Back to Journals » OncoTargets and Therapy » Volume 15
Successful Targeting of CTLA-4 in a Melanoma Clinical Case: A Long-Term “One Stop Therapeutic Shop”
Authors Colucci M , D'Alonzo V , Santangelo F, Miracco C, Valente M, Maio M, Di Giacomo AM
Received 26 July 2022
Accepted for publication 12 November 2022
Published 25 November 2022 Volume 2022:15 Pages 1409—1415
DOI https://doi.org/10.2147/OTT.S367389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Maura Colucci,1 Vincenzo D’Alonzo,1 Federica Santangelo,1 Clelia Miracco,2 Monica Valente,3 Michele Maio,1,3,4 Anna Maria Di Giacomo1,3,4
1University of Siena, Siena, Italy; 2Department of Pathology, University of Siena, Siena, Italy; 3Center for Immuno-Oncology, Medical Oncology and Immunotherapy, Department of Oncology, University Hospital, Siena, Italy; 4NIBIT Foundation Onlus, Genoa, Italy
Correspondence: Anna Maria Di Giacomo, Center for Immuno-Oncology, Medical Oncology and Immunotherapy, Department of Oncology, University Hospital of Siena, Viale Bracci, 14, Siena, 53100, Italy, Email [email protected]
Abstract: The anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) monoclonal antibody ipilimumab was the first in-class immune-checkpoint inhibitor (ICI) approved for the treatment of melanoma patients. Initially approved for metastatic cutaneous melanoma, treatment with ipilimumab subsequently demonstrated to significantly improve recurrence free survival (RFS) in fully resected, high-risk, stage III melanoma patients. Therapeutic use of ipilimumab has also allowed the initial identification and characterization of unconventional clinical and radiological patterns of response (ie, tumor flare, pseudo-progression) that may occur during ICI therapy, unlike chemotherapy or targeted therapy. As a result, the standard Response Evaluation Criteria In Solid Tumors (RECIST) and the World Health Organization (WHO) criteria conventionally utilized to assess responses to chemo/targeted therapy have been initially replaced by the immune-related (ir) Response Criteria (irRC) and then by the irRECIST, that encompass all patterns of response typical of ICI therapy, being key for the optimal comprehensive management of treated patients. Here, we report a paradigmatic clinical case of a long-term survival in a stage III melanoma patient, experiencing tumor flares during adjuvant treatment with ipilimumab, and an untreated disease relapse several years after ending therapy.
Keywords: melanoma, ipilimumab, adjuvant therapy, tumor flare, pseudo-progression, long-term survival
Background
The inhibitory receptor Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) is a key regulator of T-cell homeostasis and self-tolerance; blocking the interaction between CTLA-4 and its ligands, CD80/CD86, unleashes a strong anti-tumor immune response.1 Based on pre-clinical evidence and on encouraging results from Phase I/II studies, the randomized Phase III MDX-010 trial firstly demonstrated a significant improvement in the overall survival (OS) of metastatic melanoma patients treated with the anti-CTLA-4 monoclonal antibody (mAb) ipilimumab at 3 mg/kg.2 Likewise, the randomized phase III CA 184–024 study combining ipilimumab at 10 mg/kg with dacarbazine, demonstrated a significant improvement in OS in advanced melanoma patients compared to dacarbazine alone.3 Importantly, a 10-year pooled analysis of clinical trials with ipilimumab in metastatic melanoma showed a long-term survival in about 20% of treated patients, demonstrating that a long-lasting immunologic disease control in metastatic tumors could be achieved.4
Bringing immunotherapy to early stage disease, the randomized, double-blind, CA 184–029/European Organization for Research and Treatment of Cancer EORTC 18071 phase III trial, showed an improved recurrence-free survival (RFS) in the ipilimumab arm compared to the placebo arm (HR 0.75; 95% CI 0.64–0.90; p=0.013) in resected high-risk stage III melanoma patients.5,6 The activity of ipilimumab on RFS was confirmed at a median follow-up of 5.3 years (HR, 0.76; 95% CI, 0.64–0.89; p<0.001), demonstrating also an improved distant metastasis-free survival (DMFS) (HR, 0.76; 95% CI, 0.64–0.90; p=0.002), and a prolonged OS in the ipilimumab arm (HR, 0.72; 95.1% CI, 0.58–0.88; p=0.001).7,8
