Back to Journals » OncoTargets and Therapy » Volume 14
Successful Management of a Patient with Refractory Primary Central Nervous System Lymphoma by Zanubrutinib
Authors Cheng Q, Wang J, Lv C, Xu J
Received 3 March 2021
Accepted for publication 23 April 2021
Published 24 May 2021 Volume 2021:14 Pages 3367—3372
DOI https://doi.org/10.2147/OTT.S309408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Qiansong Cheng,1,2 Jing Wang,2 Chenglan Lv,2 Jingyan Xu2
1Department of Hematology, The Lu’an People’s Hospital, The Lu’an Hospital Affiliated to Anhui Medical University, Lu’an, Anhui, People’s Republic of China; 2Department of Hematology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China
Correspondence: Jingyan Xu
Department of Hematology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, 321 Zhongshan Road, Nanjing, 210008, People’s Republic of China
Tel +86 025 68182222
Email [email protected]
Abstract: Primary central nervous system lymphoma (PCNSL) is a rare subtype of extranodal non-Hodgkin lymphoma, and the most frequent histological type is diffuse large B‐cell lymphoma (DLBCL). Bruton’s tyrosine kinase inhibitor (BTKi) has shown clinical activity in DLBCL. We herein report a 53-year-old man who presented with binocular diplopia, gait instability, dizziness and bucking. He was diagnosed with PCNSL by cranial magnetic resonance imaging (MRI) scan and brain biopsy. Next-generation sequencing (NGS) examination identified multiple genetic abnormalities. The patient was started on a high-dose methotrexate (HD-MTX)-based protocol for two courses. However, the patient developed disease progression. The patient’s phenotypic and genetic characteristics strongly suggested BN2-DLBCL, and zanubrutinib was added to the subsequent chemotherapy regimen. The treatment was well tolerated, and complete remission (CR) was achieved after three courses of chemotherapy with the new regimen. The patient then received autologous hematopoietic stem cell transplantation after four courses of chemotherapy with the new regimen. MRI revealed stable CR. Here, we report a successful case of refractory PCNSL treated with zanubrutinib. Small molecules, such as zanubrutinib, may be selectively integrated into first-line regimens of PCNSL to enhance curative effect and reduce recurrence.
Keywords: primary central nervous system lymphoma, Bruton’s tyrosine kinase, zanubrutinib, targeted therapy
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare subtype of extranodal non-Hodgkin lymphoma (NHL). The most frequent histological type of PCNSLs is diffuse large B‐cell lymphoma (DLBCL).1,2 Treatment options of PCNSL include chemotherapy and whole-brain radiotherapy. Most chemotherapy regimens contain high-dose methotrexate (HD-MTX, ≥3 g/m2)-based chemotherapy with or without other chemotherapeutics.3
Ibrutinib is an orally administered Bruton’s tyrosine kinase inhibitor (BTKi). The drug has been approved for the treatment of chronic lymphocytic leukemia, relapsed/refractory (R/R) mantle cell lymphoma and Waldenström macroglobulinemia (WM).4 Ibrutinib has also been shown to be effective in DLBCL, especially for activated B-cell like (ABC) subtype.5 In recent years, several large-scale clinical trials have been conducted to identify genetic subtypes and explore pathogenesis of DLBCL.6,7 Here, we report a case of PCNSL (BN2-DLBCL) with disease progression after two cycles of HD-MTX-based chemotherapy. Owing to the delayed next-generation sequencing (NGS) results, zanubrutinib was added to the subsequent chemotherapy regimen. The patient responded well and achieved complete response (CR) thereafter.
Case Presentation
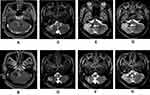
A 53-year-old man was admitted to the local hospital with complaints of binocular diplopia. Cranial magnetic resonance imaging (MRI) showed two notable nodules in the cerebellopontine angle area and the medial temporal horn of the left ventricle (Figure 1). Consistent with the results of cranial MRI, positron emission tomography/computed tomography (PET/CT) revealed a 2.1×1.6 cm mass in the cerebellopontine angle area and a 1.0×0.9 cm mass in the medial temporal horn of the left ventricle without involvement of other systems, and the maximum FDG uptake value was 27.7. The binocular diplopia worsened, and symptoms of gait instability, dizziness, bucking and numbness of the right side of the face appeared nearly one month later.
