Back to Journals » OncoTargets and Therapy » Volume 15
Successful Treatment of Primary CNS Extranodal NK/T-Cell Lymphoma with Surgery and Chemotherapy Combined with Sintilimab: A Case Report and Literature Review
Authors Qin L, Li Y, He Y, Zeng R, Pan T, Zuo Y, Xiao L, Zhou H
Received 8 October 2021
Accepted for publication 20 December 2021
Published 6 January 2022 Volume 2022:15 Pages 1—11
DOI https://doi.org/10.2147/OTT.S343400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Liping Qin,1,2,* Yajun Li,2,* Yizi He,2 Ruolan Zeng,2 Tao Pan,2 Yilang Zuo,2 Ling Xiao,3 Hui Zhou2
1Graduate Collaborative Training Base of Hunan Cancer Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421001, People’s Republic of China; 2Department of Lymphoma and Hematology, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 3Department of Histology and Embryology of School of Basic Medical Science, Central South University, Changsha, Hunan, 410013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Zhou
Department of Lymphoma and Hematology, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan, 410013, People’s Republic of China
Tel +86-73189762281
Email [email protected]
Ling Xiao
Department of Histology and Embryology of School of Basic Medical Science, Central South University, Changsha, Hunan, 410013, People’s Republic of China
Tel +86-73182650436
Email [email protected]
Abstract: Primary central nervous system extranodal natural killer/T-cell lymphoma (PCNS ENK/TCL) is an extremely rare lymphoma. Only 23 cases of PCNS ENK/TCL have been reported in the English literature. Due to the rarity of this lymphoma, an effective therapeutic strategy has not been defined. Generally, this type of lymphoma is treated with surgery, intrathecal chemotherapy, and postoperative chemoradiation therapy. The prognosis is poor. Herein, we present a case of primary brain NK/T cell lymphoma in a 50-year-old immunocompetent Chinese female and review the literature. The patient underwent intracranial tumor resection and was subsequently treated with a PD1 monoclonal antibody (Sintilimab) combined with chemotherapy. The patient survived 15 months after diagnosis. This is the first report of PCNS ENK/TCL treated with surgery and chemotherapy combined with immunotherapy and suggests an effective treatment regimen for PCNS ENK/TCL.
Keywords: primary central nervous system, extranodal natural killer/T-cell lymphoma, chemotherapy, immunotherapy, PD1
Introduction
Primary central nervous system lymphomas (PCNSLs) are aggressive non-Hodgkin extranodal lymphoma that originate from the brain parenchyma, meninges, spinal cord, or eyes excluding systemic disease; It represents 4% to 6% of all extranodal lymphomas and only 4% of all intracranial neoplasms.1,2 Among primary CNS lymphomas, more than 90% of PCNSLs are diffuse large B-cell lymphoma in immunocompetent patients,3 and less than 5% are T-cell lymphoma.2 Extranodal Natural Killer/T-cell lymphoma (ENK/TCL) originates from NK cells or cytotoxic T cells and is an aggressive lymphoma. ENK/TCL is usually related to Epstein-Barr virus (EBV) infection and is characterized by a cytotoxic phenotype, vascular damage, and prominent necrosis.4 ENK/TCL is more commonly in Asia and South America. Lesions occur mostly in the nasal cavity and the midline structure of the face. It was previously known as lethal midline granuloma.5 However, it can also occur in the upper respiratory tract, gastrointestinal tract, skin, soft tissue, and testes.4 Conversely, the central nervous system is rarely involved as the initial site for ENK/TCL lesions. Extranodal NK/TCL of the primary central nervous system (PCNS) is extremely rare. To our knowledge, only 23 cases of PCNS ENK/TCL have been reported in the English literature.6–24 To date, the understanding of this tumor is not deep enough. Herein, we present a case of PCNS ENK/TCL in a 50-year-old immunocompetent Chinese female and review of the literature.