Further supporting the efficacy of immune-checkpoint inhibitor (ICI) therapy in melanoma, blockade of the interaction between programmed cell death (PD)-1 and its ligand PD-L1 by therapeutic mAb, demonstrated a higher antitumor activity compared to ipilimumab. Indeed, the anti-PD-1 nivolumab and pembrolizumab significantly improved the OS of treatment-naive metastatic melanoma patients, reaching a 5-year survival rate of about 40%.9,10 Moreover, the randomized, double-blind, phase III KEYNOTE-054/ EORTC1325 trial, evaluating pembrolizumab administered every 3 weeks for 1 year as adjuvant treatment of patients with resected, high-risk, stage III melanoma, demonstrated a significantly longer RFS than placebo (HR, 0.57; 98.4% CI, 0.43–0.74; p<0.0001);11 these findings were confirmed at 3.5-year median follow-up (HR, 0.59; 95% CI, 0.49–0.70; p<0.0001), and an improved DMFS (HR, 0.60; 95% CI, 0.49–0.73; p<0.0001) was also reported.12 Consistently, the randomized, double-blind, Phase III, CA209-238 trial explored the efficacy of nivolumab (3 mg/kg) or ipilimumab (10 mg/kg) administered for 1 year, as adjuvant therapy in fully resected stage III/IV melanoma patients. Treatment with nivolumab resulted in a significantly longer RFS (HR, 0.72; 95% CI, 0.60–0.86) and DMFS (HR, 0.79; 95% CI, 0.63–0.99) than ipilimumab, with a more favorable safety profile.13,14
Longer-term follow-up in fully resected, high risk, stage III/IV melanoma will further assess the impact of ICI therapy in earlier stages of disease and on the induction of an efficient and long-lasting anti-tumor activity. In this scenario, we report a clinical case of an over decennial clinical success after ipilimumab monotherapy for a stage III melanoma patient, despite a delayed and untreated recurrence of disease.
Case Presentation
This clinical case illustrates an ipilimumab-induced long-term survival in a stage III melanoma patient, experiencing a medically untreated disease relapse several years after completion of adjuvant therapy.
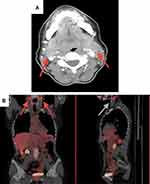
In November 2009, a 58-years-old female was diagnosed with stage IIIC cutaneous melanoma and was enrolled in the phase III randomized CA 184–029/EORTC 18071 clinical trial, receiving adjuvant ipilimumab (10 mg/kg) i.v. every 3 weeks for 4 cycles in the induction phase, and then every 12 weeks for up to 3 years in the maintenance phase.6 At the end of the induction phase (W12), tumour assessment with computed tomography (CT) scan and Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET) identified enlarged, FDG-positive, bilateral cervical lymph nodes and left palatine tonsil (Figure 1A and B). A fine needle biopsy of the palatine tonsil excluded the presence of neoplastic cell. The subsequent tumour assessment (W24) with CT scan and FDG-PET was negative; thus, this episode was classified as “tumor flare” and adjuvant treatment was resumed according to the study procedures. Maintenance phase started per protocol on August 2010.
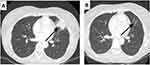
On May 2011, the W60 follow-up CT scan revealed a single lung nodule (37.9 × 34 mm) with radiologic features suggestive for an inflammatory lesion (Figure 2A). Though in the absence of clinical and laboratory signs of infection (ie, normal body temperature, C-reactive protein level and white blood cell count in the normal ranges) that would suggest for a “conventional” pneumonia, the scheduled ipilimumab dose was skipped and a further CT scan was planned after 12 weeks. In view of the full radiologic recovery of this inflammatory lung event on August 2011 (Figure 2B), treatment was resumed and completed in June 2013, when the patient entered the study follow-up phase. No additional adverse events were reported during treatment.
