Back to Journals » Drug Design, Development and Therapy » Volume 16
Sufentanil Combined with Nalbuphine via Patient-Controlled Intravenous Analgesia After Cesarean Section: A Retrospective Evaluation
Authors Wang L , Wang Y, Ma Y , Mu X, Zhang Z, Wang H , Zheng Z, Nie H
Received 28 June 2022
Accepted for publication 13 October 2022
Published 21 October 2022 Volume 2022:16 Pages 3711—3721
DOI https://doi.org/10.2147/DDDT.S380292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Lini Wang,* Yiting Wang,* Yumei Ma, Xiaoxiao Mu, Zhen Zhang, Huan Wang, Ziyu Zheng, Huang Nie
Department of Anesthesiology and Perioperative Medicine, Xijing Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huang Nie, Department of Anesthesiology and Perioperative Medicine, Xijing Hospital, Fourth Military Medical University, Changle West Road 127, Xi’an, Shaanxi, 710032, People’s Republic of China, Email [email protected]
Purpose: This retrospective study evaluated the efficacy, opioid consumption, and safety profile of two patient-controlled intravenous analgesia (PCIA) regimens (sufentanil combined with nalbuphine vs sufentanil alone) after cesarean section (CS).
Patients and Methods: Parturients (n = 1808) received sufentanil combined with nalbuphine (SN group) or sufentanil alone (S group) as PCIA after CS. The primary outcome was the numeric rating scale (NRS) pain score with movement (NRS-M) at 24 h after CS. Secondary outcomes were NRS scores at rest (NRS-R) at 24 and 48 h after CS, NRS-M at 48 h after CS, cumulative PCIA bolus times, and opioid consumption during the first 24 and 48 h postoperatively, which was measured in morphine-equivalent doses.
Results: The population comprised 993 and 815 subjects in the SN and S groups, respectively. At 24 and 48 h after CS, the respective NRS-M scores of the SN group (4.62, 3.37) were each significantly lower than those of the S group (5.18, 4.01; P < 0.01 for both). The corresponding NRS-S scores were similarly lower in the SN group (0.96, 0.19) than in the S group (2.05, 0.92; P < 0.01 for both). After adjusting for covariates, the SN group still had lower NRS-M than the S group at 24 h after CS (estimate adjusted = 0.565, P < 0.001). The PCIA bolus times were significantly lower in the SN group than in the S group. The rates of bradycardia and respiratory depression were lower in the SN group than in the S group. However, the rates of dizziness and postoperative hypotension were slightly higher in the SN group, and those of nausea/vomiting were comparable.
Conclusion: Compared with sufentanil alone, sufentanil combined with nalbuphine for PCIA provided superior analgesia in parturient women after CS.
Keywords: nalbuphine, sufentanil, patient-controlled intravenous analgesia, cesarean section
Introduction
Rates of cesarean section (CS) have increased globally in the last three decades. China has the highest rate of CS in Asia, rising from 28.8% in 2008 to 34.9% in 2014 and 36.7% in 2018.1 Postpartum pain is a major concern for women undergoing CS as it directly affects their functional recovery, physical and mental health, maternal–neonatal bonding, and breastfeeding.2 Due to inadequate pain control, at least 5.9% of patients undergoing CS reportedly experience chronic pain.3 Severe post-CS pain is also associated with several complications, such as venous thromboembolism, hyperalgesia, immune system disorder, overdose or addiction to analgesics, and postpartum depression.4 Therefore, adequate control of postpartum pain in women after CS is necessary and beneficial to both the mother and infant. However, the trauma caused by CS and the uterotonic drugs administered thereafter are obstacles to achieving adequate postoperative analgesia. There are no clear guidelines for the management of pain after CS,2 and multimodal analgesia is always recommended. Patient-controlled intravenous analgesia (PCIA) is widely used for postoperative analgesia after CS,5 also an important section of multimodal analgesia. It could provide better pain control with lower drug consumption and has a higher level of patient satisfaction, shorter hospital stay, and fewer adverse effects on pulmonary function.6
Sufentanil is widely used in PCIA as it has a rapid peak and a short half-life.7 Sufentanil offers lesser respiratory depression and better analgesic effect than fentanyl.8 However, like other opioids, sufentanil may have dose-dependent adverse effects, such as respiratory depression, nausea, vomiting, bradycardia, dizziness, and hypotension, which hamper postpartum recovery. Moreover, sufentanil alone cannot exert a satisfactory analgesic effect in parturient women because the pure µ-opiated receptor agonist cannot alleviate the visceral pain arising from the uterus. Nalbuphine is a mu (μ)-opioid receptor antagonist and kappa (κ)-opioid receptor agonist.9 Experiments and clinical trials on specific κ-opioid receptor agonists have reported that they can effectively block chemical stimulation-induced visceral pain better than pure µ-opioid receptor agonists.10 Nalbuphine, administered either via the intravenous or intrathecal route, is well known to effectively decrease opioid-induced pruritus and shivering. A combination of mixed agonist–antagonist opioids and μ-opioid receptor agonists for PCIA reportedly relieves both visceral and somatic pain effectively while decreasing the incidence of several adverse effects (vomiting, nausea, respiratory depression, and pruritus).11–13 Sufentanil combined with nalbuphine for PCIA has been used in post-CS pain management since 2017 at our hospital.
