Back to Journals » Infection and Drug Resistance » Volume 12
Successful management of Mycobacterium abscessus complex lung disease in an otherwise healthy infant
Authors Liu H, Dong F, Liu J, Liu J, Pang Y , Zhao S, Lu J , Li H
Received 17 December 2018
Accepted for publication 10 April 2019
Published 15 May 2019 Volume 2019:12 Pages 1277—1283
DOI https://doi.org/10.2147/IDR.S198461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Hui Liu,1 Fang Dong,2 Jinrong Liu,1 Jianhua Liu,3 Yu Pang,4 Shunying Zhao,1 Jie Lu,5 Huimin Li1
1Department of Respiratory Medicine, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 2Department of Laboratory Medicine, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 3Department of Pediatrics, Maternal and Child Health Care Hospital of Shunyi District, Beijing, People’s Republic of China; 4Beijing Chest Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Beijing Key Laboratory for Pediatric Diseases of Otolaryngology, Head and Neck Surgery, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China
Abstract: Mycobacterium abscessus complex (MABC) is an uncommon but increasingly important cause of invasive pulmonary disease, a condition associated with diagnostic and management challenges. MABC has mainly been reported in children with certain medical conditions, such as preexisting structural lung disorders and immunocompromised status. In this article, we describe a rare case of MABC pulmonary disease in an otherwise healthy infant. A 4-month-old female presented with cough and fever for 4 days. Computed tomography showed multiple masses and small nodules across both lungs. Isolated mycobacteria from her bronchoalveolar lavage fluid and gastric aspirate were identified as MABC by using matrix-assisted laser desorption ionization time-of-flight mass spectrometry and M. abscessus subsp. massiliense was ultimately identified by DNA sequence analysis. Prolonged treatment with a combination of azithromycin, cefoxitin, and moxifloxacin achieved a successful treatment outcome.
Keywords: nontuberculous mycobacteria, pulmonary, drug resistance, azithromycin, treatment outcome
Introduction
Mycobacterium abscessus complex (MABC) is a group of rapidly growing nontuberculous mycobacteria (NTM) which form mature colonies on solid media within 7 days.1 MABC organisms are ubiquitous in soil and water. They were first recognized as human pathogens in the 1950s,2 with pulmonary MABC infection first recognized in 1993. Since then, the majority of pulmonary disease cases have been identified in older otherwise healthy adults with no history of cigarette smoking, but who possess underlying lung airway abnormalities.3 The clinical presentation of MABC is indistinguishable from lung infection caused by other mycobacterial species.4 Moreover, in countries such as China that have a high tuberculosis burden, diagnosis of NTM infection is also difficult due to likely misidentification of sputum smear NTM bacilli as M. tuberculosis. Both the complexity of molecular techniques used to identify MABC isolates and the lack of well-established antimicrobial treatment regimens have made diagnosis and management of MABC pulmonary disease particularly challenging for pediatricians.1 Since pulmonary disease caused by MABC is rare in immunocompetent children lacking other lung pathology,5 studies are needed to better describe pediatric MABC disease patient experiences and outcomes. Here we describe a case of pulmonary disease due to MABC infection in an otherwise healthy immunocompetent infant.
