Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Successful Treatment of a Patient with Pyoderma Gangrenosum, Plaque Psoriasis and Palmoplantar Pustulosis with Adalimumab
Authors Chu Y, Liu T, Bai J, Fang H, Qiao J
Received 6 May 2022
Accepted for publication 26 July 2022
Published 20 September 2022 Volume 2022:15 Pages 1991—1995
DOI https://doi.org/10.2147/CCID.S373115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Yuqi Chu, Taoming Liu, Juan Bai, Hong Fang, Jianjun Qiao
Department of Dermatology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Jianjun Qiao, Department of Dermatology, The First Affiliated Hospital, Zhejiang University School of Medicine, No. 79 Qingchun Road, Hangzhou, 310003, People’s Republic of China, Tel +86 13735542393, Email [email protected]
Abstract: Pyoderma gangrenosum (PG) is a rare autoinflammatory skin disorder, which is characterised by rapidly developing and tender cutaneous ulcers. The treatment of PG is challenging. Palmoplantar pustulosis (PPP) is also an autoinflammatory dermatosis with sterile pustules on the palms and/or the soles. We demonstrated a 68-year-old patient with coexisting autoinflammatory diseases including PG, 1-year history of plaque psoriasis and PPP, recovered after treatment with adalimumab. We also reviewed published reports of PG-associated autoinflammatory syndromes with adalimumab.
Keywords: adalimumab, autoinflammatory syndrome, palmoplantar pustulosis, plaque psoriasis, pyoderma gangrenosum
Introduction
Pyoderma gangrenosum (PG) represents a neutrophilic dermatosis characterized by painful, sharply marginated with violaceous ulcers commonly on the lower extremities, especially the pretibial area.1 PG is a rapidly evolving and debilitating disease with severe physical and psychosocial burden.2 The pathophysiology of PG is still not clear.3 PG may be a cutaneous consequence of autoinflammation.4 PG is often associated with immune-mediated diseases that involve inflammatory bowel disease and rheumatoid arthritis.2
Palmoplantar pustulosis (PPP), a chronic and relapsing disease, is characterized by sterile and erupting pustules on the palms and/or the soles.5
Plaque psoriasis is a chronic, immune-mediated skin disease characterized by erythematous scaly patches or plaques that occur commonly on extensor surfaces.9
Severe PG is defined as a patient having multiple ulcers, or a single ulcer of 3 cm or greater, or involvement of the face.2 Patients with mild disease are initially treated with a low-dose immunosuppressive agent (such as prednisone and cyclosporine) and/or localized (topical or intralesional) therapy.2 Adverse effects may occur in patients treated with prednisone or cyclosporine, including infection, hyperglycaemia, renal dysfunction, gastrointestinal disturbance and hypertension.6
It has been suggested that imbalance of the congenital immunity including some common cytokines such as Tumor necrosis factor (TNF)-α, interleukin (IL)-17 and IL-23 may play a crucial role in the progress of both PG7 and PPP8 in recent research. Adalimumab, an anti-TNF-α antibody, was approved for the therapy of psoriasis.9 It has been showed to be effective and generally well tolerated for Japanese patients with active ulcers of PG.10
Herein, we report our successful experience of a patient of PG, plaque psoriasis and PPP with adalimumab.
Case Report
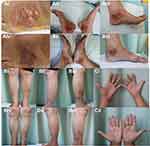
A 68-year-old female attended to our outpatient clinic with a 2-month history of persistent, rapidly developing and aching ulcers affecting her feet (Figure 1Ai–Aiii) and 1-year history of plaque psoriatic lesions symmetrically distributed on the lower extremities (Figure 1Bi–Biii). Pustules were found on her palms (Figure 1Ci). She denied history of lesions on her face and joint symptoms.