Few researches have compared the outcomes of sufentanil combined with nalbuphine and sufentanil alone for PCIA after CS. This retrospective study investigated and compared the analgesia efficacy, opioid consumption, and safety profile of sufentanil combined with nalbuphine and sufentanil alone for PCIA in a population of 1808 parturient women.
Materials and Methods
Study Cohort
The Medical Ethics Committee of First Affiliated Hospital of Air Force Medical University approved this study protocol (No. KY20212111-C-1). Owing to the retrospective design of the study, the need for individual consent was waived. The patients’ records and related information were made anonymous before this analysis. Our study complied with the Declaration of Helsinki, and this article was drafted in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
The medical records were collected of parturient women who underwent CS at Xijing Hospital between 21 September 2017 and 31 July 2020. Parturient women who underwent CS and received either sufentanil alone or sufentanil combined with nalbuphine for PCIA were enrolled. We excluded women who were given general anesthesia, switched to CS during labor analgesia, postoperatively transferred to the intensive care unit, or had missing primary outcome data (ie, numeric rating scale score with movement [NRS-M] at 24 h after CS).
Treatment Regimen
Our hospital has a standardized protocol for neuraxial anesthesia for CS. Spinal or spinal combined with epidural anesthesia is routinely used for patients with no contraindications. An epidural catheter is typically placed if procedures are anticipated to take a long time (eg, in cases wherein additional surgery besides CS is performed). Intrathecal opioids are not routinely administered in our setting.
PCIA via a patient-controlled analgesia device (AutoMed, Am3300) was started for parturient women as soon as they arrived at the ward. Women belonging to the sufentanil group (S group) received 2-µg/mL sufentanil (background infusion rate, 1 mL/h; demand dose, 0.5 mL/bolus). Women belonging to the sufentanil plus nalbuphine group (SN) received 1-µg/mL sufentanil combined with 0.4-mg/mL nalbuphine (background infusion rate, 2 mL/h; demand dose, 1 mL/per bolus). The lockout time for both groups’ PCIA was set at 10 min.
The parturient women were followed-up for 48 h postoperatively, and the following parameters were evaluated: vital signs (blood pressure, heart rate, respiratory rate), pain score, morphine-equivalent consumption, parameters of PCIA (including bolus attempts, bolus actual, volume of accumulation), sedation score (levels of sedation, LOS, 6-point scale: 0 = alert; 1 = mildly drowsy; 2 = moderately drowsy, easily arousable; 3 = very drowsy, arousable; 4 = difficult to arouse; and 5 = unarousable) at 24, and 48 h postoperatively) and adverse effects (eg, pruritus, postoperative nausea and vomiting, dizziness, and respiratory depression). During first six hours at ward postoperatively, vital signs of parturient were monitored closely with a Mindray monitor. If parturient did not have serious uncomfortable and whose vital signs were normal, the monitor would be removed after six hours postoperatively. At other times during postoperative, vital signs were manually observed and recorded by nurse.
