Back to Journals » International Journal of Women's Health » Volume 13
Suction Curettage and Foley Balloon as a First-Line Treatment Option for Caesarean Scar Pregnancy and Reproductive Outcomes
Authors Aslan M , Yavuzkir Ş
Received 4 December 2020
Accepted for publication 4 February 2021
Published 23 February 2021 Volume 2021:13 Pages 239—245
DOI https://doi.org/10.2147/IJWH.S294520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Melike Aslan, Şeyda Yavuzkir
Obstetrics and Gynecology Department, Fırat University Medicine Faculty Hospital, Elazığ, Turkey
Correspondence: Melike Aslan
Department of Obstetrics and Gynecology, Fırat University Medicine Faculty Hospital, Elazığ, Turkey
Email [email protected]
Background: Caesarean scar pregnancy (CSP) is associated with various serious pregnancy complications. This study presents the outcomes of patients treated with suction curettage and Foley balloon as the first-line treatment for CSP as well as their future outcomes in terms of successful pregnancy.
Methods: Of the 44 patients diagnosed with CSP between January 2015 and April 2019, 42 who provided consent for the termination of pregnancy and who simultaneously underwent the transabdominal ultrasound-guided suction curettage + Foley balloon treatment were included in the study. These patients were then contacted and interviewed to collect data concerning their post-treatment number of pregnancies, number of miscarriages, number of live or dead births, mode of delivery, delivery time as well as whether any abnormal placental invasion or uterine ruptures developed.
Results: Transabdominal ultrasound-guided suction curettage + Foley balloon was simultaneously performed as the first-line treatment in 42 patients with CSP. In two of these cases, wherein post-treatment level of β-hCG reached a plateau, single-dose systemic methotrexate was administered. Emergency surgical intervention, hysterectomy, massive blood transfusion and additional systemic methotrexate administration were not required. Twenty-six of 42 patients could be contacted. 18/26 were trying to conceive. 6/18 patients had secondary infertility, and 12/18 patients managed to conceive. 8/12 had caesarean delivery at full term. 1/12 was 16-week pregnant, 1/12 had tubal ectopic pregnancy and 2/12 had first-trimester abortus.
Conclusion: When administered as the first-line treatment for CSP, the suction curettage + Foley balloon treatment is a highly successful, cheap and easily performed minimally invasive method that requires only a short hospital stay, making it comfortable for patients. Compared with other uterine-sparing methods, it does not harm fertility and has positive effects on patients’ future fertility outcomes.
Keywords: caesarean scar ectopics, first-line management, suction curettage, Foley balloon, pregnancy outcomes
Introduction
Caesarean scar pregnancy (CSP) is a new variant of ectopic pregnancies. The increased number of caesarean pregnancies and the advancements in imaging techniques have rendered its diagnosis more probable. Development of the disease is still not completely known. It is believed that, due to the weak vascular support in the uterine front wall of some patients who undergo C-section, blastocyst implants itself to a previous caesarean fibrous scar and myometrium prior to the formation of decidua basalis.1 The incidence of CSP is reported to be 1/1008–1/2500 among all caesarean deliveries.2 To obtain the maximum benefit from early diagnosis and treatment, each pregnant woman with a history of previous caesarean scar should be scanned during the early weeks of her pregnancy. It can be easily diagnosed using ultrasound and Doppler imaging; however, it should be suspected first. This is because it can progress to abnormal placental invasion if misdiagnosed and not treated, which may eventually result in complications requiring even hysterectomy that is the last-line treatment, which may cause uterine rupture, massive bleeding, loss of fertility and even maternal death.2–4
There have been only five randomised studies on CSP to date, and the best evidence-based management still has to be standardised. Until then, treatment should therefore be individualised based on the clinical presentation of the specific case, β-hCG levels, imaging properties and the clinical skills of the surgeon.5
The uterus-sparing interventions allow patients to conceive again; however, there is limited information concerning the fertility and pregnancy outcomes after CSP because it is a rarely encountered variant of ectopic pregnancy.
