Back to Journals » International Medical Case Reports Journal » Volume 15
Successful Non-Operative Treatment of Enterovesical and Enterocutaneous Fistulas Due to Crohn’s Disease
Authors Li H , Xie L, Yao H , Zhang L, Liang S, Lyu W
Received 18 November 2021
Accepted for publication 1 March 2022
Published 29 March 2022 Volume 2022:15 Pages 117—124
DOI https://doi.org/10.2147/IMCRJ.S346159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Hui Li,1 Lu Xie,1 Hongdi Yao,2 Lexing Zhang,3 Sanhong Liang,1 Wen Lyu1
1Department of Gastroenterology, Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Zhejiang Chinese Medical University School, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Radiology, Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Wen Lyu, Department of Gastroenterology, Hangzhou First People’s Hospital, Zhejiang University School of Medicine, 4 Xueshi Road, Shangcheng District, Hangzhou, 310000, Zhejiang, People’s Republic of China, Email [email protected]
Background: The incidences of enterovesical and enterocutaneous fistulas are extremely low, and enterovesical and enterocutaneous fistulas are difficult to treat in patients with Crohn’s disease.
Case Summary: In this case, the patient had recurrent abdominal pain and diarrhea for more than 2 years, with fecal residue in the urine for 6 days. Pelvic magnetic resonance imaging and colonoscopy showed intestinal infection with a rectal fistula, and the initial diagnosis was severely active Crohn’s disease with an enterovesical fistula. The patient had multiple internal fistulas and infections, and strongly refused surgical conditions. The patient was given an intravenous infusion of ustekinumab and somatostatin, with anti-infective treatment, nutritional support and regulation of the intestinal flora. Drainage and debridement of the cutaneous fistula were performed. After comprehensive treatment and management, the patient’s condition achieved significant clinical remission.
Conclusion: This patient achieved clinical response and will receive follow-up for another dose of ustekinumab after 12 weeks. The patient developed enterovesical and enterocutaneous fistulas, the incidence of multiple fistulas which are low in patients with CD and are difficult to cure and prone to relapse. Only few patients achieve complete remission. At present, there is no standard and effective treatment for CD with multiple fistulas. Usually, surgery is performed for treatment. Drug therapy, especially with biological agents, should be selected as the first-line pre-operative treatment. Clinicians, especially gastroenterologists, need to improve their knowledge of these conditions and update the treatment consensus guidelines in a timely manner. Clinicians need to take into account the patient’s condition and willingness when developing an effective treatment plan.
Keywords: Crohn’s disease, enterovesical fistula, enterocutaneous fistula, ustekinumab, therapy
Introduction
Crohn’s disease (CD) is characterized by transmural inflammation of the gastrointestinal tract, which predisposes approximately 30% of patients to fistula formation.1 CD with enterovesical and enterocutaneous fistulas is difficult to treat and often recurs after clinical remission is achieved. At present, no treatment is completely effective.2 In recent years, Biological agents have been shown to be effective for the treatment of CD with fistulas.1,3 The administration of ustekinumab has been indicated to improve the clinical remission of CD with fistulas.4 In this case, the patient was diagnosed with CD complicated with multiple fistulas. Treatment was difficult, and the compliance of the patient was poor. The clinical symptoms of the patients were significantly relieved, and the fistulas were healed with combined therapy involving ustekinumab administration, fistula drainage, and debridement. This suggests that comprehensive treatment should be provided for fistulizing CD, and the timing and dosage of biological agents are particularly important. We report a patient who was diagnosed with severely active CD with enterovesical and enterocutaneous fistulas and was successfully treated with ustekinumab before the surgery.
Case Presentation
Chief Complaints
A 38-year-old patient was admitted to the hospital with recurrent abdominal pain and diarrhea for more than 2 years, with fecal residue in the urine for 6 days.