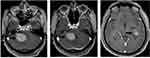 |
Figure 1 MRI scan of the brain at presentation. This MRI scan image showed two notable nodules in the cerebellopontine angle area and the medial temporal horn of the left ventricle (black arrow). |
On the basis of radiological features and symptoms, the patient was admitted to the department of neurosurgery at Nanjing Drum Tower Hospital. Resection of the cerebellopontine angle area mass was performed, but the symptoms did not improve postoperatively. Histological examination of the brain biopsy showed DLBCL. Immunohistochemical (IHC) analysis revealed the cells were positive for CD20, BCL6, MUN1, BCL2 and MYC; and negative for CD3, CD21, CD10 and CD5. The Ki-67 proliferation index was approximately 80%. In situ hybridization for EBER was negative. A diagnosis of PCNSL (non-GCB DLBCL) was made, and he was subsequently transferred to the department of hematology for further treatment.
The patient’s laboratory tests indicated a normal hemogram. Serum biochemical results were all within the normal range except for a mild increase of glucose. Viral studies for HIV and HCV were negative. A diagnosis of chronic HBV infection was made based on serological analysis, and serum HBV DNA level was 1.85×103 IU/mL. The levels of EBV DNA and CMV DNA were all below the detection limit. A staging bone marrow biopsy was performed, and there was no evidence of bone marrow involvement by lymphoma. The bone marrow aspirate indicated a normal karyotype (46, XY) without evidence of immunoglobulin heavy chain (IgH) gene rearrangement by fluorescence in situ hybridization (FISH) analysis. Disruption of BCL6 can be detected by FISH analysis in the surgical specimen. FISH for t(14;18) translocation (IgH/BCL2) and c-MYC disruption all yielded negative results. Next-generation sequencing (NGS) technology has been used to identify genes with aberrations. A panel of 222 common genes were tested (Figure S1), and the examination revealed multiple abnormalities in the surgical specimen (Table 1). Physical examination revealed slight ptosis of right eyelid, decrease of right nasolabial sulcus, disappearance of right stria frontalis and left-leaning of angulus oris.
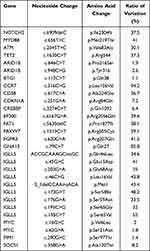 |
Table 1 Summary of Variants Detected by Next-Generation Sequencing (NGS) in Tumor Tissue |
The patient was started on a HD-MTX-based regimen for two courses (Figure 2). The initial symptoms persisted, and cranial MRI scan was performed thereafter. Even though the mass in the medial temporal horn of the left ventricle shrank slightly (not shown here), the mass in the cerebellopontine angle area clearly enlarged (Figure 3). Based on clinical and imaging features, disease progression can be determined. The patient’s phenotypic and genetic characteristics strongly suggested BN2-DLBCL.6 Owing to the poor treatment response and delayed NGS results, zanubrutinib was added to the subsequent regimen. The patient received an initial dose of 160 mg twice daily, with subsequent dose modification due to the addition of voriconazole for fungal infection. He then received two cycles of the new regimen with HD-MTX, rituximab and zanubrutinib (repeated every 28 days). The treatment was well tolerated, and symptoms were relieved gradually (Figure 2). Further cranial MRI scan was performed, and it revealed that the masses were reduced significantly. Two additional courses were given, and a CR with cicatricial changes was achieved after three courses of chemotherapy with the new regimen (Figure 3). The patient then received autologous hematopoietic stem cell transplantation with a conditioning regimen including busulfan, cyclophosphamide and thiotepa. MRI revealed stable CR, and he remained alive 11 months after diagnosis.