Herein, we intend to present the suction curettage (S&C) + Foley balloon treatment protocol and its reproductive outcomes retrospectively that was performed by residents in 42 patients with CSP who presented to our clinic within the last 5 years. This is a successful, practical, cheap, minimally invasive and comfortable (short hospital stay for patients) method not requiring high-level surgical experience as in uterine artery embolisation and laparoscopic or hysteroscopic procedures.
Materials and Methods
This retrospective study was conducted after receiving informed and signed consent forms from the patients and approved by the local ethics committee of Fırat University with 2020 as the approval year, 06–22 as the approval number and conducted in accordance with the ethical standards of the Institutional and National Committee on Human Experimentation and with the Helsinki Declaration of 1975, which revised in 2000.6 The patients who were presented to our clinic between January 2015 and April 2019 were scanned, and 44 of them were diagnosed with CSP according to the diagnostic criteria. Of these, 42 were included in the study after they provided consent for the termination of pregnancy and following the confirmation of their diagnoses via postoperative histopathological diagnosis. Two of these patients diagnosed with CSP did not consent for the termination of pregnancy and decided to sustain their pregnancy. Demographical and clinical data were recorded.
Transvaginal sonographic examinations were performed using Voluson’s ultrasound device Voluson® E6 (GE Medical Systems, Zipf, Austria). The data on patients’ age, number of C-sections, admission haemoglobin (Hb) value (gr/dL), β-hCG value (IU/L), clinical symptoms, amount of blood transfusion and duration of hospital stay were recorded.
Ultrasound criteria of CSP: 1) Visualization of an empty uterus with empty, closed cervical canal, 2) The gestational sac embedded in previous scar(s) (Figure 1) 3) Thin or absent myometrial layer between the GS and urinary bladder, 4) Rich vascular pattern, and arterio-venous malformation (AVM) around the GS.7,8
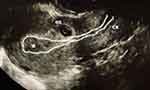 |
Figure 1 Transvaginal ultrasound showing cesarean scar pregnancy. *Empty endometrium with empty, closed cervix. Abbreviations: GS, gestational sac located in previous scar; CX, cervix. |
Achieving a β-hCG level of 0 IU/L, transvaginal USG imaging not indicating a gestational sac and no-vascularisation around it were accepted as indicators of a successful treatment, whereas blood loss of >300 mL during the procedure or the follow-up period after S&C, requirement for emergency surgical intervention, β-hCG values reaching a plateau or being in an upward trend, transvaginal USG imaging indicating a larger gestational sac and increased vascularisation surrounding the sac were deemed indicative of treatment failure.
After informing the patients about the risks of hysterectomy, bleeding and bladder injury that transabdominal ultrasound-guided S&C + Foley balloon entailed, Karman cannula number 4 was used to access the sac level via the cervix with the patients under general anaesthesia and in lithotomy position, and evacuation was performed until the gestational sac was no longer visible. Subsequently, 16 Fr catheter was placed on the sac level and inflated using saline until the bleeding stopped. Simultaneously, 10 IU oxytocin was intravenously administered. β-hCG was checked at the postoperative 24th hour and the Foley balloon was removed. Patients without any complication and pain and who were haemodynamically stable with minimal bleeding or no bleeding at all were tested to observe their β-hCG levels daily throughout their hospital stay and then advised to have their β-hCG levels tested once a week after being discharged. They were advised to go to the hospital in cases of severe abdominal pain, massive bleeding and increased level of β-hCG. The patients whose post-treatment β-hCG reached a plateau were administered a single 50-mg/m2 dose of intramuscular methotrexate. Their β-hCG levels were checked on days 4 and 7. Patients with a haemoglobin value of <7 gr/dL were transfused with 2 units of blood. The 42 patients who underwent the S&C + Foley balloon procedure were called using their phone numbers which were previously recorded, and 26 of these were contacted. These patients were asked about their post-treatment number of pregnancies, number of miscarriages, number of live or dead births, mode of delivery, delivery time, recurrence of scar pregnancy and whether any abnormal placental invasion or uterine rupture developed. These data were recorded.