History of Present Illness
The patient had developed abdominal distension, abdominal pain, diarrhea and mucus in the stool 2 years prior to admission. The symptoms of the patient had worsened 1 year prior, and he went to Zhejiang Provincial People’s Hospital and was diagnosed with Crohn’s disease. The patient was given mesalazine orally. After discharge, the patient stopped taking mesalazine. The patient developed diarrhea that occurred more than 10 times per day, accompanied by dysuria, fecal residue in the urine, and decreased urine volume 6 days prior to admission.
History of Past Illness
The patient underwent anal fistula surgery 1 year prior to admission.
Physical Examination
The patient was malnourished and was positive for subumbilical tenderness.
Laboratory Examinations
The auxiliary examinations after admission showed that the white blood cell count was 9.9 * 10 ^ 9/L, neutrophil proportion was 66.1%, hemoglobin level was 98 g/L, CRP level was 139.9 mg/L, and serum albumin level was 24.5 g/L. Laboratory examinations showed the following: autoantibody (-), immunization (-), tuberculosis antibody (-), and T-SPOT (-). Test for inflammatory bowel disease-associated antibodies showed positivity for ASCA IgG, ASCA IgA, and F12Y IgG and negativity for AYMA IgG, AYCA IgA, and pANCA. The calprotectin level was greater than 1800 ug/g.
Imaging Examinations
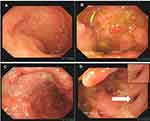
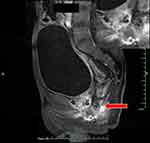
Colonoscopy revealed the normal mucosa in the terminal ileum (Figure 1A), and paving stone-like changes in the colon, ulcers in the local mucosa (Figure 1B and C), and fistulas 25 cm away from the anus on November 30, 2020 (Figure 1D). The colonoscopic biopsy pathology showed chronic mucosal inflammation with erosion, focal inflammatory exudation, necrosis, infiltration of a large number of plasma cells and neutrophils in the stroma, no granuloma formation, and mild dysplasia of the local glands. The patient underwent enhanced pelvic magnetic resonance imaging, and the results showed multiple abnormal signal shadows in and around the prostate and urethra, an unclear boundary between the lesions and the anorectal canal, and infection with fistula formation (Figure 2). Capsule endoscopy revealed chronic inflammation of the small intestine with villous atrophy.
Further Diagnostic Work-Up
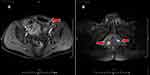
The patient was admitted to our hospital again for 5 days due to swelling and pain in the left lower abdomen. There was a palpable mass 10 cm in size in the left lower abdomen of the patient, with obvious swelling and tenderness, increased skin temperature, and local tenderness (Figure 3A). The white blood cell count was 17.6 * 10 ^ 9/L, and the CRP level was 139.9 mg/L. B-ultrasound showed a hypoechoic mass and abundant blood supply in the left lower abdomen, with a range of approximately 6.0 * 2.4 * 3.5 cm. Considering the presence of the inflammatory mass and the unclear boundary between the hypoechoic mass and the abdominal cavity, a local sinus was suspected of having formed. Enhanced pelvic magnetic resonance imaging suggested local wall thickening of the sigmoid colon and rectum and inflammatory bowel disease; infection of the left lower abdominal wall with subcutaneous pneumatosis and local communication with the sigmoid colon, suggesting fistula formation; and multiple abnormal signals around the anus and corpus cavernosum, suggesting sinus and fistula formation with infection (Figure 4A and B).
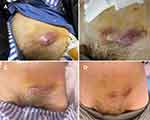 |
Figure 3 Enterocutaneous fistula. (A) A cutaneous fistula was found in the left abdomen. (B and C) The cutaneous fistula was treated. (D) The cutaneous fistula was healed. |
Final Diagnosis
The final diagnosis was severely active CD with enterovesical and enterocutaneous fistulas.