Assessments
The primary outcome was the numeric rating scale (NRS) score with movement (NRS-M) at 24 h after CS. The secondary outcomes included NRS scores at rest (NRS-R) and at 24 and 48 h after CS, NRS-M at 48 h after CS, and the cumulative PCIA bolus times and opioid consumption within 24 and 48 h postoperatively measured in morphine-equivalent consumption. Postoperative pain was scored on a scale of 0 to 10 on the NRS, with nil (0) representing no pain and 10 representing the worst imaginable pain. Using the already available opioid conversion tables, intravenous opioid doses were converted to IV morphine-equivalent consumption. Additional secondary outcomes included postoperative hospitalization, time to remove the urinary catheter, and PCIA-related adverse effects within 48 h after CS.
Data Collection
The evaluated demographic variables included age, gestational weeks, weight, number of prior cesarean deliveries, and comorbidities. Perioperative variables included CS combined with concomitant procedures (eg, myomectomy of the uterus or removal of an ovarian tumor or cyst), surgery duration, anesthesia method, blood loss, blood transfusion, and intraoperative medication. PCIA-related adverse effects were defined a priori as postoperative respiratory depression (respiratory rate < 10 breaths/min or oxygen saturation below 90% without oxygen inspired), dizziness, hypotension (systolic blood pressure <90 mmHg), bradycardia (heart rate < 60 beats/min), nausea, vomiting, and pruritus. The time to remove the urinary catheter was defined as the time passed since the end of the CS until the removal of the urinary catheter. Data were compiled from the medical record system and postoperative analgesic follow-up database.
Statistical Analysis
Both Shapiro–Wilk and Kolmogorov–Smirnov tests were applied for normality tests a priori to the presentation of descriptive statistics. Continuous variables with symmetric distribution are shown as the mean (standard deviation); conversely, continuous variables with skewed distribution are shown as the median (interquartile range). The categorical variables are displayed as frequencies and percentages.
To accommodate skewed distributions, the non-parametric Wilcoxon rank sum test was used to compare crystalline liquid volume, blood transfusion, blood loss, and urine output between groups. Between-group comparisons for the following parameters were conducted using the chi-squared test: the number of prior cesarean deliveries, comorbidities, combined operation, intrathecal morphine administration, colloidal liquid administration, transversus abdominis plane (TAP) block, NSAIDs, and other categorical variables. No imputations for missing data were performed as the percentage of missing data was less than 5% for all outcomes.
The primary outcome was evaluated with multiple linear regression model. The model was built following the logic of univariate analysis, multiple regression (saturated model) and the final optimal regression (via stepwise selection, backward regression). The factors identified in the final model were those with P-values <0.2 in the univariate analyses or with clinical significance. Multicollinearity was evaluated using the variance inflation factor, wherein independence was assumed at variance inflation factor <10. A logistic regression analysis was then conducted to investigate the occurrence of inadequate analgesia (NRS score ≥ 4) adjusted for relevant confounders. Comparison of between groups was performed using t-test. Effect sizes for outcome variables were described by difference with 95% confidence intervals.
All hypothesis tests were two-sided at a 5% significance level. SPSS software (Version 26.0, SPSS, Chicago, IL, USA) was used to perform all statistical analyses.
Results
Demographic and Perioperative Characteristics
Overall, 2014 parturient women who visited our hospital between 21st September 2017 and 31st July 2020 were evaluated for eligibility, and 1808 of these women were included in the final analysis (Figure 1). There were 993 and 815 women in SN and S groups, respectively.
 |
Figure 1 Case screening flow chart. 1808 parturient women were included in our study, 993 of them in sufentanil-nalbuphine group, and 815 patients in sufentanil group. Baseline characteristics of parturients are shown in Table 1. |
The rate of comorbidities was significantly lower in the SN group (54.61%) than in the S group (56.82%, P = 0.004; Table 1); conversely, the operative time was significantly longer in the former (P < 0.001). Compared with the S group, the rates of NSAID use and TAP were lower in the SN group (P < 0.001, P = 0.001); however, dexamethasone was administered to significantly more patients in the SN group (P = 0.001). In addition, the ratio of colloidal liquid administration was lower in the SN group (8.36%) than in the S group (16.93%; P < 0.001). In addition, there were significant differences in blood loss and urine output between the two groups (P = 0.001, P < 0.001). The groups were comparable in terms of the duration of postoperative hospital stay and time of indwelling catheter.