The SPSS 16 package was used for statistical analysis. Descriptive statistics are given as mean, standard deviation, frequency and percentage values.
Results
The data is presented in Tables 1 and 2. The mean age of patients was 32 (22–43) years. The gestational week was between 5 and 9 + 4 weeks. Nine (20.45%) of the patients presented with abdominal pain and vaginal bleeding, 26 (59.09%) presented with vaginal bleeding, and nine (20.45%) were diagnosed incidentally with no complaint at all.
 |
Table 1 Patient and Procedure Characteristics |
 |
Table 2 Reproductive Outcomes in Women with a Previous Cesarean Scar Pregnancy |
A total of 42 patients simultaneously underwent transabdominal ultrasound-guided S&C + Foley balloon treatment. In two of these patients, wherein post-treatment level of β-hCG reached a plateau, single-dose systemic methotrexate was administered. Furthermore, two patients with a haemoglobin value of <7 gr/dL were transfused with 2 units of blood. Uterine rupture was not observed in any patient. Emergency surgical intervention, hysterectomy, massive blood transfusion and additional systemic methotrexate administration were not required in any patient.
Two patients with positive fetal cardiac activity did not consent to the procedure and chose to sustain their pregnancy. One of these patients was diagnosed with placenta percreta and underwent elective delivery and caesarean hysterectomy in week 35.
The other patient was diagnosed with intrauterine fetal death, placenta previa totalis, abnormal placental invasion in week 36. She underwent elective caesarean delivery. Firmly adherence of placenta to the myometrium confirmed the diagnosis of abnormal placental invasion, caesarean hysterectomy was performed without complication. Pathology result reported as placenta increta.
Only 26 of the patients included in the present study could be contacted, whereas 16 did not answer. Moreover, 18 of these 26 patients were trying to conceive, whereas eight of them were taking contraceptive measures. Six of 18 patients had secondary infertility, and 12 of 18 patients that were trying to conceive managed to conceive. Eight of these 12 patients who conceived had caesarean delivery at full term, two of whom had a history of one missed abortion each. One of these two patients was diagnosed with a pregnancy implanted close to the scar line; however, the patient did not wish to undergo any intervention and instead choose to have a spontaneous abortion with no complications. One patient (1/12) was 16-week pregnant, 1/12 had a tubal ectopic pregnancy and 2/12 had their pregnancies terminated with first-trimester abortion, whereas there was no patient with second-trimester abortion or preterm delivery (Figure 2).
 |
Figure 2 Flowchart of follow-up data for 42 women with cesarean scar pregnancies. |
The mean ages of patients who conceived again, who delivered at full term, whose pregnancies were terminated with abortion and of infertile patients were 37.58 (28–46), 36 (28–40), 41.5 (35–46) and 38.5 (31–46) years, respectively.
Discussion
CSP is one of the rarely encountered forms of ectopic pregnancies. In parallel with the increasing rate of caesarean deliveries and advancements in transvaginal ultrasound imaging, the rate of CSP diagnoses has also increased. The most suitable treatment method for CSP is uncertain, and there is no standard treatment approach because the clinical experiences of CSPs are presented as case series in the literature and because of the lack of randomised controlled studies. Patients with symptoms of bleeding and haemodynamic instability need surgical intervention. These interventions might be performed laparoscopically or via laparotomy and the type of surgery might possibly incorporate hysterectomy. Treatment may be in the form of dilatation and curettage or methotrexate administration in a stable patient. Other options include wedge resection of ectopic pregnancy via laparotomy or laparoscopy, ectopic pregnancy resection by hysteroscopic excision, local injection of 5 mEq potassium chloride into the sac, selective arterial embolisation of the uterine arteries together with curettage and/or methotrexate administration and treatment with local or systemic methotrexate administration.9–12
Despite the high number of methods to treat CSP, no global treatment protocol concerning the best and standard treatment has still been published. In this retrospective study, we simultaneously performed S&C + Foley balloon in 42 of 44 patients with CSP. Two of these patients were administered systemic methotrexate treatment because their β-hCG levels reached a plateau. All these patients were successfully treated. Two other patients chose to sustain their pregnancy because they were positive for fetal cardiac activity. Two patients diagnosed with abnormal placental invasion underwent caesarean hysterectomy in the third trimester.