Case Presentation
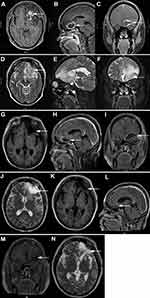
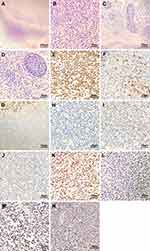
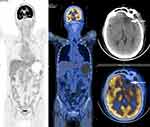
A 50-year-old Chinese female presented with worsening vision loss in the right eye and weakness in both lower limbs for approximately 11 months, She also reported headache, no fever, night sweats, dizziness, and vomiting. She had normal vision in the left eye and had a history of hypertension and ectopic pregnancy. The patient also had a history of cholecystectomy and right breast cyst surgery. There was no other relevant family and personal medical history. Physical examination revealed that the patient was conscious, spoke clearly, with the tongue in the middle position. The bilateral frontal lines and the nasolabial groove were symmetrical. The bilateral pupils were equally large and round, with a diameter of 3 mm. The patient’s left and right visual acuity was 1.0 and 0.1, respectively. The intraocular pressure on both sides was 16 mmHg. There was no evidence of lymphadenopathy or hepatosplenomegaly. A magnetic resonance imaging (MRI) scan was performed that revealed a mass located in the left frontal lobe with a large cross section of 4.3×3.2 cm (Figure 1A–F). The lesion exhibited slightly longer T1 and slightly longer T2 signals, with internal density that was not uniform (Figure 1A–F). Due to the mass effect, the midline structure and the left ventricle were compressed and shifted to the right (Figure 1C). The MRI revealed that the upper margin of the intracranial segment of the left optic nerve was closely associated with the intracranial tumor, while no obvious abnormality was observed in the right optic nerve (Figure 1D). Magnetic resonance spectroscopy (MRS) of the brain showed an increased choline peak and a suppressed N-acetylaspartate peak (Figure 2). The patient underwent intracranial tumor resection. The tumor was yellowish-white in color, hard in texture, without capsule, and had a rich in blood supply, with significant surrounding edema. The histological examination revealed that the brain parenchyma was diffusely infiltrated by atypical lymphocytes and showed extensive necrosis (Figure 3A). The tumor cells were of medium size with irregularly folded nuclei and indistinct nucleoli. The cytoplasm was moderate and mitotic figures were easily identified (Figure 3B). The tumor cells presented an angiocentric (Figure 3C) and angiodestructive growth pattern (Figure 3D). The EnVision method was applied for immunohistochemical staining,25 and revealed that the tumor cells were positive for CD3 (Figure 3E), CD4 (Figure 3F), CD7, and CD8. The NK cell marker CD56 was partially positive (Figure 3G). PD1 was weakly positive (Figure 3M) and PD-L1 was positive (Figure 3N); however, B cell markers, including CD20 (Figure 3H) and CD79a, were negative. In addition, cytotoxic markers such as granzyme B (Figure 3I) and TIA-1 (Figure 3J) were also positive for tumor cells. The Ki-67 proliferation index was positive in about 80% of tumor cells (Figure 3K). Additionally, tumor cells were positive for Vim, LCA, and negative for CD30, CK, S100, Syn, GFAP, olig-2, ALKp80, and EMA. Small RNAs encoded by EBV (EBERs) were detected by in situ hybridization (ISH) in the nucleus of lymphoma cells (Figure 3L). Through histopathological analysis, the diagnosis of ENK/TCL was established. Subsequent laboratory tests revealed that platelets, hemoglobin level, and white blood cell count were all within the reference range. The results of the blood biochemical examination were normal. Syphilis serologies, hepatitis C virus, and hepatitis B virus were all negative. The patient was immunocompetent and HIV negative. The erythrocyte sedimentation rate (ESR) was elevated at 82 mm/hour (normal range 0–20 mm/hour). Quantitative polymerase chain reaction (qPCR) analysis was positive for EBV DNA (5.96×102 copies/mL) in peripheral blood. The lymphocyte subsets showed a decrease in the CD4+/CD8+ ratio and B lymphocytes (CD3-CD19+). Cerebrospinal fluid (CSF) analysis showed an elevated white blood cell count (53×106/L, normal range 0–5×106/L), with pleocytosis (54×106/L, normal range 0–8×106/L) and slightly elevated protein (628.6 mg/L, normal range 150–450 mg/L). The CSF cytologic examination was negative for neoplastic cells, and flow cytometry immunophenotyping analysis was normal. The real-time qPCR analysis of the CSF was negative for EBV DNA. A bone marrow biopsy, bone marrow smear, and bone marrow flow cytometry immunophenotyping analysis was negative for lymphomatous involvement. The whole-body positron emission photography/computed tomography (PET/CT) scan revealed that the left frontal lobe foci showed postoperative changes with sparse fluorodeoxyglucose uptake, but no foci of increased uptake elsewhere in the body (Figure 4). Magnetic resonance imaging (MRI) scan after surgery and before chemotherapy revealed a residual cavity was formed in the left frontal lobe, a small amount of fluid was accumulated in the left frontal subdural, a long shadow of T1 and long T2 signals was observed in the left frontal lobe, the edge was enhanced by enhanced scanning. No abnormal signal areas and abnormal enhancement foci were observed in other brain parenchyma (Figure 1G–J). From the combined radiological and clinicopathological analysis, the patient was diagnosed with PCNS ENK/TCL. The patient underwent six cycles of PD1 monoclonal antibody (Sintilimab) and high-dose methotrexate and pegaspargase and thiotepa chemotherapy regimen (Sintilimab 200 mg, methotrexate 3.5 g/m2, pegaspargase 2000 IU/m2, thiotepa 30 mg/m2) plus an intrathecal methotrexate (15 mg) and cytarabine (50 mg) and dexamethasone (5 mg) in the first two cycles. After treatment, her right eye visual acuity recovered. The subsequent MRI obtained 8 months later revealed the extent of abnormal enhancement foci and the residual cavity in the left frontal lobe was smaller than before (Figure 1K–N). The EBV DNA from peripheral blood returned to normal after treatment. The main adverse events during treatment were hematologic toxicity: grade 1 neutropenia, grade 3 anemia, and grade 4 thrombocytopenia. The efficacy was evaluated as complete response and the patient remained alive 15 months after her diagnosis. For subsequent treatment, we planned to use Sintilimab as maintenance therapy, but the patient refused further treatment.
 |
Figure 2 Magnetic resonance spectroscopy (MRS) of the brain. The lesion in the left frontal lobe showed an increased choline peak and a suppressed N-acetylaspartate peak. |
Discussion
PCNSL is confined to the brain parenchyma, eyes, spinal cord, or meninges, with no evidence of systemic involvement at the time of diagnosis. PCNSL is a specific form of non-Hodgkin lymphoma and represents 4% to 6% of all extranodal lymphomas,26 and only 4% of all intracranial neoplasms.2 Among primary CNS lymphomas, more than 90% are diffuse large B-cell lymphoma (DLBCL).3 PCNS ENK/TCL is distinctly rare, with only 23 cases reported in the English literature so far. Among these cases, aggressive clinical behavior and a rapid progressive clinical course were reported, and patients have a poorer prognosis than extracranial NK/TCL.6–24 PCNSLs are often accompanied by nonspecific clinical symptoms such as dizziness, headache, vomiting, and blurred vision. Our patient presented with loss of visual acuity and weakness of the limbs, which were consistent with the nonspecific symptoms of primary CNS lymphoma. Morphologically, NK/TCL shows obvious necrosis and angiodestructive growth pattern, and there was diffuse lymphocyte infiltration. The morphology of tumor cells varies greatly. The typical immunophenotype of NK/TCL is cytoplasmic CD3+, CD56+, EBER+, and cytotoxic markers (perforin, granzyme B, and TIA-1) are positive.27 Recently, research showed that most NK/T cell lymphoma were from the T cell lineage, and half lacked expression of TCR protein.28 The rearrangement of the TCR gene is an important diagnostic molecular marker for this lymphoma.