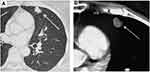
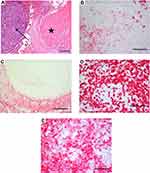
On January 2017, a control CT scan detected a single pulmonary nodule (7x6 mm) that significantly increased (12.5 × 8.5 mm) 4 months later; thus, also based on its morphologic features (Figure 3A and B) suspicious for a metastatic lesion, a wedge pulmonary resection was performed on June 2017. Notably, histopathologic report depicted scattered residual melanoma cell nests surrounded by a rich mononuclear cell infiltrate and plentiful necrosis; immunohistochemistry revealed a T cell infiltration mostly composed of CD4+ and CD8+ cells (Figure 4A–D).
No further treatments were administered from June 2017 and the last CT scan performed on January 2022 did not show signs of disease relapse.
Discussion
Unprecedented clinical results have been achieved with therapeutic mAb against CTLA-4 and/or PD-1/PD-L1, firstly in melanoma and subsequently in solid tumours of different histology. ICI treatment promotes an immune-mediated tumour eradication by inhibiting negative signalling pathways that down-regulate anti-tumor T-cell activity. Based on their unique mechanism of action, responses to ICI can be delayed and/or show peculiar features, such as tumor flares and pseudo progressions, that unlike conventional RECIST and WHO criteria are appropriately captured by irRC and irRECIST.15–17 Tumor flares may mimic disease progression and comprise lymph nodes enlargement, skin nodules, and pulmonary infiltrates, with or without systemic symptoms.18 First described in hematologic malignancies treated with immunomodulatory agents such as imatinib or thalidomide/lenalidomide, tumor flares were also reported during ICI therapy and require awareness by treating physician to properly evaluate potential tumor progression(s).19 Along this line, pseudo progressions were described as the growth of pre-existing lesions, or the appearance of new ones, followed by tumour regression, potentially due to T-cell infiltration.20 Although pseudo progressions occur in a small subgroup of subjects, their potential clinical consequences are relevant for the most appropriate management of patients in the course of ICI therapy. Indeed, 9.7% of patients with metastatic melanoma who received ipilimumab were classified as progressors according to WHO criteria but as responders according to irRC; moreover, while responders by both criteria had a median survival of 31.2 months, median OS in responders by irRC alone was not reached.20,21 Thus, the decision to discontinue therapy at first evidence of disease progression must rely exclusively on clinical factors including worsening of the performance status, disease-related symptoms, tumour growth rate and, in the absence of such signs, therapy should be continued until disease progression is confirmed at a further evaluation.22,23
The patient described in this report experienced a biopsy-confirmed tumor flare and pneumonia while on adjuvant therapy, both suggestive for a significant immune activation mediated by ipilimumab. Furthermore, while off therapy, she developed an irRC-confirmed single site recurrence of disease, that was surgically removed and showed limited residues of neoplastic cells, a major CD4+ and CD8+ T lymphocytes infiltrate, and abundant tumour cell necrosis all suggestive of an ongoing anti-tumor response. These findings were supported by similar observations demonstrating, that the increase of a CD8+ T lymphocytes infiltrate, results in a better response to therapy, during ipilimumab treatment.24–26
The main teachings of this clinical case are two-folds: first, in the presence of disease recurrence or progression confirmed by irRC or irRECIST, that would rule out “conventional” pseudo progression, one cannot exclude that the increasing volume of new and/or pre-existing lesions can be due to immune infiltration by immune cells; second, that under the comprehensive circumstances of this patient case history further medical therapy could be avoided since an ongoing and long-lasting active anti-tumor immune response, primed by previous ICI therapy, is capable to provide long-term disease control in melanoma.
Limitations
We acknowledge that observations generated in a single case limited the extent of interpretation of results, however the long-lasting active anti-tumor immune response we observed in a melanoma patient after adjuvant ipilimumab therapy, clearly demonstrated its ability to provide a durable disease control.
