolus administration, minimising preparation time and workload. Furthermore, unused reconstituted solutions are typically discarded after use; however, this study demonstrates that these may be utilised for subsequent administrations, reducing wastage and costs.
Future assays investigating the stability of co-amoxiclav should consider analysis at a range of concentrations to account for patient populations with specific dosing requirements, such as those on fluid restriction due to reduced renal clearance. Further investigations are warranted to understand the stability of co-amoxiclav in various infusion devices such as elastomeric pumps and intravenous infusion bags.
Conclusion
Resistance to common infections has previously been mitigated by the discovery of novel antibiotics. However, with the current scarcity of newly developed compounds, this study focused on optimising the administration of broad-spectrum co-amoxiclav by determining its shelf-life in a range of temperatures and diluents. Results suggest co-amoxiclav shelf-life is longer than previously determined, rendering it suitable for administration via prolonged infusion in terms of stability. Multifactor analysis indicated that co-amoxiclav stability was significantly influenced by diluent and storage temperature. Findings from this study aid in ameliorating current dosing regimens to optimise antibiotic efficacy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi:10.2147/IDR.S173867
2. Ventola CL. The antibiotic resistance crisis: causes and threats. P&T J. 2015;40:277–283.
3. Piddock LJV. The crisis of no new antibiotics-what is the way forward? Lancet Infect Dis. 2012;12:249–253. doi:10.1016/S1473-3099(11)70316-4
4. World Health Organization. Antimicrobial Resistance - Global Report on Surveillance; 2014.
5. Craig WA, Ebert SC. MINIREVIEW continuous infusion of B-lactam antibiotics. Antimicrob Agents Chemother. 1992;36:2577–2583. doi:10.1128/AAC.36.12.2577
6. Fawaz S, Barton S, Whitney L, Swinden J, Nabhani-Gebara S. Stability of meropenem after reconstitution for administration by prolonged infusion. Hosp Pharm. 2018;1–7. doi:10.1177/0018578718779009
7. Lodise TP, Lomaestro BM, Drusano GL. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on β-lactam antibiotics - insights from the society of infectious diseases pharmacists. Pharmacotherapy. 2006;26:1320–1332. doi:10.1592/phco.26.9.1320
8. Roberts JA, Kruger P, Paterson DL, Lipman J. Antibiotic resistance–what’s dosing got to do with it? Crit Care Med. 2008;36:2433–2440. doi:10.1097/CCM.0b013e318180fe62
9. Lorente L, Lorenzo L, Martfn MM, Jimenez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40:490–945. doi:10.1345/aph.1
10. Manning L, Wright C, Ingram PR, et al. Continuous infusions of meropenem in ambulatory care: clinical efficacy, safety and stability. PLoS One. 2014;9:7–9. doi:10.1371/journal.pone.0102023
11. Levison ME, Levison JH. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am. 2009;23:1–29. doi:10.1016/j.idc.2009.06.008.Pharmacokinetics
12. Fournier A, Goutelle S, Yok-Ai Q, et al. Population pharmacokinetic study of amoxicillin-treated burn patients hospitalized at a swiss tertiary-care center. Antimicrob Agents Chemother. 2018;62:1–12. doi:10.1128/AAC.00505-18
13. Kambia NK, Merite N, Dine T, et al. Stability studies of amoxicillin/clavulanic acid combination in polyolefin infusion bags. Eur J Hosp Pharm. 2010;16:30–37.
14. Nur AO, Hassaon AAA, Gadkariem EA, Osman Z, Ali GKM. Stability of co-amoxiclav reconstituted injectable solution. Eur J Pharm Med Res. 2015;2:109–123.
15. Vega EM, Manzo RH, Sola N. Improving the stability of potassium clavulanate in admixture with amoxicillin. Hosp Pharm. 2008;15:183–185.
16. Carlier M, Verstraete AG, De Waele JJ, Stove V. Stability of amoxicillin and amoxicillin/clavulanic acid reconstituted in isotonic saline. J Chemother. 2017;29:54–56. doi:10.1179/1973947815y.0000000052
17. Moriyama B, Henning SA, Neuhauser MM, Danner RL, Walsh TJ. Continuous-infusion β-lactam antibiotics during continuous venovenous hemofiltration for the treatment of resistant gram-negative bacteria. Ann Pharmacother. 2009;43:1324–1337. doi:10.1345/aph.1L638
18. Patel PR, Cook SE. Stability of meropenem in intravenous solutions. Am J Heal Pharm. 1997;54:412–421. doi:10.1093/ajhp/54.4.412
19. Kheirolomoom A, Kazemi-Vaysari A, Ardjmand M, Baradar-Khoshfetrat A. The combined effects of pH and temperature on penicillin G decomposition and its stability modeling. Process Biochem. 1999;35:205–211. doi:10.1016/S0032-9592(99)00052-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.