We report a case of PCNS ENK/TCL in a 50-year-old immunocompetent Chinese female. To our knowledge, this is the first case of this tumor treated with an immune checkpoint inhibitor. Furthermore, we checked the protein expression of programmed cell death 1 (PD1) and programmed cell death ligand 1 (PD-L1) by immunohistochemistry (Figure 3M and N). The description of this case will contribute to a better understanding of this rare type of lymphoma and accumulate more clinical and pathological data.
The previously reported cases PCNS ENK/TCL, including demographics, pathological features, and outcome, are presented in Table 1. Twenty-three cases were collected from the English literature and summarized.6–24 These patients were pathologically diagnosed as primary CNS ENK/TCL, excluding secondary and invasive NK cell leukemia. According to previously reported cases, overall survival in patients with PCNS ENK/TCL is not high. The highest survival reported was 139 months to date, and the median overall survival was 6 months.6–24 There were nineteen immunocompetent (IC) cases and two immunosuppressive (IS) cases of which one was infected with HIV and the another was a posttransplant patient. Most of the patients were from Asia, Africa, and Spain, and the age of these patients was between 13 and 77 years (median, 53 years). There were 13 males and 10 females, and the male-to-female ratio was 1.3:1. In terms of IC cases, age ranged between 13 and 77 years (median, 54 years), with 10 males and 9 females. The lesions of all reported cases were restricted to the CNS; however, the tumor was broadly distributed throughout the CNS, including the brain parenchyma, orbital, meninges, spinal cord and pituitary gland, cauda equina, indicating that there was no predilection site. The locations of previously reported cases of PCNS ENK/TCL are summarized in Table 1. Most cases were diagnosed by surgical resection, including the case reported herein. Among all reported cases, treatment information was available for eighteen patients. All these patients received different treatments: seven underwent surgical excision, twelve underwent MTX chemotherapy, and twelve received radiotherapy. Although most cases received combination treatment, some patients received radiotherapy or chemotherapy alone, while none of the patients received surgical intervention alone. However, the case we reported was treated with surgical excision, chemotherapy, and immunotherapy. For all patients, the prognosis was poor as shown in Table 1, with the overall survival of the IC cases being 1–29 months (median, 7 months). Histologically, they showed vascular damage and necrosis accompanied by vascular-centered infiltration. Expression of various markers included CD3 (20/21); CD56 (15/20); CD5 (1/8); CD45 (6/6); cytotoxic molecules (TIA-1/granzyme B/perforin) (12/12), and EBER (18/18). T cell clonality was detected in 42% (5/12) of the cases examined. Some of the included cases revealed that the majority of NK/T-cell lymphoma were of the T-cell lineage, and half lacked TCR protein expression.28 In our case, immunohistochemical staining showed the tumor cells were positive for CD3, CD4, TIA-1, granzyme B, CD56 and were negative for CD20. In addition, PD1 was weakly positive and PD-L1 was positive. EBV-encoded small RNAs (EBERs) were detected in the nucleus of lymphoma cells. However, T-cell clonality was not evaluated in our case.