Early diagnosis and management is of critical importance because of the serious consequences of CSP. The clinical presentation of a patient and the USG findings are relevant in making the diagnosis. One-third of the incidentally diagnosed cases are clinically asymptomatic.3 Most patients present with non-specific symptoms. Moreover, the most common clinical symptom is vaginal bleeding.13,14 Overall, 24.6% of cases present with slightly severe abdominal pain and/or vaginal bleeding.15 In the present study, 9 (20.45%) patients presented with abdominal pain and vaginal bleeding, 26 (59.09%) presented with vaginal bleeding, and 9 (20.45%) were diagnosed incidentally with no complaint at all. In our study, clinical symptoms of patients were found similar to previous studies.
The mean gestational age when the diagnosis is made varies between 5 and 16 weeks.13 In the present study, 42 patients with unruptured CSP at a gestational week of 5 to 9 + 4 weeks who were haemodynamically stable and had a distinct myometrial thickness between the bladder and the CSP sac simultaneously underwent S&C + Foley balloon treatment.
Decreased myometrial thickness observed in the first-trimester transvaginal ultrasound examination was associated with abnormal placental adhesions that have high morbidity rates and have 4–5 times higher risk of placenta percreta.16 A study by Timor-Tritsch et al17 highlights that placenta accreta can occur due to progression of CSP and CSP is a precursor of adherent placenta. The same study also revealed that CSP and early placenta accreta have the same histology. Although two of our patients were indeed diagnosed with CSP during the first trimester (weeks 7–9) and properly informed about the potential complications, they rejected the termination of pregnancy and sustained their pregnancy. These two patients eventually underwent caesarean hysterectomy after being diagnosed with placenta percreta during the last trimester.
The factors that affect scar-healing of caesarean incisions include improper closure of uterine incision, postoperative infection, existing health problems like diabetes mellitus and connective collagen tissue disorders, factors that reduce blood flow to the scar tissue such as smoking and predisposing factors such as short time interval from previous caesarean pregnancies.17–19
Adenomyosis, in-vitro fertilisation, history of dilatation curettage and manual removal of placenta are potential risk factors. In addition to C-section, surgeries such as myomectomy, metroplasty and hysteroscopy are other risk factors.4,11,20
The number of previous C-sections was not associated with CSP. Overall 43 patients included in the present study had a history of at least one C-section and a maximum of four, whereas one patient did not have a history of C-section but of metroplasty surgery.
As in many other studies in the literature, Uçar et al21 argued that curettage should not be used as the first-line treatment in cases of CSP and it should be accompanied by other treatments rather than being preferred as the primary treatment. They supported it by noting that CSP has risks of very serious bleeding and uterine rupture because it was shown to have a very weak contraction component due to the scar. The study by Rotas et al22 also reported a high failure rate of 76.1% when curettage was preferred as the first-line treatment option, although it is less invasive.3,23,24 However, in our opinion, the above-mentioned failure rate is associated with the sharp curettage procedures possibly performed using metal curette in the CSP case series that were managed with curettage in the literature. This is because most of the studies managed with curettage did not include the necessary details concerning the Dilatation and curettage (D&C) procedure according to our observations. Therefore, we suspect that the D&C procedures might have been performed using metal curette following dilatation. In the present study, however, the pregnancy material was aspirated via TAUSG-guided vacuum curettage without performing dilatation, and the success rate was 95%.
In addition, there are studies that argue the opposite and that manage cases only with S&C without the need for additional treatments.25 Polat et al26 report a success rate of 84.2% in their study where S&C was used as the first-line treatment. The 42 patients with CSP included in the present study underwent S&C + Foley balloon as the first-line treatment, and none of these developed abundant vaginal bleeding or uterine rupture. Two patients were transfused with 2 units of blood, but the haemoglobin values of these patients at presentation were <9 gr/dL. We believe that CSP should be managed by performing USG-guided Foley balloon curettage as the first-line treatment.