Case report
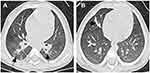
A 4-month-old female infant, born prematurely at 30 weeks and weighing 1,550 g, was hospitalized after 4 days of cough and fever. Her past history included a controlled premature birth by cesarean section due to placenta previa. She suffered from a brief period of mechanical ventilation and administration of exogenous surfactant after being born. After admitted, mild bilateral lung coarse rales were found on physical examination. Laboratory tests results showed a white blood cell count of 22.9×109 cells/L containing 58.2% neutrophils and a C-reactive protein concentration of 28 mg/L. Chest radiography exhibited patchy shadows localized to the right lung and lower left lung (Figure 1A). Subsequent computed tomography (CT) detected multiple masses and small nodules across both lungs with mediastinal lymph node involvement (Figure 1B and C). The patient was then started on intravenous ceftriaxone. Bronchoscopy revealed minimal overlying airway secretions and airway patency extending from the upper respiratory tract to the lungs; 3–9 acid-fast bacilli organisms/100 high-power fields were found in bronchoalveolar lavage fluid (BALF) and gastric aspirate by Ziehi-Neelsen staining. Thus, the patient was suspected of harboring pulmonary tuberculosis infection and treated with isoniazid, rifampin, and pyrazinamide. Although both tuberculin skin test and interferon gamma (IFN-γ) release assays were negative, mycobacterial liquid cultures of bronchoalveolar lavage and gastric aspirate were positive at 118 and 86 hrs of culture, respectively. Isolated mycobacteria were identified (with a confidence level of 84%) as MABC using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (Vitek MS system, bioMerieux, France), supporting a diagnosis of MABC pulmonary disease. Immunologic evaluation results, including serum immunoglobulin levels and flow cytometric lymphocyte phenotyping were as follows: IgG 4.96 g/L (normal range 1.8-8 g/L), IgM 1.00 g/L (normal range 0.2-1 g/L), IgA 0.225 g/L (normal range 0.08–0.8g/L), IgE <5.00 IU/mL (normal range ≤15 IU/mL), CD3+ cells 69.4% (normal range 55–82%), CD4+ cells 53.9% (normal range 55–57%), CD8+ cells 14.9% (normal range 11–25%), B cell 23.2% (normal range 11–45%), which were basically normal. However, natural killer (NK) cell level was 3%, which is below normal range 7–40%. In the review after 3 months, NK cell level raised to 10%. Whole exon sequencing (WES) was then performed and detected no pathogenic mutations, ruling out genetic disorders such as cystic fibrosis and primary immunodeficiency disease as factors contributing to exacerbation of MABC pulmonary disease in our patient. The subject family has no other case of MABC infection or immune-compromised individuals. According to expert consensus regarding diagnosis and treatment of NTM diseases,6 the therapy was modified to include oral clarithromycin (7.5 mg/kg, q12 h), linezolid (10 mg/kg, q8 h), and intravenous imipenem (15 mg/kg, q6 h). Due to the possibility of other mycobacterial infections, rifampicin (15 mg/kg/day) and ethambutol (15 mg/kg/day) were also administered. Subsequently, the patient’s temperature returned to normal after 1 week and her cough gradually reduced, prompting cessation of intravenous imipenem administration. She was discharged after 25 days of hospitalization.
After an additional 3 months of outpatient therapy, her chest CT remained unchanged and the patient exhibited recurrence of fever with wheezing. The white blood cell count was 17.6×109 cells/L, of which 47.4% were neutrophils, with a C-reactive protein concentration of 30 mg/L. Bronchoscopy revealed an excrescent obstruction of the sub-branch of the right lower bronchi. Acid-fast staining microscopy and mycobacterial cultures of BALF and gastric aspirate were negative. The isolates that had been preserved during the first hospital stay were sent to the National Tuberculosis Clinical Laboratory of Beijing Chest Hospital for drug susceptibility testing (DST) and further strain identification. DST was carried out according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Final tested drug concentrations were as follows: clarithromycin 0.0625–32 μg/mL, amikacin 0.5–256 μg/mL, moxifloxacin 0.0625–32 μg/mL, cefoxitin 0.5–256 μg/mL, linezolid 0.25–128 μg/mL, imipenem 0.25–128 μg/mL, meropenem 0.25–128 μg/mL, and doxycycline 0.0625–32 μg/mL.
The minimum inhibitory concentration (MIC) of each drug was determined using the CLSI-recommended broth microdilution method performed in 96-well plates. DST demonstrated that isolates were susceptible to clarithromycin (MIC 0.25–0.5 μg/mL), amikacin (MIC 1–2 μg/mL), moxifloxacin (MIC 1 μg/mL), cefoxitin (MIC 4–8 μg/mL), and linezolid (MIC 1 μg/mL), with intermediate susceptibility to imipenem (MIC 8 μg/mL) and resistance to meropenem (MIC 64 μg/mL) and doxycycline (MIC >32 μg/mL). The isolates had no inducible resistance to clarithromycin, as they were susceptible to clarithromycin at both days 3 and 14.