Abbreviations
CTLA-4 T, Cytotoxic T lymphocyte-associated protein 4; ICI, Immune Checkpoint Inhibitors; RECIST, Response Evaluation Criteria In Solid Tumors; WHO, World Health Organizations; irRC, immune-related Response Criteria; mAb, monoclonal antibody; DMFS, distance metastasis free survival; CT, computed tomography; OS, overall survival; RFS, Recurrence free survival; EORTC, European Organization for Research and Treatment of Cancer; PET, positron emission tomography; FDG, Fluorodeoxyglucose; H&E, hematoxylin and eosin; PD-1, Programmed death-1; PD-L1, Programmed death-Ligand 1.
Ethics Approval and Informed Consent
No institutional approval was required to publish the case details.
Consent for Publication
Informed consent was obtained in both written and verbal forms from patient to publish this case report and any accompanying images.
Acknowledgments
The authors wish to acknowledge the patient who participated in this study and her family.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
AMDG has served as a consultant and/or advisor to Incyte, Pierre Fabre, Glaxo Smith Kline, Bristol-Myers Squibb, Merck Sharp Dohme, and Sanofi and has received compensated educational activities from Bristol Myers Squibb, Merck Sharp Dohme, Pierre Fabre and Sanofi; MM has served as a consultant and/or advisor to Roche, Bristol-Myers Squibb, Merck Sharp Dohme, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, Glaxo Smith Kline, Sciclone, Sanofi, Alfasigma, and Merck Serono; and own shares in Epigen Therapeutics, Srl. MC, VD, FS, CM and MV have no conflicts of interest to declare for this work.
References
1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060
2. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
3. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi:10.1056/NEJMoa1104621
4. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J ClinOncol off J Am Soc Clin Oncol. 2015;33(17):1889–1894. doi:10.1200/JCO.2014.56.2736
5. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Correction to Lancet Oncol 2015; 16: 52230. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2015;16(6):e262. doi:10.1016/S1470-2045(15)70271-8
6. Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10. doi:10.1016/j.ejca.2019.07.001
7. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi:10.1056/NEJMoa1611299
8. Smithy JW, Shoushtari AN, Adjuvant PD-1. Blockade in resected melanoma: is preventing recurrence enough? Cancer Discov. 2022;12(3):599–601. doi:10.1158/2159-8290.CD-21-1593
9. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi:10.1056/NEJMoa1412082
10. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. doi:10.1001/jama.2016.4059
11. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi:10.1056/NEJMoa1802357
12. Eggermont AMM, Blank CU, Mandalà M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643–654. doi:10.1016/S1470-2045(21)00065-6
13. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi:10.1056/NEJMoa1709030
14. Weber J, Larkin J, Mandalá M, et al. Five-year outcomes with adjuvant nivolumab versus ipilimumab in resected stage IIIB–C or IV melanoma (CheckMate 238).
15. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi:10.1016/S1470-2045(17)30074-8
16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
17. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi:10.1158/1078-0432.CCR-09-1624
18. NIH. National Cancer Institute, DCTD division of cancer treatments & diagnosis. common terminology criteria for adverse events v3.0 (CTCAE); 2006.
19. Taleb BA. Tumour flare reaction in cancer treatments: a comprehensive literature review. Anticancer Drugs. 2019;30(9):953–958. doi:10.1097/CAD.0000000000000814
20. Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33(31):3541–3543. doi:10.1200/JCO.2015.61.6870
21. Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. doi:10.1200/JCO.2015.64.0391
22. Wang GX, Kurra V, Gainor JF, et al. Immune checkpoint inhibitor cancer therapy: spectrum of imaging findings. Radiographics. 2017;37(7):2132–2144. doi:10.1148/rg.2017170085
23. Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. doi:10.1007/s00262-008-0642-y
24. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi:10.1056/NEJMoa1406498
25. Harder N, Schönmeyer R, Nekolla K, et al. Automatic discovery of image-based signatures for ipilimumab response prediction in malignant melanoma. Sci Rep. 2019;9(7449). doi:10.1038/s41598-019-43525-8
26. Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9(204). doi:10.1186/1479-5876-9-204
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.