Medical treatment is also an option in CSP. According to Li et al,27 the success rate of systemic MTX administration alone is <38.5%. Combined (local and systematic) MTX administration appears to be more successful. However, slow resolution, long hospital stay, ongoing risk of uterine rupture and haemorrhage as well as drug-related side effects are the disadvantages. In the present study, S&C + Foley balloon treatment required a mean hospital stay of 2 days. There was a need for additional systemic MTX treatment only in two patients.
The pregnancy rate in the present study was 66.7%. This result is similar to the study by Wang et al,28 whereas the rates of pregnancy were 83% and 87.5% in similar studies by Ben Nagi et al29 and Gao et al,30 respectively. The secondary infertility rate in cases included in the present study that could not conceive despite their efforts following the S&C + Foley balloon procedure was 23.07%. The post-CSP infertility rate was 14.3% by Gao et al.30 We attributed this difference between the infertility rates to the mean age of our secondary infertile patients being 38.5. We believe that the high rate of miscarriages during the first trimester (33.3%) can be associated with maternal age, similar to the study by Gao et al.30
Some studies revealed that CSP can recur in future pregnancies.29 One of the patients included in the present study was diagnosed with a pregnancy implanted close to the scar during the period ` CSP; however, we believe that this might be a misdiagnosis because the patient had spontaneous abortion with no complications.
Abnormal placental invasion was not observed in any patient who had caesarean delivery after undergoing S&C + Foley balloon procedure.
Conclusion
Patients with CSP are at risk of spontaneous abortion, secondary infertility, recurrent CSP, placenta accreta, and postpartum bleeding in consecutive pregnancies.
The present study reports that reproductive outcomes are not influenced negatively in CSP cases following the S&C + Foley balloon procedure, and the risk of complications such as first or second-trimester spontaneous abortion, uterine rupture, preterm delivery and recurring scar implantation is low. We recommend that the S&C + Foley balloon method be considered as the first-line treatment in patients with CSP because it can be easily performed and is a successful, cheap and minimally invasive method requiring a short hospital stay.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114(3):253–263. doi:10.1111/j.1471-0528.2006.01237.x
2. Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. Cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2003;21(3):310. doi:10.1002/uog.55
3. Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247–253. doi:10.1002/uog.974
4. Marchiolé P, Gorlero F, de Caro G, Podestà M, Valenzano M. Intramural pregnancy embedded in a previous cesarean section scar treated conservatively. Ultrasound Obstet Gynecol. 2004;23(3):307–309. doi:10.1002/uog.981
5. Gonzalez N, Tulandi T. Cesarean scar pregnancy: a systematic review. J Minim Invasive Gynecol. 2017;24(5):731–738. doi:10.1016/j.jmig.2017.02.020
6. Riis P. Perspectives on the fifth revision of the declaration of helsinki. JAMA. 2000;284(23):3045–3046. doi:10.1001/jama.284.23.3045
7. Timor-Tritsch IE, Monteagudo A, Santos R, et al. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):
8. Osborn DA, Williams TR, Craig BM. Cesarean scar pregnancy: sonographic and magnetic resonance imaging findings, complications, and treatment. J Ultrasound Med. 2012;31(9):1449–1456. doi:10.7863/jum.2012.31.9.1449
9. Graesslin O, Dedecker F