DNA sequence analysis of the ribosomal gene internal transcribed spacer (ITS) and rpoB, and hsp65 genes was performed to identify the subspecies of causative agent. Bacterial DNA extracted using the boiling method was used as a template for PCR. PCR primer pairs were as follows: 5‘-AAGTCGTAACAAGGTARCCG-3‘ and 5‘-TCGCCAAGGCATCCACC-3‘ for the ribosomal gene ITS region, 5‘-GGCAAGGTCACCCCGAAGGG-3‘ and 5‘-AGCGGCTGCTGGGTGATCATC-3‘ for rpoB, and 5ʹ-ATCGCCAAGGAGATCGAGCT-3ʹ and 5ʹ-AAGGTG-CCGCGGATCTTGTT-3ʹ for hsp65. PCR products were purified and sequenced by TsingKe Biotech Corp. (Beijing, China). Species identification was accomplished using BLAST searching that ultimately identified patient isolates as M. abscessus subsp. massiliense (with sequence identity of 99%).
After consultation with physicians specializing in NTM disease from Beijing Chest Hospital, we treated the patient with intravenous cefoxitin (40 mg/kg q8 h), oral azithromycin (10 mg/kg/d), and moxifloxacin (10 mg/kg/d). After 3 months of treatment using this regimen, chest radiography revealed improvement, with white blood cell count (8.65×109 cells/L) and C-reactive protein concentration (<8 mg/L) both returning to normal ranges (4–10×109 cells/L and 0–8 mg/L, respectively). Cefoxitin was discontinued after 4 months of use.
Monitoring for adverse drug events was performed monthly. No obvious adverse drug reactions were observed. Blood platelet counts ranged from 152×109 cells/L to 298×109 cells/L (normal range 100–400×109 cells/L). Ranges of aspartate aminotransferase were 12–38 U/L (normal range 5–40 U/L), of alanine aminotransferase were 8–26 U/L (normal range 5–40 U/L), of serum total bile acid were 5–8 µmol/L (normal range 0–10 µmol/L), of urea were 1.8–5.2 mmol/L (normal range 1.7–7.1 mmol/L), and of creatinine were 32.1–52.2 µmol/L (normal range 30–80 µmol/L). The ECG (electrocardiogram) was normal. After 12 months of antibiotic therapy, bronchoscopy revealed no excrescent obstruction or airway patency. Chest CT showed that most lung lesions had been absorbed, with strip shadows visible in both lungs (Figure 2A and B). Moxifloxacin was discontinued; however, based upon the recommendation of NTM disease specialists, the patient continued to receive oral azithromycin and cefprozil for 4 months, with cessation of all treatment after 16 months of antibiotic therapy. Upon examination at 3 and 6 months after completion of therapy, the patient was in good clinical condition. The recent laboratory tests results showed a white blood cell count of 8.2×109 cells/L containing 49.6% neutrophils and a C-reactive protein concentration <8 mg/L. The patient will continue to receive regular follow-up by mycobacterial disease physicians.
Discussion
MABC is the most important group of rapidly growing mycobacteria involved in pulmonary infections. Pediatric patients with chronic lung disease and immune defects are more susceptible than healthy individuals to pulmonary MABC infection.7 Because MABC pulmonary disease is rare in immunocompetent children in the absence of lung pathology, only two MABC lung infection cases have been previously reported in healthy children.8,9 The first case involved a 6-month-old boy presenting with MABC pneumonia with no known prior history of medical illness. He was treated using long-term combined antibiotic therapy, and repeated attempts to culture organisms from BALF failed to confirm mycobacterial infection. Although he suffered additional episodes of bacterial pneumonia, no IFN-γ receptor deficiency was detected. The other case involved a 2-year-old boy who presented with wheezing. His chest CT and bronchoscopy showed a mass obstructing the right mainstem bronchus. He was treated with ciprofloxacin and clarithromycin followed by surgical resection. After 18 months of therapy, cultures were negative. To our knowledge, this current report is the first report in China of a case of MABC pulmonary disease in an immunocompetent infant lacking preexisting lung disease.