 |
Table 1 Demographic and Perioperative Characteristics, Values are Mean (SD) Unless Stated Otherwise |
Pain Scores and Opioid Consumption
Pain Scores at Rest and with Movement (NRS-R and NRS-M)
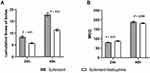
The SN group had lower NRS scores than the S group, both with movement and at rest (Figure 2). At 24 and 48 h after CS, the NRS-R scores of the SN group (0.96 ± 1.17 and 0.19 ± 0.58, respectively) were significantly lower than those of the S group (2.05 ±1.08 and 0.92 ± 1.24; P < 0.01 for both; Figure 2A). At 24 and 48 h after CS, the NRS-M scores of the SN group (4.62 ± 1.27 and 3.37 ± 1.11) were significantly lower than those of the S group (5.18 ± 1.27, 4.01 ± 1.16; P < 0.01 for both; Figure 2B). An NRS score ≥4 indicated inadequate analgesia. Notably, at 24 h after CS, the rate of inadequate analgesia was significantly lower in the SN group (84.29%) than in the S group (89.93%; P < 0.001).
The following covariates showed a P-value <0.2 in the univariate linear regression model and were included in the multivariate analysis (Table 2): comorbidities, combined operation, surgical duration, intraoperative opioid administration, NSAID administration, TAP block, blood loss, dexamethasone use, and the volumes of crystalline liquid and urine output. Other clinical covariates entered into the multivariate analysis were as follows: repeated CS, blood transfusion, and use of epidural morphine. According to the multivariate linear regression analysis, at 24 h after CS, the following factors were associated with lower NRS-M: sufentanil combined with nalbuphine administered via PCIA, epidural morphine, intravenous NSAID used, and dexamethasone. Even after adjusting for the covariates, the NRS-M of the SN group was lower than that of the S group (Table 3).
 |
Table 2 Multiple Linear Regression Analysis of the NRS with Movement at 24 h |
 |
Table 3 NRS with Movement at 24 h After CS |
Regarding inadequate analgesia (NRS score ≥ 4), at 24 h after CS, the following were associated with a lower rate of inadequate analgesia (Table 4): sufentanil combined with nalbuphine administered via PCIA, epidural morphine, and the use of NSAIDs (odds ratio [OR] = 0.582, P < 0.001; OR = 0.396, P = 0.014; OR = 0.537, P < 0.001, respectively).
 |
Table 4 Binary Logistic Regression Analysis of Inadequate Analgesia at 24h with Movement |
Bolus Attempts and Opioid Consumption
At both 24 and 48 h after CS, the cumulative number of bolus attempts in the SN group (5.74 ± 7.89 and 11.33 ± 13.36, respectively) was significantly smaller than that in the S group (8.34 ± 13.21 and 17.84 ± 19.85; P < 0.01 for both, Figure 3A).
Concerning opioid consumption, the morphine-equivalent consumption during the 24 h after surgery was slightly higher in the SN group (91.09 mg) than in the S group (80.64 mg; P < 0.01); however, the consumption was comparable between the two groups during the 48 h after surgery (Figure 3B).
Adverse Effects
The rates of postoperative respiratory depression and bradycardia were significantly lower for the SN group (nil and 0.91%, respectively) than for the S group (0.73% and 3.31%; P = 0.014 for both; Table 5). However, the SN group experienced higher rates of postoperative hypotension and dizziness (10.88% and 5.4%) than the S group (6.03% and 2.09%; P < 0.001, P = 0.002). The rates of postoperative nausea and vomiting for the two groups did not markedly differ. Pruritus was not observed in any of the women.
 |
Table 5 Adverse Effects in Parturient Women Within 48h After Surgery |
Discussion
This retrospective study evaluated the efficacy and safety of two PCIA regimens after CS: 1-μg/mL sufentanil combined with 0.4-mg/mL nalbuphine (SN) vs 2-μg/mL sufentanil (S) alone. It was found that the combined sufentanil–nalbuphine regime had the better analgesic effect: the NRS scores were lower in the SN group both at rest and with movement, and the rates of inadequate analgesia (NRS score ≥ 4) were lower. Furthermore, compared to the S group, the SN group had lower rates of respiratory depression and bradycardia but higher incidence of dizziness and hypotension. The reason about higher incidence of postoperative hypotension may be the lower utilization rate of colloidal solution in SN group than that in S group during surgery. Of course, this is also a conjecture, which needs to be confirmed by further prospective studies.