Laboratory investigations revealed elevated levels of C-reactive protein (14.1 mg/L; normal, <8 mg/L) and erythrocyte sedimentation rate (74 mm/H; normal, <20 mm/H). The outcome of syphilis antibody and T cell spot test for tuberculosis infection was negative. We have scraped some flakes for fungal culture to rule out fungal infections. Other laboratory investigations, including complete blood count, liver functions and renal functions were unremarkable. Histopathology of the ulcer near the right medial malleolus showed dense neutrophils which confined to the dermis (Figure 2), with no indication of cutaneous vasculitis, mycobacterium tuberculosis, parasitic infections or fungal infections. The clinical and histopathological findings above were consistent with PG. Based on the erythematous scaly plaques distributed on the lower extremities, diagnosis of plaque psoriasis was made. The patient also had pustules on her palms leading to the clinical diagnosis of PPP.
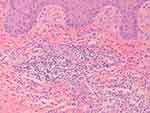 |
Figure 2 Histopathology of the ulcer near the right medial malleolus. (40×) H & E stain showing dense neutrophilic infiltrate in the dermis. |
She was treated with adalimumab with 80 mg subcutaneously at week 0 and then 40 mg every 2 weeks. In the third month, the lesions of PG (Figure 1Aiv–Avi), psoriasis (Figure 1Biv–Bvi) and PPP (Figure 1Cii) improved markedly. The Psoriasis Area Severity Index score improved from 2.4 to 0.2. The body surface area of psoriasis reduced from 1.5% to 0.5%. She did not report any adverse effects during the period of therapy.
Discussion
The coexistance of PG and PPP is rarely reported in literature. Only six cases in Japanese population8 and one case from Nepal11 have been reported till date. To our knowledge, our case firstly described the coexistence of plaque psoriasis, PPP and PG.
PPP is a kind of inflammatory disease that affects the palms and/or the soles with sterile pustules, which are debilitating and usually resistant to treatment.5 PPP is a limited form of pustular psoriasis and presents both in isolation and in patients with a history of plaque psoriasis .12
Case series and case reports may have revealed the efficacy and safety of adalimumab for the treatment of pyoderma gangrenosum-associated autoinflammatory syndromes (Supplementary Table 1). Adalimumab was successful for treating 3 patients with PAPA (pyogenic arthritis, pyoderma gangrenosum and acne), 3 patients with PASS (pyoderma gangrenosum, acne, suppurative hidradenitis and ankylosing spondylitis), 3 patients with PsAPASH (psoriatic arthritis, pyoderma gangrenosum, acne, suppurative hidradenitis), 1 patient with PAPASH (pyoderma gangrenosum, acne, psoriasis, arthritis and suppurative hidradenitis) and 6 patients with PASH (pyoderma gangrenosum, acne, suppurative hidradenitis). On a pathogenetic basis, these conditions share the common pathway, which involves the imbalance of the innate immunity that results in increased levels of IL-1 family, induction of TNF-α and subsequent sterile neutrophilic infiltration in the skin.2 The involvement of TNF-α could justify the treatment of blocking TNF-α in these patients.
It has been reported that PG responds well to biologics against IL-1β,13 IL-12,14 IL-17,15 IL-23,16 IL-1 receptor,17 IL-6 receptor18 and most commonly TNF.19 The T helper (Th)17/TNF-α axis leading to neutrophil infiltration has been shown in the pathogenetic basis of psoriasis,20 PPP8 and PG.2 It is shown in recent studies that TNF‐α, IL‐17 and IL‐23 are increased in lesional skin of PG and PPP. IL-17-positive lymphocytes were observed in biopsy samples obtained from a leg ulcer and plantar pustule.8 These findings indicate that the Th 17/TNF-α axis may perform key roles in both PG and PPP.
Physicians need to consider the presence of coexisting diseases when they are selecting the treatment. Methotrexate, cyclosporine and biologics are potential therapeutic modalities for this patient. However, the possibility of therapeutic failures and side-effects of methotrexate and cyclosporine make the clinicians to shift them to alternative long-term therapies. Cyclosporine can cause significant side effects, such as hypertension and nephrotoxicity, while methotrexate can lead to hepatic and hematological toxicities, limiting their long-term use.5 Therefore, we tried treating this patient with adalimumab and the outcome was satisfactory.
In summary, we reported a case of PG, plaque psoriasis and PPP successfully treated with adalimumab. Further clinical observations will be necessary to assess the prevalance of this clinical entity and the efficacy of adalimumb.