Clinical symptoms secondary to MABC infection in both adults and children include the following symptoms: fever, increased cough with sputum production or hemoptysis, and weight loss.1 In addition to clinical symptoms, radiographic features consistent with NTM pulmonary disease are required for diagnosis. A high-resolution CT scan is more specific than traditional radiographic films for detection of nodules, cavitations, tree-in-bud patterns, focal consolidation and bronchiectasis, which are the most common findings in adult patients1,10,11 Nevertheless, the mycobacterial culture method, when used in combination with clinical and radiographic findings, is the gold standard for diagnosis of MABC lung disease,12 with either positive cultures obtained from two separate occasions of expectorated sputum or a single positive culture from bronchoalveolar lavage or transbronchial biopsies required for definitive diagnosis.1,12 Currently, MABC can be divided into three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii. Subspecies identification of MABC isolates is important, due to inherent differences in inducible macrolide resistance between M. abscessus subsp. abscessus and M. abscessus subsp. massiliense.13,14 Because bacterial species identification using biochemical assays and other conventional methods cannot reliably discriminate among MABC subspecies, in the past 10 years new DNA sequence-based technologies have been developed for this purpose. However, most pediatric referral center laboratories lack resources to perform sequence-based testing, thus requiring testing to be performed by national reference laboratories.15 In this study, our patient met clinical, radiographic, and microbiologic criteria for diagnosis of NTM lung disease, as outlined by the American Thoracic Society (ATS). Patient isolates were further identified as M. abscessus subsp. massiliense using sequence analysis targeting the ribosomal ITS region and rpoB and hsp65 genes.
Rough and smooth colony MABC phenotypes can arise from a single parental strain to cause disease with different clinical outcomes.16–18 The smooth variant “masks” underlying glycosylated lipoproteins involved in host immune recognition of bacilli by expressing glycopeptidolipid (GPL) on the outermost side of the cell wall to block bacteria-host lipid-based cellular interactions. By preventing MABC from being recognized by host innate immune surveillance mechanisms and promoting biofilm formation, GPL facilitates bronchiectatic colonization of lung airways. GPL then undergoes a spontaneous or temperature-induced loss of expression that results in ‘unmasking’ of bacilli to permit TLR2-based recognition by macrophages and airway epithelial cells followed by release of pro-inflammatory cytokines such as TNFα (from macrophages) and IL-8 (from respiratory tract epithelial cells).16 In contrast to M. tuberculosis, this phagosomal escape mechanism is unrelated to ESX-1, since MABC lacks an ESX-1 homolog.17 However, rough variants acquire a toxic phenotype characterized by growth of serpentine cells and macrophage apoptosis while they rapidly proliferate to perpetuate infection, a process similar to that employed by virulent M. tuberculosis.16
Disease due to MABC, a rarely detected pulmonary pathogen in the pediatric population, requires confirmation of virulence as a cause of disease after ruling out host immunodeficiency disorders via detailed immunological evaluation. Host defense against mycobacterial infection depends on both the ability of monocytes to produce interleukin (IL)-12 and the ability of activated T-cells to produce IFN-γ.19 Therefore, disseminated NTM disease is a definite manifestation of infection in children with immune deficiencies, such as HIV infection or abnormalities in the IL-12/IFN-γ pathway. In our patient, immunoglobulin levels and lymphocyte subsets were basically normal and WES result showed no mutation in genes known to cause primary immunodeficiency disease, ruling out a definitive immune deficiency as the cause of her disease. To explain why this patient contracted NTM infection, two possible risk factors could be postulated as predisposing factors: premature delivery and a possible predisposition to MABC lung disease, although this patient has yet to be diagnosed with any subtle immune abnormality as a risk factor.
