Back to Journals » Clinical Ophthalmology » Volume 8
Successful treatment of neovascular age-related macular degeneration following single bevacizumab failure using aflibercept in a vitrectomized eye
Authors Hahn P
Received 26 August 2014
Accepted for publication 17 September 2014
Published 17 October 2014 Volume 2014:8 Pages 2129—2131
DOI https://doi.org/10.2147/OPTH.S73265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Paul Hahn
Duke Eye Center, Duke University Medical Center, Durham, NC, USA
Abstract: Intravitreal anti-vascular endothelial growth factor (VEGF) pharmacotherapy in vitrectomized eyes remains a challenge due to the reduced half-life of these agents. Aflibercept may have stronger binding activity and a longer intravitreal half-life compared to bevacizumab and ranibizumab, but its use in postvitrectomy eyes has not been reported. We present a case of an 89-year-old female, with recurrent choroidal neovascularization 10 years following prior macular translocation vitrectomy surgery for neovascular age-related macular degeneration, successfully treated with monthly aflibercept injections initiated following poor response to a single initial bevacizumab injection. This report suggests that aflibercept may be an important treatment option for vitrectomized eyes requiring anti-VEGF treatment.
Keywords: anti-VEGF, vitrectomized, aflibercept, neovascular age-related macular degeneration
Introduction
The treatment of neovascular age-related macular degeneration (NVARMD) and other retinal diseases has been transformed by the development of intravitreal anti-vascular endothelial growth factor (VEGF) pharmacotherapy. These agents effectively stabilize and can even reverse the natural course of vision loss. Reduced efficacy of these agents has been observed in vitrectomized eyes, in which more rapid diffusion and clearance of intravitreal therapies compared to nonvitrectomized eyes poses a challenge in treatment of retinal diseases in eyes having undergone vitrectomy.1
Aflibercept, the newest of the anti-VEGF agents, may have stronger binding activity with a longer half-life compared to bevacizumab and ranibizumab.2 Given these potential pharmacokinetic advantages, aflibercept may be better suited for treatment of retinal diseases in vitrectomized eyes.
Case description
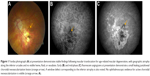
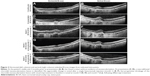
An 89-year-old Caucasian female presented with acute-onset metamorphopsia and vision loss in the left eye. She had a history of NVARMD, with a longstanding, stable disciform scar and finger-counting vision in the right eye and a history of macular translocation surgery (with concurrent cataract extraction and subsequent strabismus surgery and silicone oil removal) 10 years prior in the left eye. Over the ensuing 10 years following macular translocation, she maintained regular follow-up examinations, with stable left-eye vision at the 20/32–20/50 level without active choroidal neovascularization (CNV) or need for subsequent treatment. Three months following an examination recording 20/40 vision and stable findings (10 years following macular translocation), she reported awakening with “blurry, dark, and wavy” vision loss in the left eye. On exam, her visual acuity measured 20/100. Her anterior-segment examination including intraocular pressure was unremarkable. Her fundus examination demonstrated geographic atrophy along the inferior arcade, consistent with prior macular translocation surgery, without any ophthalmoscopically visible heme, fluid, or exudates (Figure 1). Fluorescein angiography demonstrated leakage consistent with CNV (Figure 1), and optical coherence tomography (OCT) imaging demonstrated a subretinal CNV lesion without associated fluid (Figure 2). This lesion was not present on OCT imaging at her recent visit 3 months prior.
Given these findings, prompt anti-VEGF treatment to the left eye was recommended, and the patient was treated that day with intravitreal injection of bevacizumab 1.25 mg. She returned 4 weeks later as scheduled reporting worsening of her visual acuity, which had deteriorated to 20/160. Her ophthalmoscopic and imaging findings remained essentially unchanged. Given the absence of appropriate response in a functionally monocular patient, the decision was made to switch treatment to aflibercept 2.0 mg. One month after a single aflibercept injection, her visual acuity dramatically improved to 20/80. An additional aflibercept injection was administered, and her visual acuity further improved 1 month later to 20/50. Ongoing monthly aflibercept injections were administered, with improvement of visual acuity to 20/32 after four consecutive aflibercept injections. OCT imaging was repeated at 3-month intervals after aflibercept initiation, resulting in fibrosis and moderate shrinkage of her CNV.
Discussion
While intravitreal pharmacotherapy has revolutionized the clinical treatment of retinal diseases, management of eyes following prior vitrectomy has been challenging due to the altered pharmacokinetics of intravitreal agents, which have a shorter half-life and reduced efficacy in the vitrectomized eye. Investigations with a sustained-release dexamethasone intravitreal implant (Ozurdex; Allergan, Irvine, CA, USA) have demonstrated successful treatment of macular edema associated with central retinal vein occlusion, uveitis, and diabetic retinopathy in vitrectomized eyes,3–5 but novel anti-VEGF treatment options beyond higher or more frequent dosing have not been described. Aflibercept, the newest of the anti-VEGF agents, has stronger binding activity and extended half-life compared to bevacizumab and ranibizumab, and successful treatment of diseases recalcitrant to prior bevacizumab or ranibizumab injections has been reported.6 Similarly, this pharmacokinetic profile may be favorable for the treatment of vitrectomized eyes despite the challenges initially identified with bevacizumab and ranibizumab. To the author’s knowledge, there are no reports of intravitreal treatment in vitrectomized eyes using aflibercept described in the literature.
In the case reported herein, a monocular patient with acute loss of vision and new-onset CNV secondary to NVARMD in a vitrectomized eye was poorly responsive with worsening visual acuity after a single injection with bevacizumab, but demonstrated dramatic improvement in visual acuity after a single aflibercept injection, with continued improvement in retinal architecture and return to baseline visual acuity following serial aflibercept injections. It is certainly possible that the favorable response seen in this patient would have occurred with ongoing bevacizumab injections. However, given the stronger binding activity and extended half-life with a similar safety profile, the author advocates consideration of aflibercept for treatment of postvitrectomy eyes. This case represents proof of concept that the theoretical pharmacokinetic advantages of aflibercept may allow for successful treatment of vitrectomized eyes, and further studies are warranted to test this hypothesis.
Disclosure
The author reports no conflicts of interest in this work.
References
Yanyali A, Aytug B, Horozoglu F, Nohutcu AF. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol. 2007;144(1):124–126. | ||
Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina. 2012;32(3):434–457. | ||
Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–923. | ||
Reibaldi M, Russo A, Zagari M, et al. Resolution of persistent cystoid macular edema due to central retinal vein occlusion in a vitrectomized eye following intravitreal implant of dexamethasone 0.7 mg. Case Rep Ophthalmol. 2012;3(1):30–34. | ||
Adán A, Pelegrín L, Rey A, et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina. 2013;33(7):1435–1440. | ||
Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond). 2014;28(7):895–899. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.