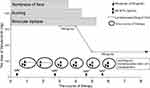 |
Figure 2 Treatment procedure, and timeline of symptoms during the treatment. MRI1, first MRI scan; MRI2, second MRI scan; MRI3, third MRI scan; MRI4, fourth MRI scan; bid, twice daily. |
Discussion
PCNSL is a rare extranodal non-Hodgkin lymphoma (NHL), typically with DLBCL histology and inferior prognosis to other nodal lymphomas.2 Based on cell of origin (COO), the majority of PCNSLs (over 85%) can be classified as ABC subtype, which is associated with frequent mutations in the B-cell receptor (BCR) signaling pathway and inferior outcomes.8 HD-MTX, which served as the foundation of all first-line treatment regimens, has improved prognosis of PCNSLs significantly during the past decades. Other drugs found to be safe and useful when combined with HD-MTX are rituximab, temozolomide, thiotepa, vincristine, cytarabine and procarbazine.9 However, outcome for patients with R/R PCNSL is poor, and treatment options for these patients remain limited.
DLBCLs constitute a heterogeneous group of malignancies regardless of subtypes based on COO, termed germinal center B-cell-like (GCB), ABC and unclassified.10 Nonetheless, this classification cannot fully explain the heterogeneous responses to standard scheme (R-CHOP) or targeted therapy. Due to the progress of genomic technologies, large-scale structural and functional analysis can be implemented to detect genes with recurrent aberrations. Genetic classification of DLBCL provides a novel and evolving understanding of tumor pathogenesis and potential therapeutic targets. In the past few years, several integrative analyses have been conducted to identify genetic subtypes and uncover therapeutic vulnerabilities of DLBCL based on tumor genetics.6,7,11 A study by Schmitz and colleagues identified four genetic subtypes on the basis of genetic aberrations, termed MCD subtype (co-occurrence of MYD88L265P and CD79B mutations), BN2 subtype (BCL6 fusions and NOTCH2 mutations), N1 subtype (NOTCH1 mutations) and EZB subtype (EZH2 mutations and BCL2 translocations).6 Recently, Wright and colleagues adopted an algorithm to determine the probability of a specific DLBCL belonging to a particular subtype, and identified seven genetic subtypes based on its genetic features. In addition to the above-mentioned four subtypes, the A53 subtype (aneuploid with TP53 inactivation) and ST2 subtype (SGK1 and TET2 mutations) were identified, and the EZB subtype can be subdivided into EZB-MYC+ and EZB-MYC− types.7 Considerable researches have focused attention on BTK as a potential therapeutic target in DLBCL, and the BTKi exhibited potential therapeutic effects on DLBCL, especially in MCD, BN2 and A53 subtypes.6,7
Recently, a large number of genomic researches using archival tissue banks have been undertaken. These investigations have identified BCR and Toll-like receptor (TLR) signaling pathways as being critical in the pathogenesis of PCNSL.8 These pathways may potentially be targeted at different signaling nodes. BTK linked the BCR and TLR with nuclear factor kappa B (NF-κB), and a first-in-class BTKi (ibrutinib), which targeted BCR signaling pathway at the central nodule, has produced promising results in PCNSLs.12,13 Zanubrutinib is a novel, highly potent, second-generation BTKi. As compared with ibrutinib, this novel BTKi possesses a highly selective target-binding profile and exhibits fewer off-target effects on other related kinases.14 Based on less off-target toxicity, treatment with zanubrutinib is generally well tolerated as compared with ibrutinib, particularly in older patients. A recent report has shown that zanubrutinib has a good effect on Bing–Neel syndrome, which is characterized by lymphoplasmacytic cell infiltration of the CNS in patients with WM. This report confirmed that zanubrutinib can cross the BBB and exert clinical efficacy in CNS.15
R/R PCNSL patients have a dire prognosis: a median OS of 2–3 months, regardless of the lines of salvage treatment, was found in a large-scale prospective cohort study.16 Several recent studies indicated that a relatively high objective response rate can be achieved for R/R PCNSL patients who received ibrutinib, alone or combined with other therapies.12,17,18 To the best of our knowledge, no case has previously been reported with treatment of these patients with zanubrutinib. In the present research, we described a newly diagnosed PCNSL patient with BN2-DLBCL subtype. HD-MTX-based regimens were given as first-line chemotherapy. Due to the large lesion and severe symptoms, lenalidomide, which can inhibit NF-κB and PI3K/AKT pathway, was added to the regimens. The patient developed disease progression after two cycles of chemotherapy. Owing to the poor response and delayed NGS