Treatment
The patient was administered an intravenous infusion of ustekinumab 260 mg on December 4, 2020. Anti-infective treatment and nutritional support were given. The symptoms of the patient significantly improved. The patient was admitted due to the development of an extraintestinal fistula on January 18, 2021. The patient had multiple internal fistulas and infections, and lacked surgical conditions. Moreover, after undergoing an anal fistula surgery, the patient refused to undergo surgery again, and conservative drug treatment was administered. Conservative drug treatment was administered. Fasting was suggested, and somatostatin was administered to inhibit the secretion of digestive juice. The patient was given an intravenous infusion of ustekinumab 90 mg on February 8, 2021, with anti-infective treatment, nutritional support, and regulation of the intestinal flora. Drainage and debridement of the cutaneous fistula were performed and treated with dressing changes.
Outcome and Follow-Up
After discharge, the patient was given mesalazine orally, supplemented by enteral nutrition support. The cutaneous fistula was treated with a dressing change (Figure 3B and C). The patient’s clinical symptoms significantly improved, CRP had successfully declined from 139.9 mL/L to 25 mL/L, and the cutaneous fistula healed (Figure 3D). The patient had been suggested to another dose of ustekinumab after 12 weeks.
Discussion
Enterovesical and enterocutaneous fistulas are serious complications of CD and are difficult to treat. They can occur spontaneously or postoperatively and are associated with high morbidity and mortality. Its management requires combined medical and surgical strategies to prevent abscess formation and induce healing. However, extended or repeated intestinal resections may result in short bowel syndrome and the need for intestinal transplantation. Treatment of enterovesical and enterocutaneous fistulas in CD still represents a major challenge for gastroenterologists and surgeons. Biologic agents have improved the medical treatment of CD-related fistulas, but many patients still require surgical intervention. Optimal management of fistulas is still being debated. In the case of multiple fistulas in CD, surgery is a safe and effective treatment. Although the success of medical treatment for fistulas in CD has so far been modest, some scholars consider medical therapy the first choice.5,6 Taxonera et al carried out a study, contained 97 entero-urinary fistulas, evaluated the clinical remission rate of medical therapy for entero-urinary fistulas in CD.7 They concluded that medical therapy (infliximab and adalimumab) was successful, with 45% of patients (33 entero-urinary fistulas) achieving sustained remission without need for surgery, which suggested that medical therapy made it possible to avoid surgery. There was a study to evaluate the outcomes of medical therapy in a cohort of CD with entero-urinary fistulas patients. Thirty-five percent of patients (33 in 97) received anti-TNF therapy. Of these, 45% achieved sustained remission (median follow-up 35 months) without needing surgery, which made it possible to avoid surgery. Anti-TNF agents have undoubtedly represented a major advance in the therapy of CD, however, these therapies are associated with a number of drawbacks, which include a substantial rate of primary/secondary nonresponse and the potential risk of local and systemic adverse effects. Hence, there is considerable interest in the development of novel pharmaceutical agents to treat fistulizing CD.
Previous studies have shown a positive effect of medical therapy on fistulizing CD.8,9 The management of fistulizing CD has two objectives: to induce fistula tract remission and to control infection. Enterocutaneous fistulas are, in most cases, a late surgical complication, and the indication and timing for treatment are due to their output volume.10 Abscesses may be present alone or in association with enteric fistulas. The initial approach is conservative, and a percutaneous drainage should be a good treatment of a bridge to elective surgery. In this case, we employed ustekinumab to induce fistula remission and anti-infective treatment. After drainage and debridement of the cutaneous fistula, the patient achieved clinical remission. Medical therapy appears to have benefitted the patient with enterovesical and enterocutaneous fistulas in this case.
