Back to Journals » OncoTargets and Therapy » Volume 12
Successful treatment with cryoablation in a patient with bone metastasis in the mid-shaft femur: a case report
Authors Kita K, Nakamura T , Yamanaka T, Yoshida K, Hagi T, Asanuma K, Nakatsuka A, Sudo A
Received 22 November 2018
Accepted for publication 21 February 2019
Published 17 April 2019 Volume 2019:12 Pages 2949—2953
DOI https://doi.org/10.2147/OTT.S195634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Kouji Kita,1 Tomoki Nakamura,1 Takashi Yamanaka,2 Keisuke Yoshida,1 Tomohito Hagi,1 Kunihiro Asanuma,1 Atsuhiro Nakatsuka,2 Akihiro Sudo1
1Department of Orthopaedic Surgery, Mie University Graduate School of Medicine, Tsu, Japan; 2Department of Radiology, Mie University Graduate School of Medicine, Tsu, Japan
Background: Treatment of metastatic bone tumors is challenging due to the morbidity associated with patients with metastasis. The present case report described a patient with successful treatment of bone metastasis using cryoablation with plate and cementation to prevent fracture for bone metastasis of leiomyosarcoma in the mid-shaft of the femur.
Case report: The metastatic tumor was located at intramedullary lesion of the femur. At first, cryoablation was performed under local anesthesia. After one week after cryoablation, curettage and fixation with plate and cementation were performed to prevent fracture. Tumor cells were not observed in the histopathological findings of the curettage tissue. Four years after cryoablation, there was no recurrence and the patient could walk without any support.
Conclusion: We suggest that a tumor with limited cancellous bone and of a small size may undergo cryoablation. The prevention of fracture after cryoablation should be considered.
Keywords: leiomyosarcoma, bone metastasis, cryoablation
Introduction
Treatment of metastatic bone tumors is challenging due to the morbidity associated with patients with metastasis. Leiomyosarcoma is known to metastasize to distant sites in up to 30% of the cases.1 Survival after metastasis is poor, and the 5-year post-metastatic survival rate was reported to be 15% and 40%.2–4 However, if the metastases can be completely resected, the prognosis may be improved. The present case report described a patient with bone metastasis of leiomyosarcoma in the mid-shaft of the femur. Generally, resection of segmental bone defects after intercalary resection of the femur is performed with endoprosthesis,5 distraction osteogenesis,6 allograft,7 and autologous graft,8 which are all highly invasive procedures.
Cryosurgery as surgical adjuncts in the treatment of aggressive benign bone tumors was reported and demonstrate that the ability of cryosurgery to eradicate tumors while avoiding the need for extensive resections and reconstruction procedures.9 Cryoablation for bone metastasis was reported to reduce pain, and its utility as the curative treatment for metastasis is well-known.10–12 Here, we report a case with successful treatment of bone metastasis using cryoablation with plate and cementation to prevent fracture. The approval of institutional review board was waived because of the nature of retrospective nature. The written informed consent to publish case details in the present report was obtained from the patient.
Case report
A 49-year-old woman visited the clinic due to a mass in her right thigh. The patient first noticed the mass four years prior to presentation. After needle biopsy was performed, she was referred to our hospital with a diagnosis of leiomyosarcoma.
A physical examination confirmed the mass with a diameter of 4 cm on the lateral side of the right thigh. There was no adhesion with skin and no tenderness.
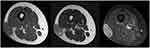
Magnetic resonance imaging (MRI) identified a subcutaneous soft tissue mass, which exhibited low signal intensity on T1-weighted images and heterogeneous signal intensity on T2-weighted images. The tumor was enhanced by a gadolinium injection (Figure 1). Computed tomography (CT) of the chest, abdomen, and pelvis did not demonstrate any distant metastasis. All routine blood laboratory data were normal. Wide tumor resection was performed. Adjuvant chemotherapy and radiotherapy were not administered because of the small (<5 cm) and superficial tumor.
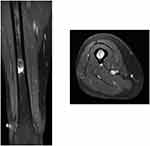
One year and 3 months postoperatively, the patient developed lung nodules. The maximum size of the lung nodules was 5 mm. Therefore, excisional biopsy was performed. The histopathological diagnosis was consistent with lung metastasis of leiomyosarcoma. Positron emission tomography (PET)–CT revealed the high FDG uptake with a maximum standardized uptake value of 4.4 in the lung and the right mid-shaft of the femur. On MRI, the tumor size on the mid-shaft of the right femur was 2 cm (Figure 2). The cortical bone was maintained, and therefore, the patient did not present any symptoms. We diagnosed the patient with lung and bone metastases. Therefore, systemic chemotherapy using doxorubicin and ifosfamide was administered. Next, we planned to treat the bone metastasis of the right femur. First, cryoablation under CT guidance was performed (Figures 3 and 4). Ablation needles were inserted into the proximal and distal side of the tumor. Cryoablation was performed using an argon gas–based system (CryoHit, Galil Medical) under CT-fluoroscopic guidance (Aquilion ONE, Canon Medical System, Ootawata Japan). After administration of local anesthesia with 1% lidocaine (Xylocaine, AstraZeneca International), after tract was holed by electric drill, 17-gauge cryoprobes (IceRod; Galil Medical, Japan) were placed into the tumor according to their size and shape. Cryoablation was then performed with two cycles of 15-min freeze and 5-min thaw.13 To monitor the size of the ice ball, CT scans were obtained at the end of each freezing cycle. After one week after cryoablation, curettage and fixation with plate and cementation were performed to prevent fracture. Tumor cells were not observed in the histopathological findings of the curettage tissue (Figures 5 and 6). After cryoablation, radiofrequency ablation was performed regularly for lung metastases. Four years after cryoablation, there was no recurrence and the patient could walk without any support.