results, lenalidomide was removed, and zanubrutinib was added to the subsequent chemotherapy regimen. The patient responded well to the treatment and achieved CR after three cycles of chemotherapy. Lionakis et al demonstrated in a clinical trial with PCNSL patients that increased aspergillosis was detected with ibrutinib alone or combined with chemotherapy.13 The patient in our case also developed pulmonary fungal infection during treatment. The underlying mechanism of this phenomenon is that BTKi may impair fungal immune surveillance, and this effect can be exacerbated by co-administration of chemotherapy. The significance of the larger proportion of NGS results detected in our case is unclear. If the patient had not responded to zanubrutinib, other treatment options of hypomethylating agents and/or histone acetyltransferases inhibitor could be selected for targets of TET2, CREBBP and EP300.19,20
Due to the low incidence and difficulty in clinical diagnosis, it is difficult to undertake large clinical trials to determine the optimal regimen of patients with PCNSL. Recently, several large-scale clinical trials have been conducted to identify genetic subtypes and explore pathogenesis of DLBCL. These studies provide a wealth of information on DLBCL. As the majority of PCNSLs are of B‐cell origin and the most frequent histological type (over 90%) is DLBCL, this information could be put in order in clinical practices to enable the application of precision and personalized medicine for PCNSL. In conclusion, we report a first, successful case of refractory PCNSL treated with zanubrutinib according to the NGS results. Over the next few years, we will likely see small molecules, such as zanubrutinib, being selectively integrated into first-line regimens of PCNSL to enhance curative effect and reduce recurrence.
Ethical Approval
Ethical approval was obtained from the Institutional Research Ethics Committee.
Patient Informed Consent
Written informed consent was obtained from the patient for the publication of his case details and images.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. doi:10.1038/bjc.2011.357
2. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418. doi:10.1200/JCO.2017.72.7602
3. Mendez JS, Grommes C. Treatment of primary central nervous system lymphoma: from chemotherapy to small molecules. Am Soc Clin Oncol Educ Book. 2018;38(38):604–615. doi:10.1200/EDBK_200829
4. Younes A, Ansell S, Fowler N, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol. 2017;14(6):335–346. doi:10.1038/nrclinonc.2016.205
5. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. doi:10.1038/nm.3884
6. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi:10.1056/NEJMoa1801445
7. Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551–568. doi:10.1016/j.ccell.2020.03.015
8. Batchelor TT. Primary central nervous system lymphoma: a curable disease. Hematol Oncol. 2019;37(Suppl 1):15–18. doi:10.1002/hon.2598
9. Royer-Perron L, Hoang-Xuan K. Management of primary central nervous system lymphoma. Presse Med. 2018;47(11–12 Pt 2):e213–e244. doi:10.1016/j.lpm.2018.04.016
10. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi:10.1038/35000501
11. Reddy A, Zhang J, Davis NS, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171(2):481–494. doi:10.1016/j.cell.2017.09.027
12. Lewis KL, Chin CK, Manos K, et al. Ibrutinib for central nervous system lymphoma: the Australasian lymphoma alliance/MD Anderson Cancer Center experience. Br J Haematol. 2020;192(6):16. doi:10.1111/bjh.16946
13. Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843.e5. doi:10.1016/j.ccell.2017.04.012
14. Guo Y, Liu Y, Hu N, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of bruton’s tyrosine kinase. J Med Chem. 2019;62(17):7923–7940. doi:10.1021/acs.jmedchem.9b00687
15. Wong J, Cher L, Griffiths J, et al. Efficacy of zanubrutinib in the treatment of bing-neel syndrome. Hemasphere. 2018;2(6):e155. doi:10.1097/HS9.0000000000000155
16. Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. doi:10.1093/neuonc/now033
17. Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the Phase II ‘proof-of-concept’ iLOC study by the Lymphoma Study Association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi:10.1016/j.ejca.2019.05.024
18. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. doi:10.1158/2159-8290.CD-17-0613
19. Traina F, Visconte V, Elson P, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelodysplasia and related neoplasms. Leukemia. 2014;28(1):78–87. doi:10.1038/leu.2013.269
20. Lu SX, Abdel-Wahab O. Genetic drivers of vulnerability and resistance in relapsed acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2016;113(40):11071–11073. doi:10.1073/pnas.1613836113
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.