 |
Table 1 Demographics, Pathological Features, and Outcome of Primary Central Nervous System Extranodal NK/T-Cell Lymphoma Cases Reported |
Due to the rarity of this lymphoma, an effective therapeutic strategy has not been established. Generally, this lymphoma is treated by surgery, intrathecal chemotherapy, and postoperative chemoradiation therapy. Currently, the standardized therapeutic strategy for CNS lymphoma includes radiation therapy and high-dose MTX.29 The use of whole brain radiation therapy (WBRT) in PCNSL treatment is controversial, especially in patients over 60 years of age, due to its limited efficacy and late-onset neurotoxicity.1 In our case, the patient underwent surgical excision and was received six cycles of PD1 monoclonal antibody (Sintilimab), high-dose methotrexate, pegaspargase, thiotepa chemotherapy regimen plus intrathecal chemotherapy. The patient tolerated the treatment well and continued to show radiographical and clinical improvements, although her outcome is not known. Considering the patient’s good response to chemotherapy and the delayed neurotoxicity of WBRT, WBRT was not considered. Due to the short survival period of standard treatment and the highly aggressive course of this tumor, some clinicians recommend bone marrow or peripheral stem cell transplantation to consolidate.29 Nonetheless, there are no reliable data for NK/T-cell lymphoma, and this treatment strategy is usually used for the treatment of B-cell lymphoma. Due to the difficulty in performing clinical trials for PCNS ENK/T-cell lymphoma, there is still a lack of standardized treatments. Some studies have reported the overexpression of the PD-L1 protein in ENK/TCL by immunohistochemistry,30–32 and refractory NK/T-cell lymphoma with PD-L1 overexpression was sensitive to anti-PD1 antibody treatment.33 One possible mechanism for NK/T-cell lymphoma to escape effector T cell immune targeting is to suppress antitumor immunity by PD1 ligation with PD-L1. Blocking the PD1/PD-L1 signaling pathway with anti-PD1 antibody can restore effector T cells targeting NK/T-cell lymphoma cells, which is a potential therapeutic strategy. A study carried out in Asian countries that included seven patients who were refractory or relapsed after the SMILE chemotherapy regimen underwent a median of seven cycles of a single agent, pembrolizumab (an anti-PD-L1 antibody), and reported that two patients achieved a partial response (PR), and five patients achieved a complete response (CR). The objective response rate (ORR) was 100%.33 Two independent studies demonstrated that three patients with refractory or relapsed ENK/TCL achieved a CR after therapy with pembrolizumab.34,35 Another study reported that three cases with refractory or relapsed ENK/TCL achieved a clinical response with low-dose nivolumab (an anti-PD-1 antibody).36 A pilot study showed that nivolumab achieved objective clinical and radiographic responses in four patients with relapsed/refractory PCNSL and one secondary CNS lymphoma, including four CR and one PR.37 These results indicated that anti-PD1/PD-L1 monoclonal antibody may be an effective treatment for PCNS ENK/TCL. In our case, we verified the expression of PD1 and PD-L1 and revealed that PD1 was weakly positive (Figure 3M) and PD-L1 was positive (Figure 3N). Therefore, for this patient, we chose a chemotherapy regimen containing the PD1 monoclonal antibody (Sintilimab). Our patient’s good outcome may also be attributed to the reduction of the tumor mass by resection before high-dose MTX, which may have enhanced the antitumor efficacy of MTX, while the surgical disruption of the blood-brain barrier may have resulting in increasing doses of drug entering the brain. However, our case report still has certain limitations due to the lack of molecular profiling, which may be important to determine responses to PD-1 immunotherapy in NK/TCL.38
Conclusion
We report a new case of PCNS ENK/TCL in a 50-year-old immunocompetent Chinese female, and summarized the clinicopathological features of previously reported cases. Although most of primary CNS lymphoma are B-cell lymphoma, NK/T-cell lymphoma also do occur. Therefore, NK/T-cell lymphoma should be included in the differential diagnosis of primary CNS lymphoma, and excluding direct invasion of extracranial NK/T-cell lymphoma. Surgery and chemotherapy combined with immunotherapy may be an effective strategy for the treatment of PCNS ENK/TCL. Further studies are needed to verify the efficacy and safety of this treatment strategy.
Ethics and Consent Statement
Written informed consent has been provided by the patient to have the case details and any accompanied images published. An institutional approval was not required for a case report.