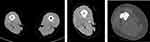
 | Figure 2 Magnetic resonance imaging (MRI) revealed the intramedullary metastasis at femur. |
 | Figure 3 After tract was holed by electric drill, ablation needles were inserted into the proximal and distal side of the tumor. |
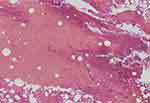 | Figure 5 In the histopathological findings of curettage tissue, tumor cells were not observed. |
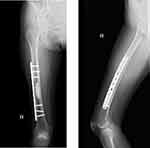 | Figure 6 On X-ray, the femur was reinforced by plate and cement. Left: AP view, right: lateral view. |
Discussion
In the present case, cryoablation proved to be a useful method to treat bone metastasis in a patient with leiomyosarcoma. Moreover, the reinforcement using plate and cementation may be effective to prevent fracture, and the patient was able to maintain activity for up to 4 years.
Generally, resection of a segmental bone defect after intercalary resection of the femur is performed via endoprosthesis,5 distraction osteogenesis,6 allograft,7 and autologous graft.8 However, these are highly invasive procedures and revisional surgery may be considered due to loosening, infection, pseudo-joints, and bone absorption.5 If the primary bone sarcoma is located in the mid-shaft of a long bone, these procedures may be indicated. However, these procedures should be considered with caution in patients with bone metastasis due to the poor associated mortality.
Cryoablation was first described in the 1800s and has since evolved into a mainstay therapy within dermatology and its percutaneous application was subsequently modified for musculoskeletal tumors.14–16 Marcove et al described the first report of 20 primary and metastatic bone tumors in 1968.16 They reported that the relief from pain was complete in a large part of the patients. Cryoablation has an advantage in that it can treat irregular and/or large tumors by using multiple synergistic probes using live CT monitoring; an ice ball is used to confirm the treated lesion and prevent complications. Furthermore, it can be applied repeatedly. Deschamps et al reported that the local control rate after cryoablation of bone metastasis was 67% at 1 year if the size was less than 3 cm in diameter.10 They also suggested that a size of 2 cm was a good prognostic factor for local control. McMenomy et al reported that local control was acquired in 13 (68%) of 19 bone metastases.11 They also reported that 32 of 33 treated soft-tissue metastases were locally controlled. They explained that the differences between these results may be because of the occasional underappreciation of the extent of the bone metastasis or limited visualization of the ice ball within bone on CT at the time of the cryoablation procedure. In addition, the ice ball margin can be difficult to appreciate in sclerotic lesions or those without large, distinct osteolytic or soft tissue components.11 In the present case, we considered that the metastasis may be controlled because the tumor was 2 cm in size and the cortical bone was not destroyed. In addition to local effect, cryoimmunology has shown promising results in several cancers after cryosurgery and is expected to influence the next generation of tumor immunotherapy.17–19 The cells that die via necrosis after freezing release preserved intracellular organelles, antigens and damage associated molecular patterns (DAMPs).20 Dendritic cells (DCs) that phagocytize DAMPs activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappa B) pathway, which then promotes the expression of co-stimulatory CD80/86 molecules.21,22 DCs that present antigens on major histocompatibility complex (MHC) molecules and show co-stimulators stimulate T-cells and promote systemic immune response.23–25
The rate of fracture after cryotherapy has been reported as being between 6.5% and 16.7%.9,26–28 To reduce the possibility of fracture, reconstruction of large defects to support the tumor cavity is recommended because frozen bone shows trabecular necrosis with disruption of the osteoid seams and extensive marrow necrosis.9 Frozen bone re-ossifies slowly and often incompletely. Deschamps et al recommended cementation after cryotherapy to prevent fracture.10 Mirels et al recommended performing internal fixation prophylactically in cases with a high probability of pathological fracture.29 In the present case, we used a plate and cement to prevent fracture after cryotherapy, according to previous reports. Four years after cryoablation, there was no recurrence and function was maintained, although further follow-up should be necessary for the evaluation of local control. In conclusion, cryoablation may be a safe and effective treatment for bone metastasis from soft tissue sarcoma. We suggest that a tumor with limited cancellous bone and of a small size should undergo cryoablation, although a large-scale study should be necessary for further validation.
Disclosure
The authors report no conflict of interest in this work.