Ideal analgesia after CS requires relief from both somatic and visceral pain7 without negative effects for parturient women or infants. Sufentanil is a pure µ-opioid receptor agonist, whereas nalbuphine is a κ-opioid receptor agonist and μ-opioid receptor antagonist. The effect of opiates on visceral pain is reportedly related to the κ-opioid receptor agonism.14 In a combined sufentanil–nalbuphine regimen used for PCIA to relieve the postoperative pain in patients undergoing CS, sufentanil is mainly for somatic pain, whereas nalbuphine primarily addresses visceral pain. Nalbuphine is considered comparable to morphine in analgesic efficacy, whereas sufentanil has about 1000 times higher analgesic efficacy than morphine.15
The combination of a pure µ-receptor agonist (sufentanil) with a mixed agonist–antagonist, such as nalbuphine, has shown complicated effects. Loomis et al16 reported that although nalbuphine at low doses appeared to act as a µ-opiate receptor agonist, at high doses, it behaved like an antagonist of a µ-opiate receptor agonist. In the present study, the low-dose nalbuphine possibly acts on sufentanil and makes it more potent. Therefore, patients in the SN group reported less pain.
In a randomized controlled study with PCIA comprising hydromorphone (0.05 mg/mL) combined with low, middle, or high concentrations of nalbuphine (0.5, 0.7, and 0.9 mg/mL), all treatment groups experienced similar analgesic efficacy, with the parturient women either at rest or with movement.4 In that study, the low-concentration group used the same concentration of nalbuphine that was used in the SN group in the present study.7 The author also reported that compared with low- and middle-concentration groups, the patients in the high-concentration group showed the highest sedation level, the lowest uterine cramping pain scores, and the highest rates of urinary retention; they also took the longest time until the first flatus. Therefore, to avoid extreme effects, they recommended the middle concentration regimen (0.05-mg/mL hydromorphone combined with 0.7-mg/mL nalbuphine) for PCIA after CS. In a future study, we will investigate whether this middle concentration of nalbuphine combined with sufentanil is superior to the concentration used in the present study.
Maternal–neonatal bonding and breastfeeding after CS deserves attention. Jacqz-Aigrain et al17 reported that a breastfed neonate ingested 0.59% ± 0.27% of the maternal daily dose of nalbuphine and that it is safe to breastfeed the infant during nalbuphine PCIA. The ceiling effect of respiratory depression also favors nalbuphine as an alternative to sufentanil for PCIA.18 Compared with the use of sufentanil alone for PCIA, nalbuphine combined with sufentanil for PCIA after CS could reduce sufentanil consumption and decrease NRS scores. This translates into a more comfortable experience and shortens the bed rest time of postpartum women, thus making it highly beneficial for maternal–neonatal bonding and breastfeeding.
Additionally, epidural morphine, TAP block, and dexamethasone infusion have a positive effect on analgesia after CS. A previous study19,20 reported significantly improved pain scores in obstetric patients receiving TAP block. NSAIDs amplify the analgesic effect of the opioid on both somatic and visceral pain to different extents and decreased the demand ratio for patient-controlled analgesia and 24-h morphine consumption after CS by approximately 30%.21 These interventions are now recommended for patients after CS as components of the multimodal analgesia strategy. Investigating whether nalbuphine combined with sufentanil achieves a satisfactory analgesic effect within the multimodal analgesia background has more merit than only comparing the effects of different regimes of PCIA.
Limitations
This study has several limitations. As a retrospective study, some residual confounding may have been inevitable Logistic regression analysis was employed to eliminate the influence of confounding factors on the primary outcome. However, certain factors that may affect the analgesia outcome were not considered, such as changes in surgical techniques over time, differences between surgeons, and the psychological state of parturient women.22,23 Secondly, although we analyzed and compared NRS within 48 h of performing CS, there was no distinction made between visceral pain and somatic pain. Finally, only one dosage of nalbuphine was used in this study. The ideal dosage of nalbuphine needs to be determined by comparing outcomes using multiple doses in a future study.
Conclusion
Compared with sufentanil alone, sufentanil combined with nalbuphine for PCIA provided superior analgesia after CS for the parturient women in this population.
Acknowledgment
We thank Medjaden Inc. for scientific editing of this manuscript.