Funding
This work was supported by Changsha Municipal Natural Science Foundation (No. kq2014206); the Natural Science Foundation of Hunan Province National Health Commission (No. 20201659); the grants from the Hunan Provincial Science and Technology Department (No. 2017SK2130); the National Cancer Center (grant number NCC2017A20); the “Scientific Research Climbing Plan” of Hunan Cancer Hospital (grant number ZX2020003). the Hunan Provincial Natural Science Foundation of China (No. 2021JJ30425); the Natural Science Foundation of Hunan Province National Health Commission (No. 202203045455).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418. doi:10.1200/jco.2017.72.7602
2. Biccler JL, Savage KJ, Brown PDN, et al. Risk of death, relapse or progression, and loss of life expectancy at different progression-free survival milestones in primary central nervous system lymphoma. Leuk Lymphoma. 2019;60(10):2516–2523. doi:10.1080/10428194.2019.1594219
3. Le M, Garcilazo Y, Ibanez-Julia MJ, et al. Pretreatment hemoglobin as an independent prognostic factor in primary central nervous system lymphomas. Oncologist. 2019;24(9):e898–e904. doi:10.1634/theoncologist.2018-0629
4. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
5. Harabuchi Y, Takahara M, Kishibe K, Nagato T, Kumai T. Extranodal natural killer/T-cell lymphoma, nasal type: basic science and clinical progress. Front Pediatr. 2019;7:141. doi:10.3389/fped.2019.00141
6. Morita M, Osawa M, Naruse H, Nakamura H. Primary NK/T-cell lymphoma of the cauda equina: a case report and literature review. Spine. 2009;34(24):E882–E885. doi:10.1097/BRS.0b013e3181b29de6
7. Cai B, Hu JJ, Tang QX, Lin W, Wang N. Primary meningeal NK/T cell lymphoma masquerading as tuberculous meningitis. Neurol Sci. 2014;35(9):1467–1469. doi:10.1007/s10072-014-1783-8
8. Imai A, Takase H, Imadome KI, et al. Development of extranodal NK/T-cell lymphoma nasal type in cerebrum following Epstein-Barr virus-positive uveitis. Intern Med. 2017;56(11):1409–1414. doi:10.2169/internalmedicine.56.7573
9. Cobo F, Talavera P, Busquier H, Concha A. CNK/T-cell brain lymphoma associated with Epstein-Barr virus in a patient with AIDS. Neuropathology. 2007;27(4):396–402. doi:10.1111/j.1440-1789.2007.00784.x
10. Kaluza V, Rao DS, Said JW, de Vos S. Primary extranodal nasal-type natural killer/T-cell lymphoma of the brain: a case report. Hum Pathol. 2006;37(6):769–772. doi:10.1016/j.humpath.2006.01.032
11. Liao B, Kamiya-Matsuoka C, Gong Y, Chen M, Wolf BA, Fowler NH. Primary natural killer/T-cell lymphoma presenting as leptomeningeal disease. J Neurol Sci. 2014;343(1–2):46–50. doi:10.1016/j.jns.2014.05.015
12. Li LF, Taw BB, Pu JK, Hwang GY, Lui WM, Leung GK. Primary central nervous system natural killer cell lymphoma in a Chinese woman with atypical (11)C-choline positron emission tomography and magnetic resonance spectrometry findings. World Neurosurg. 2015;84(4):1176 e1175–1179. doi:10.1016/j.wneu.2015.06.063
13. Yan J, Liu W, Wang X, et al. Primary central nervous system extranodal natural killer/T-cell lymphoma, nasal type colliding with meningioma. World Neurosurg. 2018;120:17–26. doi:10.1016/j.wneu.2018.08.065
14. Liu JK, Sayama C, Chin SS, Couldwell WT. Extranodal NK/T-cell lymphoma presenting as a pituitary mass. Case report and review of the literature. J Neurosurg. 2007;107(3):660–665. doi:10.3171/JNS-07/09/0660
15. Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004;17(9):1097–1107. doi:10.1038/modpathol.3800157
16. Ogura R, Aoki H, Natsumeda M, et al. Epstein-Barr virus-associated primary central nervous system cytotoxic T-cell lymphoma. Neuropathology. 2013;33(4):436–441. doi:10.1111/neup.12005
17. Li D, Fu F, Lian L. Primary central nervous system extranodal nasal-type natural killer/T-cell lymphoma with CD20 expression. Neuropathology. 2018;38(2):198–204. doi:10.1111/neup.12438