An optimal therapeutic regimen and treatment duration for MABC lung disease have not yet been established. Because MABC is naturally resistant to conventional first-line antituberculous drugs, combination therapy using an oral macrolide and DST results-based parenteral administration of amikacin with cefoxitin, imipenem, or linezolid for weeks to months is a currently accepted practice. This treatment is then followed by oral antimicrobial therapy for at least 12 months after cultures remain negative. This regimen has been recommended by both the ATS/Infectious Diseases Society of America and numerous experts.1 However, some MABC isolates are susceptible to linezolid or moxifloxacin, as shown in several studies of adults that demonstrate higher treatment response rates to combination antibiotic therapy for patients with M. abscessus subsp. massiliense lung disease than for those with M. abscessus subsp. abscessus lung disease. This observation is likely due to the presence of a functional ribosomal methyl transferase gene, erm(41), in M. abscessus subsp. abscessus, which results in inducible macrolide resistance, while M. abscessus subsp. massiliense possesses a nonfunctional erm(41) gene lacking inducible resistance.20–22
When pediatricians decide to initiate long-term antibiotic treatment for MABC lung disease, they should select drugs based on prior experience or on in vitro DST results to carefully weigh treatment benefits against risks. For example, azithromycin has unique pharmacokinetic properties that permit administration of single daily dosages, as well as an intermittent treatment option, to better support patient compliance when compared to drugs such as clarithromycin. Moreover, azithromycin has been more effective than clarithromycin against M. abscessus subsp. massiliense in experimental models.23 Although amikacin also exhibits adequate in vitro activity against MABC isolates, it was not used to treat our patient, due to risks of potential serious side effects. Such side effects include ototoxicity and nephrotoxicity, the former observed in a 4-year-old boy with MABC central nervous system infection who developed profound hearing loss at the end of the second month of amikacin treatment.24 After DST of isolates from our patient confirmed moxifloxacin susceptibility, this drug was orally administered. Meanwhile, cefoxitin and imipenem, two β-lactams currently recommended as accepted MABC treatment guidelines, represent a largely unstudied class of potentially useful antibiotics for MABC infection treatment.25 Cefoxitin was selected according to DST results. Moreover, as compared to imipenem, cefoxitin showed superior pharmacokinetic parameters and narrower activity spectrum, which further justified cefoxitin more suitable for long-term treatment of this patient.26 The patient in current study received oral cefprozil in combination with azithromycin during the continuation phase on the advice of NTM experts, with treatment resulting in further absorption of lung lesions.
After our patient was confirmed to have infection with M. abscessus subsp. massiliense, she was treated with oral azithromycin for 13 months in combination with intravenous cefoxitin for 4 months, oral moxifloxacin for 9 months, and oral cefprozil for 4 months to achieve clinical recovery confirmed using chest imaging. No obvious adverse drug reactions, such as neutropenia, hepatotoxicity, tendinopathy, or ECG QT interval prolongation, were observed during treatment.
Conclusion
In this report, we suggest that MABC lung disease may occur in healthy infants in the absence of significant predisposition to lung disease. Due to the absence of innate immunity in infants, the prevalence of acquired mycobacterial drug resistance, and the lack of established therapeutic regimens, pediatric MABC lung disease treatment is challenging. Improvements in molecular techniques for identification of MABC subspecies and in DST are both needed to improve the curative rate. Here, prolonged treatment with a combination of azithromycin, cefoxitin, and moxifloxacin achieved a successful treatment outcome for an otherwise healthy infant with lung disease caused by M. abscessus subsp. massiliense.
Ethics Statement
The study was approved by the institutional review board of Beijing Children’s Hospital, Capital Medical University. Written informed consent has been provided by the patient’s parents to have the case details and any accompanying images published.
Acknowledgments
This work was supported by the Clinical Research Special Fund of Wu Jieping Medical Foundation (320.6750.160.56). The authors thank Prof. Naihui Chu from Beijing Chest Hospital for her help in caring for study patient.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
2. Lee MR, Sheng WH, Hung CC, Yu C-J, Lee L-N, Hsueh P-R. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21(9):1638–1646. doi:10.3201/2109.141634
3. Griffith DE, Girard WM, Wallace RJ
4. Epson E, Cassidy M, Marshall-Olson A, Hedberg K, Winthrop KL. Patients with nontuberculous mycobacteria: comparison of updated and previous diagnostic criteria for lung disease. Diagn Microbiol Infect Dis. 2012;74(1):98–100. doi:10.1016/j.diagmicrobio.2012.05.035
5. Nolt D, Michaels MG, Wald ER. Intrathoracic disease from nontuberculous mycobacteria in children: two cases and a review of the literature. Pediatrics. 2003;112(5):e434. doi:10.1542/peds.112.5.e434
6.