Consent for Publication
Informed consent for publication of the case details and connected images was gained from the patient. Institutional approval from the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine has been obtained to publish the case details (Approved number: IIT-2022-004).
Funding
National Natural Science Foundation of China (82173400 to JQ, 81972931 to HF), the Medical and Health Science and Technology Project of Health Commission of Zhejiang Province (2020KY558 to JQ).
Disclosure
The authors report no conflicts of interest.
References
1. Ashchyan HJ, Nelson CA, Stephen S, et al. Neutrophilic dermatoses: pyoderma gangrenosum and other bowel- and arthritis-associated neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:1009–1022. doi:10.1016/j.jaad.2017.11.063
2. Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6(81). doi:10.1038/s41572-020-0213-x
3. Saternus R, Schwingel J, Muller CSL, Vogt T, Reichrath J. Ancient friends, revisited: systematic review and case report of pyoderma gangrenosum-associated autoinflammatory syndromes. J Transl Autoimmun. 2020;3:100071. doi:10.1016/j.jtauto.2020.100071
4. Satoh TK, Mellett M, Contassot E, French LE. Are neutrophilic dermatoses autoinflammatory disorders? Br J Dermatol. 2018;178:603–613. doi:10.1111/bjd.15105
5. Freitas E, Rodrigues MA, Torres T. Diagnosis, screening and treatment of Patients with Palmoplantar Pustulosis (PPP): a review of current practices and recommendations. Clin Cosmet Investig Dermatol. 2020;13:561–578. doi:10.2147/CCID.S240607
6. Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958–h2958. doi:10.1136/bmj.h2958
7. Fischer-Stabauer M, Boehner A, Eyerich S, et al. Differential in situ expression of IL-17 in skin diseases. Eur J Dermatol. 2012;22:781–784. doi:10.1684/ejd.2012.1854
8. Ohtsuka M, Yamamoto T. Rare association of pyoderma gangrenosum and palmoplantar pustulosis: a case report and review of the previous works. J Dermatol. 2014;41:732–735. doi:10.1111/1346-8138.12543
9. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–1960. doi:10.1001/jama.2020.4006
10. Yamasaki K, Yamanaka K, Zhao Y, et al. Adalimumab in Japanese patients with active ulcers of pyoderma gangrenosum: twenty-six-week Phase 3 open-label study. J Dermatol. 2020;47:1383–1390. doi:10.1111/1346-8138.15533
11. Pukar C, Sudha A, Punam P. Simultaneous occurrence of pyoderma gangrenosum and palmoplantar pustular psoriasis: is it an association or coincidental findings? Clin Case Rep. 2021;9(1):410–415. doi:10.1002/ccr3.3544
12. Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with palmoplantar pustulosis in the United States. JAMA Dermatol. 2022;158:68–72. doi:10.1001/jamadermatol.2021.4635
13. Kolios AG, Maul J-T, Meier B, et al. Canakinumab in adults with steroid-refractory pyoderma gangrenosum. Br J Dermatol. 2015;173:1216–1223. doi:10.1111/bjd.14037
14. Goldminz AM, Botto NC, Gottlieb AB. Severely recalcitrant pyoderma gangrenosum successfully treated with ustekinumab. J Am Acad Dermatol. 2012;67:e237–e238. doi:10.1016/j.jaad.2012.04.045
15. McPhie ML, Kirchhof MG. Pyoderma gangrenosum treated with secukinumab: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20940430. doi:10.1177/2050313X20940430
16. John JM, Sinclair RD. Tildrakizumab for treatment of refractory pyoderma gangrenosum of the penis and polymyalgia rheumatica: killing two birds with one stone. Australas J Dermatol. 2020;61:170–171. doi:10.1111/ajd.13196
17. Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol. 2009;161:1199–1201. doi:10.1111/j.1365-2133.2009.09404.x
18. Solovan C, Nicula Proca A, Ursoniu S. Pros and cons of biological therapy in psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):e74–e75. doi:10.1111/jdv.13735
19. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525–531. doi:10.2340/00015555-2008
20. Martin DA, Towne JE, Kricorian G, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133:17–26. doi:10.1038/jid.2012.194
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.