References
1. Rubenzik M, Limthongkul B, Humphreys TR. Leiomyosarcoma. Mohs Micrographic Surgery. 2012;23:279–285. doi:10.1007/978-1-4471-2152-7_23
2. Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcoma. Cancer. 2009;115:3774–3781. doi:10.1002/cncr.24420
3. van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: the European organization for research and treatment of cancer-soft tissue and bone sarcoma group study of 255 patients. Cancer. 1996;77:675–682.
4. Smith R, Pak Y, Kraybill W, Kane JM
5. Grzegorz G. Oncological and functional results after surgical treatment of bone metastases at the proximal femur. BMC Surg. 2018;18:5. doi:10.1186/s12893-018-0336-0
6. Watanabe K, Tsuchiya H, Yamamoto N, et al. Over 10-year follow-up of functional outcome in patients with bone tumors reconstructed using distraction osteogenesis. J Orthop Sci. 2013;18:101–109. doi:10.1007/s00776-012-0327-4
7. Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi:10.1097/01.blo.0000150371.77314.52
8. Harrington KD. The use of hemipelvic allografts or autoclaved grafts for reconstruction after wide resections of malignant tumors of the pelvis. J Bone Joint Surg Am. 1992;74(3):331–341. doi:10.2106/00004623-199274030-00003
9. Malawer MM, Dunham W. Cryosurgery and acrylic cementation as surgical adjuncts in the treatment of aggressive (benign) bone tumors. Clin Orthop Relat Res. 1991;262:42–57.
10. Deschamps F, Farouil G, de Baere T. Percutaneous ablation of bone tumors. Diagn Interv Imaging. 2014;95:659–663. doi:10.1016/j.diii.2014.04.004
11. McMenomy BP, Kurup AN, Johnson GB, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Inter Radiol. 2013;24:207–213. doi:10.1016/j.jvir.2012.10.019
12. Susa M, Kikuta K, Nakayama R, et al. CT guided cryoablation for locally recurrent or metastatic bone and soft tissue tumor: initial experience. BMC Cancer. 2016;16:798. doi:10.1186/s12885-016-2852-6
13. Yamanaka T, Yamakado K, Yamada T, et al. CT-guided percutaneous cryoablation in renal cell carcinoma: factors affecting local tumor control. Vasc Interv Radiol. 2015;26(8):1147–1153. doi:10.1016/j.jvir.2015.04.031
14. Prohaska J, Bardi T. Cryotherapy. Tampa, FL: Stat Peals Publishing; 2018.
15. Menendez LR, Tan MS, Kiyabu MT, Chawla SP. Cryosurgical ablation of soft tissue sarcomas: a phase I trial of feasibility and safety. Cancer. 1999;86:50–57.
16. Marcove RC, Miller TR, Cahan WC. The treatment of primary and metastatic bone tumors by repetitive freezing. Bull N Y Acad Med. 1968;44(5):532–544.
17. Sabel MS, Nehs MA, Su G. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005;90:97–104. doi:10.1007/s10549-004-3289-1
18. Murakami H, Demura S, Kato S, et al. Systemic antitumor immune response following reconstruction using frozen autografts for total en bloc spondylectomy. Spine J. 2014;14:1567–1571. doi:10.1016/j.spinee.2013.09.030
19. Ablin RJ, Soanes WA, Gonder MJ. Prospects for immunotherapy in cases of metastasizing carcinoma of the prostate. Cryobiology. 1971;8:271–279.
20. Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi:10.1016/j.cryobiol.2008.10.126
21. Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death release heat shock proteins, which deliver a partial maturation signal to dendritic cells and active the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546.
22. Bottero V, Withoff S, Verma IM. NF-kappa B and the regulation of hematopoiesis. Cell Death Differ. 2006;13:785–797. doi:10.1038/sj.cdd.4401888
23. Mehta A, Okle R, Sheth RA. Thermal ablative therapies and immune checkpoint modulation: can locoregional approaches effect a systemic response? Gastroenterol Res Pract. 2016;2016:9251375. doi:10.1155/2016/9251375
24. Den Brok MH, Sutmuller RP, Nierkens S, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumor immunity. Br J Cancer. 2006;13:785–797.
25. Redondo P, Del Olmo J, Lopez-Diaz de Cerio A, et al. Imiquimod enhances the systemic immunity attained by local cryosurgery destruction of melanoma lesions. J Invest Dermatol. 2007;127:1673–1680. doi:10.1038/sj.jid.5700777
26. van der Geest IC, de Valk MH, de Rooy JW, et al. Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol. 2008;98:421–426. doi:10.1002/jso.21122
27. Mohler DG, Chiu R, Mccall DA, Avedian RS. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res. 2010;468:2765–2773. doi:10.1007/s11999-010-1445-y
28. Pritsch T, Bickels J, Wu CC, Squires HM, Malawer MM. The risk for fractures after curettage and cryosurgery around the knee. Clin Orthop Relat Res. 2007;458:159–167. doi:10.1097/BLO.0b013e318038fc3d
29. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fracture. Clin Orthop. 1989;249:256–264.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.