7. Lopez-Varela E, Garcia-Basteiro AL, Santiago B, et al. Non-tuberculous mycobacteria in children: muddying the waters of tuberculosis diagnosis. Lancet Respir Med. 2015;3(3):244–256. doi:10.1016/S2213-2600(15)00062-4
8. Do PC, Nussbaum E, Moua J, Chin T, Randhawa I. Clinical significance of respiratory isolates for mycobacterium abscessus complex from pediatric patients. Pediatr Pulmonol. 2013;48(5):470–480. doi:10.1002/ppul.22638
9. Freeman AF, Olivier KN, Rubio TT, et al. Intrathoracic nontuberculous mycobacterial infections in otherwise healthy children. Pediatr Pulmonol. 2009;44(11):1051–1056. doi:10.1002/ppul.21069
10. Floto RA, Olivier KN, Saiman L, et al. US cystic fibrosis foundation and european cystic fibrosis society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1–i22. doi:10.1136/thoraxjnl-2015-207360
11. Han D, Lee KS, Koh WJ, Yi CA, Kim TS, Kwon OJ. Radiographic and CT findings of nontuberculous mycobacterial pulmonary infection caused by mycobacterium abscessus complex. Am J Roentgenol. 2003;181(2):513–517. doi:10.2214/ajr.181.2.1810513
12.
13. Koh WJ, Stout JE, Yew WW. Advances in the management of pulmonary disease due to mycobacterium abscessus complex. Int J Tuberc Lung Dis. 2014;18(10):1141–1148. doi:10.5588/ijtld.14.0134
14. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64(3):309–316. doi:10.1093/cid/ciw724
15. Sabin AP, Ferrieri P, Kline S. Mycobacterium abscessus complex infections in children: a review. Curr Infect Dis Rep. 2017;19(11):46. doi:10.1007/s11908-017-0597-2
16. Ryan K, Byrd TF. Mycobacterium abscessus: shapeshifter of the mycobacterial world. Front Microbiol. 2018;9:2642. doi:10.3389/fmicb.2018.02642
17. Bernut A, Herrmann JL, Ordway D, Kremer L. The diverse cellular and animal models to decipher the physiopathological traits of mycobacterium abscessus infection. Front Cell Infect Microbiol. 2017;7:100. doi:10.3389/fcimb.2017.00517
18. Ripoll F, Pasek S, Schenowitz C, et al. Non mycobacterial virulence genes in the genome of the emerging pathogen mycobacterium abscessus. PLoS One. 2009;4(6):e5660. doi:10.1371/journal.pone.0005660
19. Lamb GS, Starke JR. Mycobacterium abscessus complex infections in children: a review of current literature. J Pediatric Infect Dis Soc. 2018;7(3):e131–e144. doi:10.1093/jpids/piy047
20. Jeon K, Kwon OJ, Lee NY, et al. Antibiotic treatment of mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180(9):896–902. doi:10.1164/rccm.200905-0704OC
21. Harada T, Akiyama Y, Kurashima A, et al. Clinical and microbiological differences between mycobacterium abscessus and mycobacterium massiliense lung diseases. J Clin Microbiol. 2012;50(11):3556–3561. doi:10.1128/JCM.01175-12
22. Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of mycobacterium massiliense from mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183(3):405–410. doi:10.1164/rccm.201003-0395OC
23. Choi GE, Shin SJ, Won CJ, et al. Macrolide treatment for mycobacterium abscessus and mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012;186(9):917–925. doi:10.1164/rccm.201111-2005OC
24. Lamb GS, Del Valle Mojica C, Srinivas N, et al. Central nervous system infections caused by mycobacterium abscessus: ventricular shunt infection in two pediatric patients and literature review. Pediatr Infect Dis J. 2018. doi:10.1097/INF.0000000000002146
25. Story-Roller E, Maggioncalda EC, Cohen KA, Lamichhane G. Mycobacterium abscessus and β-lactams: emerging insights and potential opportunities. Front Microbiol. 2018;9:2273. doi:10.3389/fmicb.2018.02273
26. Lavollay M, Dubée V, Heym B, et al. In vitro activity of cefoxitin and imipenem against mycobacterium abscessus complex. Clin Microbiol Infect. 2014;20(5):O297–O300. doi:10.1111/1469-0691.12405
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.