Traditionally, T helper cell 1 (Th1) is recognized as the major immune effector of mediating CD. Then, a new set of T helper cells producing IL-17 (Th17) have been described. The description of effector Th17 cells by Harrington et al11 came after the initial description of the novel cytokine IL-23 which shared the same p40 subunit with IL-12.12 IL-12 mediates the development of Th1 cells. The p40 protein subunit is shared between the two cytokines and is the target molecule for ustekinumab. Ustekinumab should be given for moderate-to-severe Crohn’s disease patients who failed previous treatment with corticosteroids, thiopurines, methotrexate, or anti-TNF inhibitors or who have had no prior exposure to anti-TNF inhibitors (strong recommendation, high level of evidence).13 Ustekinumab has been recently approved in the EU and the USA as an intravenous induction and subcutaneous maintenance therapy for adult patients with moderately to severely active CD with failure of or intolerance to treatment with immunomodulators, corticosteroids or at least one TNF antagonist.4,14 Its efficacy has been demonstrated in landmark clinical trials for induction and maintenance of response and remission in CD patients, independent of their previous exposure to anti-TNF agents. Ustekinumab appears to be associated with rapid and sustained clinical effect, with additional support for potential mucosal healing. Ustekinumab may also be considered in the first-line setting, especially in frail patient.15
There is no evidence to firmly support the positioning of one biologic over another. However, there are these studies focusing on the moderate to severe CD. Feagan et al evaluated the clinical response of ustekinumab in two populations with moderately to severely active CD.16 The patients in the study included patients who were primary or secondary nonresponse to TNF antagonists or had unacceptable side effects, and patients who were conventionally therapy failed or had unacceptable side effects. They found that patients receiving intravenous ustekinumab had a significantly higher rate if response than did those receiving placebo, especially those who never receiving TNF antagonists had the highest clinical response. Another study showed that the rate of clinical remission was higher in TNF-antagonist-naïve patients compared with TNF-antagonist-refractory patients in the similar serum concentration of ustekinumab. In the first real-life cohort of 38 patients with severe CD who failed with anti-TNF and were treated with subcutaneous ustekinumab, dose escalation to every 4 weeks from every 8 weeks was required in almost half of the patients and was successful in achieving clinical response in 61.1%.17 A study of fistulizing CD in 27 trials (21 trials focused on perianal fistulizing CD suggested that ustekinumab was highly efficacious with regard to clinical fistula remission. They also found that the clinical remission rate of fistulas treated with ustekinumab combined with anti-infective therapy was higher than that of fistulas treated with ustekinumab alone. The study by Matthew J. Lee et al suggested that a higher proportion of the patients who received ustekinumab achieved fistula remission than those who received the placebo.18 A Japanese case report described the administration of ustekinumab to a patient with CD with an enterocutaneous fistula who achieved clinical remission, complete closure of the fistulas, and resolution of drainage without abscess formation after infliximab treatment failure.19 Another study that evaluated the efficacy of ustekinumab for active perianal fistulizing CD showed that 41% and 17% of the patients had achieved fistula response and remission at weeks 8, 24, and 52, respectively.20
The potential use of ustekinumab is broadening the therapeutic repertoire to address the needs of individuals who may have a contraindication to anti-TNF therapy, do not respond to anti-TNF therapy at the time of induction or eventually lose their response to therapy. Meanwhile, more studies are needed to evaluate dosing escalation or de-escalation in addition to the timing of therapy switches, and long-term efficacy and safety data in fistulizing CD patients are still needed.
Conclusion
Despite significant progress in fistulizing CD treatment with the use of new biologic medications targeting different inflammatory pathways, a challenging proportion of patients do not respond or lose response to treatment. Perforating CD necessitates a multidisciplinary approach involving, behind the gastroenterologist and the surgeon, the radiologist, the urologist, the gynecologist and the nutritionist in order to obtain the best tailored treatment. This case presented above shows the success of a multimodal treatment strategy for severely active CD with multiple fistulas. The fistulas were successfully cured with ustekinumab combined with antibiotic treatment. Surgery has been temporarily avoided, and the quality of life of the patient has improved. This case provides guidance for the systematic clinical treatment of fistulizing CD. And individualized treatments are needed for each patient and each fistula type. In this case, follow-up is needed to assess the long-term clinical remission of fistulas.