10. Ghezzi F, Laganà D, Franchi M, Fugazzola C, Bolis P. Conservative treatment by chemotherapy and uterine arteries embolization of a cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;103(1):88–91. doi:10.1016/S0301-2115(02)00003-9
11. Lee CL, Wang CJ, Chao A, et al. Laparoscopic management of an ectopic pregnancy in a previous caesarean section scar. Hum Reprod. 1999;14(5):1234–1236. doi:10.1093/humrep/14.5.1234
12. Deans R, Abbott J. Hysteroscopic management of cesarean scar ectopic pregnancy. Fertil Steril. 2010;93(6):1735–1740. doi:10.1016/j.fertnstert.2008.12.099
13. Riaz RM, Williams T, Craig B, Myers D. Cesarean scar ectopic pregnancy: imaging features, current treatment options, and clinical outcomes. Abdom Imaging. 2015;40(7):2589–2599. doi:10.1007/s00261-015-0472-2
14. Zhang Y, Gu Y, Wang JM, Li Y. Analysis of cases with cesarean scar pregnancy. J Obstet Gynaecol Res. 2013;39:195–202. doi:10.1111/j.1447-0756.2012.01892.x
15. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6):592–593. doi:10.1046/j.1469-0705.2000.00300-2.x
16. O’Brien JM, Wetham D, Fecteau C, Jansen J, Hiles M. Augmenting myometrial healing after cesarean delivery: use of an adjuvant biologic graft placement in an ovine model. Am J Perinatol. 2011;28:543–550. doi:10.1055/s-0031-1272973
17. Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383–395. doi:10.1002/uog.13282
18. Holland MG, Bienstock JL. Recurrent ectopic pregnancy in a cesarean scar. Obstet Gynecol. 2008;111:541–545. doi:10.1097/01.AOG.0000287295.39149.bd
19. Fylstra DL. Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv. 2002;57:537–543. doi:10.1097/00006254-200208000-00024
20. Ofili-Yebovi D, Ben-Nagi J, Sawyer E, et al. Deficient lower-segment cesarean section scars: prevalence and risk factors. Ultrasound Obstet Gynecol. 2008;31(1):72–77. doi:10.1002/uog.5200
21. Uçar MG, Ilhan TT, Kebapçılar AG. Treatment-resistant cesarean scar pregnancy, a case report and brief review of literature. Med J Aegean Clin. 2015;53(3).
22. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373e1381. doi:10.1097/01.AOG.0000218690.24494.ce
23. Neiger R, Weldon K, Means N. Intramural pregnancy in a cesarean section scar. A case report. J Reprod Med. 1998;43:999–1001.
24. Ayoubi JM, Fanchin R, Meddoun M, Ferinandez H, Pons JC. Conservative treatment of complicated cesarean scar pregnancy. Acta Obstet Gynecol. 2001;80:469–70. doi:10.1034/j.1600-0412.2001.080005469.x
25. Arslan M, Pata O, Dilek TU, Aktas A, Aban M, Dilek S. Treatment of viable cesarean scar ectopic pregnancy with suction curettage. Int J Gynaecol Obstet. 2005;89(2):163–166. doi:10.1016/j.ijgo.2004.12.038
26. Polat I, Ekiz A, Acar DK. Suction curettage as first line treatment in cases with cesarean scar pregnancy: feasibility and effectiveness in early pregnancy. J Matern Fetal Neonatal Med. 2016;29(7):1066–1071. doi:10.3109/14767058.2015.1034100
27. Li N, Zhu F, Fu S, Shi X. Transvaginal ultrasound-guided embryo aspiration plus local administration of low-dose methotrexate for caesarean scar pregnancy. Ultrasound Med Biol. 2012;38(2):209–213. doi:10.1016/j.ultrasmedbio.2011.10.012
28. Wang Q, Peng H-L, He L, Zhao X. Reproductive outcomes after previous cesarean scar pregnancy: follow up of 189 women. Taiwan J Obstet Gynecol. 2015;54(5):551–553. doi:10.1016/j.tjog.2015.08.006
29. Ben Nagi J, Ofili-Yebovi D, Sawyer E, Aplin J, Jurkovic D. Successful treatment of a recurrent cesarean scar ectopic pregnancy by surgical repair of the uterine defect. Ultrasound Obstet Gynecol. 2006;28(6):855–858. doi:10.1002/uog.3843
30. Gao L, Huang Z, Zhang X, Zhou N, Huang X, Wang X. Reproductive outcomes following cesarean scar pregnancy - a case series and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2016;200:102–107. doi:10.1016/j.ejogrb.2016.02.039
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