18. Guan H, Huang Y, Wen W, Xu M, Zan Q, Zhang Z. Primary central nervous system extranodal NK/T-cell lymphoma, nasal type: case report and review of the literature. J Neurooncol. 2011;103(2):387–391. doi:10.1007/s11060-010-0384-5
19. Prajapati HJ, Vincentelli C, Hwang SN, Voloschin A, Crocker I, Dehkharghani S. Primary CNS natural killer/T-cell lymphoma of the nasal type presenting in a woman: case report and review of the literature. J Clin Oncol. 2014;32(8):e26–e29. doi:10.1200/JCO.2012.47.6796
20. Okada A, Harada Y, Inoue T, Okikawa Y, Ichinohe T, Kiuchi Y. A case of primary extranodal natural killer/T-cell lymphoma in the orbit and intraocular tissues with cerebrospinal fluid involvement. Am J Ophthalmol Case Rep. 2018;11:37–40. doi:10.1016/j.ajoc.2018.05.002
21. Jiang X, Yin W, Song J, Chen X, Zhao C, Wen F. Primary central nervous system extranodal NK/T cell lymphoma, nasal type, with antecedent hemophagocytic syndrome in a child. Pediatr Dev Pathol. 2014;17(6):482–486. doi:10.2350/14-02-1441-CR.1
22. Shimatani Y, Nakano Y, Tsuyama N, et al. Extranodal NK/T-cell lymphoma, nasal type, manifesting as rapidly progressive dementia without any mass or enhancing brain lesion. Neuropathology. 2016;36(5):456–463. doi:10.1111/neup.12285
23. Miyata-Takata T, Takata K, Kato S, et al. Clinicopathological analysis of primary central nervous system NK/T cell lymphoma: rare and localized aggressive tumour among extranasal NK/T cell tumours. Histopathology. 2017;71(2):287–295. doi:10.1111/his.13223
24. Li X, Yu H, Fu X, et al. Clinical analysis of patients with primary and secondary extranodal natural killer/T-cell lymphoma of central nervous system. Hematol Oncol. 2021. doi:10.1002/hon.2894
25. Sabattini E, Bisgaard K, Ascani S, et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51(7):506–511. doi:10.1136/jcp.51.7.506
26. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. doi:10.1038/bjc.2011.357
27. Allen PB, Lechowicz MJ. Management of NK/T-cell lymphoma, nasal type. J Oncol Pract. 2019;15(10):513–520. doi:10.1200/jop.18.00719
28. Hong M, Lee T, Young Kang S, Kim SJ, Kim W, Ko YH. Nasal-type NK/T-cell lymphomas are more frequently T rather than NK lineage based on T-cell receptor gene, RNA, and protein studies: lineage does not predict clinical behavior. Mod Pathol. 2016;29(5):430–443. doi:10.1038/modpathol.2016.47
29. Yang H, Xun Y, Yang A, Liu F, You H. Advances and challenges in the treatment of primary central nervous system lymphoma. J Cell Physiol. 2020;235(12):9143–9165. doi:10.1002/jcp.29790
30. Jo JC, Kim M, Choi Y, et al. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/ T-cell lymphoma, nasal type. Ann Hematol. 2017;96(1):25–31. doi:10.1007/s00277-016-2818-4
31. Kim WY, Jung HY, Nam SJ, et al. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016;469(5):581–590. doi:10.1007/s00428-016-2011-0
32. Panjwani PK, Charu V, DeLisser M, Molina-Kirsch H, Natkunam Y, Zhao S. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum Pathol. 2018;71:91–99. doi:10.1016/j.humpath.2017.10.029
33. Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi:10.1182/blood-2016-12-756841
34. Lai J, Xu P, Jiang X, Zhou S, Liu A. Successful treatment with anti-programmed-death-1 antibody in a relapsed natural killer/T-cell lymphoma patient with multi-line resistance: a case report. BMC Cancer. 2017;17(1):507. doi:10.1186/s12885-017-3501-4
35. Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11(1):15. doi:10.1186/s13045-018-0559-7
36. Chan TSY, Li J, Loong F, Khong P-L, Tse E, Kwong Y-L. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2018;97(1):193–196. doi:10.1007/s00277-017-3127-2
37. Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. doi:10.1182/blood-2017-01-764209
38. Lim JQ, Huang D, Tang T, et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia. 2020;34(12):3413–3419. doi:10.1038/s41375-020-1000-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.