Informed Consent Statement
Informed written consent was obtained from the patient for publication of this report. There is no institutional approval is required to publish the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study, acquisition of data, analysis and interpretation; took part in drafting, revising and critically reviewing the article; All authors have given final approval of the version to be published, have agreed on the journal to which the article has been submitted, and have agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Kaimakliotis P, Simillis C, Harbord M, Kontovounisios C, Rasheed S, Tekkis PP. A systematic review assessing medical treatment for rectovaginal and enterovesical fistulae in Crohn’s disease. J Clin Gastroenterol. 2016;50:714–721. PMID: 27466166. doi:10.1097/MCG.0000000000000607
2. Schwartz DA, Maltz BE. Treatment of fistulizing inflammatory bowel disease. Gastroenterol Clin North Am. 2009;38:595–610. PMID: 19913204. doi:10.1016/j.gtc.2009.07.009
3. Panes J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652–664. PMID: 28790453. doi:10.1038/nrgastro.2017.104
4. Engel T, Yung DE, Ma C, et al. Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis. 2019;51:1232–1240. PMID: 31202609. doi:10.1016/j.dld.2019.05.002
5. Zhang W, Zhu W, Li Y, et al. The respective role of medical and surgical therapy for enterovesical fistula in Crohn’s disease. J Clin Gastroenterol. 2014;48:708–711. PMID: 24457944. doi:10.1097/mcg.0000000000000040
6. Present DH. Urinary tract fistulas in Crohn’s disease: surgery versus medical therapy. Am J Gastroenterol. 2002;97:2165–2167. PMID: 12358227. doi:10.1111/j.1572-0241.2002.05967.x
7. Taxonera C, Barreiro-de-acosta M, Bastida G, et al. Outcomes of medical and surgical therapy for entero-urinary fistulas in Crohn’s disease. J Crohns Colitis. 2016;10:657–662. PMID: 26786982. doi:10.1093/ecco-jcc/jjw016
8. Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–1207. doi:10.1016/j.crohns.2014.04.005
9. Attauabi M, Burisch J, Seidelin JB. Efficacy of ustekinumab for active perianal fistulizing Crohn’s disease: a systematic review and meta-analysis of the current literature. Scand J Gastroenterol. 2021;56:53–58. PMID: 33264569. doi:10.1080/00365521.2020.1854848
10. Sampietro GM, Casiraghi S, Foschi D. Perforating Crohn’s disease: conservative and surgical treatment. Dig Dis. 2013;31:218–221. PMID: 24030229. doi:10.1159/000353373
11. Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. PMID: 16200070. doi:10.1038/ni1254
12. Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. PMID: 11114383. doi:10.1016/s1074-7613(00)00070-4
13. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. PMID: 29610508. doi:10.1038/ajg.2018.27
14. Engel T, Kopylov U. Ustekinumab in Crohn’s disease: evidence to date and place in therapy. Ther Adv Chronic Dis. 2016;7:208–214. doi:10.1177/2040622316653306
15. Armuzzi A, Ardizzone S, Biancone L, et al. Ustekinumab in the management of Crohn’s disease: expert opinion. Dig Liver Dis. 2018;50:653–660. PMID: 29610019. doi:10.1016/j.dld.2018.02.017
16. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. PMID: 27959607. doi:10.1056/NEJMoa1602773
17. Kopylov U, Afif W, Cohen A, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease–the McGill experience. J Crohns Colitis. 2014;8:1516–1522. PMID: 24996483. doi:10.1016/j.crohns.2014.06.005
18. Lee MJ, Parker CE, Taylor SR, et al. Efficacy of medical therapies for fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1879–1892. PMID: 29374617. doi:10.1016/j.cgh.2018.01.030
19. Madarame A, Kimura H, Kunisaki R. Successful treatment with ustekinumab for enterocutaneous fistulas in Crohn’s disease. J Crohns Colitis. 2020;14:569–570. PMID: 31616911. doi:10.1093/ecco-jcc/jjz161
20. Attauabi M, Burisch J, Seidelin JB. Efficacy of ustekinumab for active perianal fistulizing Crohn disease: a double-center cohort study. Inflamm Bowel Dis. 2021;27:e37–e8. PMID: 33200769. doi:10.1093/ibd/izaa297
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.