Funding
The authors have no funding in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qiao J, Wang Y, Li X., et al. A Lancet Commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet. 2021;397:2497–2536.
2. Sutton CD, Carvalho B. Optimal pain management after cesarean delivery. Anesthesiol Clin. 2017;35:107–124.
3. Nikolajsen L, Sørensen HC, Jensen TS, et al. Chronic pain following cesarean delivery. Acta Anaesthesiol Scand. 2004;48:111–116.
4. Huang CY, Li SX, Yang MJ, et al. A comparative study of three concentrations of intravenous nalbuphine combined with hydromorphone for post-cesarean delivery analgesia. Chin Med J. 2020;133:523–529.
5. Wu Z, Zhao P, Peng J, et al. A patient-controlled intravenous analgesia with tramadol ameliorates postpartum depression in high-risk woman after cesarean section: a randomized controlled trial. Front Med. 2021;8:679159.
6. Chi X, Li M, Mei W, Liao M. Comparison of patient-controlled intravenous analgesia with sufentanil versus tramadol in post-cesarean section pain management and lactation after general anesthesia - A prospective, randomized, double-blind, controlled study. J Pain Res. 2017;10:1521–1527.
7. Han L, Su Y, Xiong H, et al. Oxycodone versus sufentanil in adult patient-controlled intravenous analgesia after abdominal surgery: a prospective, randomized, double-blinded, multiple-center clinical trial. Medicine. 2018;97:e11552.
8. Bailey PL, Streisand JB, East KA, et al. Differences in magnitude and duration of opioid-induced respiratory depression and analgesia with fentanyl and sufentanil. Anesth Analg. 1990;70:8–15.
9. Miller RD, Cohen NH, Eriksson LI, et al. Miller’s Anesthesia.
10. Pasternak GW. Molecular biology of opioid analgesia. J Pain Symptom Manage. 2005;29:S2–9.
11. Schultz-Machata AM, Becke K, Weiss M. Nalbuphine in anesthesia. Der Anaesth. 2014;63:135–143.
12. Van Niel JC, Schneider J, Tzschentke TM. Efficacy of full m-opioid receptor agonists is not impaired by concomitant buprenorphine or mixed opioid agonists/antagonists-preclinical and clinical evidence. Drug Res. 2016;66:562–570.
13. Yeh YC, Lin TF, Lin FS, et al. Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain. Br J Anaesth. 2008;101:542–548.
14. Rivière PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141:1331–1334.
15. Sun Z, Zhu Z, Yang G, et al. The 95% effective dose of nalbuphine in patient-controlled intravenous analgesia for patients undergoing laparoscopic total hysterectomy compared to equivalent sufentanil. Medicine. 2020;99:e20424.
16. Loomis CW, Penning J. A study of the analgesic interaction between intrathecal morphine and subcutaneous nalbuphine in the rat. Anesthesiology. 1989;71:704–710.
17. Jacqz-Aigrain E, Serreau R, Boissinot C, et al. Excretion of ketoprofen and nalbuphine in human milk during treatment of maternal pain after delivery. Ther Drug Monit. 2007;29:815–818.
18. Deng C, Wang X, Zhu Q, et al. Comparison of nalbuphine and sufentanil for colonoscopy: a randomized controlled trial. PLoS One. 2017;12:e0188901.
19. Cole J, Hughey S, Longwell J. Transversus abdominis plane block and intrathecal morphine use in cesarean section: a retrospective review. Reg Anesth Pain Med. 2019;13:rapm-2019–100483.
20. Mallan D, Sharan S, Saxena S, et al. Anesthetic techniques: focus on transversus abdominis plane (TAP) blocks. Local Reg Anesth. 2019;12:81–88.
21. Hsu HW, Cheng YJ, Chen LK, et al. Differential analgesic effect of tenoxicam on the wound pain and uterine cramping pain after cesarean section. Clin J Pain. 2003;19:55–58.
22. Chen Y, Ye X, Wu H, et al. Association of postpartum pain sensitivity and postpartum depression: a prospective observational study. Pain Ther. 2021;10:1619–1633.
23. Ren L, Chen Q, Min S, et al. Labor Analgesia reduces the risk of postpartum depression: a cohort study. Transl Neurosci. 2021;12:396–406.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.