Back to Journals » OncoTargets and Therapy » Volume 11
Successful cisplatin-etoposide chemotherapy-based treatment of a primary small cell neuroendocrine carcinoma of the tonsil with multiple metastases: a case report
Authors Wang X, Chen Q, Meng J
Received 6 May 2018
Accepted for publication 23 July 2018
Published 3 September 2018 Volume 2018:11 Pages 5391—5395
DOI https://doi.org/10.2147/OTT.S173231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Xing Wang,1,2 Qiming Chen,1 Jian Meng1
1Department of Oromaxillofacial-Head and Neck Surgery, Affiliated Xuzhou Hospital, College of Medicine, Southeast University, Xuzhou, People’s Republic of China; 2Department of Oral Medicine, Peking University School and Hospital of Stomatology, Beijing, People’s Republic of China
Abstract: Extrapulmonary small cell neuroendocrine carcinoma (SNEC) is an extremely rare and highly malignant tumor with a poor prognosis. Multiple metastases of SNEC are even more rare, and patients with locally advanced and metastatic disease generally face a poor outcome. To date, only a few cases of SNEC have been reported. Here, we describe a rare case of a 70-year-old female patient with SNEC of the tonsil who presented with multiple metastases and had achieved a complete response (CR) of the primary lesion and cervical lymph nodes for more than 1 year after receiving palliative chemotherapy. Initially, the patient presented with a 2-month history of throat pain. Magnetic resonance imaging and computed tomography revealed a soft mass with moderate enhancement on the left tonsil, which was confirmed by incisional aspiration biopsy. She was additionally sent for a positron emission tomography scan to evaluate small metastases in the left cervical lymph node, right lung, multiple mediastinal lymph nodes, and the fourth lumbar (L4) vertebra body metastases. Histopathological examination of the SNEC confirmed a nested, typical endocrine appearance with small round cells containing ovoid-shaped nuclei and high mitotic activity. Immunohistochemically, the tumor cells were positive for cytokeratin 8/18+, synaptophysin+, CD56+, and Ki-67 (>50%). The patient received 6 cycles of cisplatin combined with etoposide and was subsequently placed under close observation (>12 months). To date, she has achieved a CR of the primary lesion and cervical lymph nodes. In summary, we have described a case of successful treatment after chemotherapy for SNEC and have elucidated professional knowledge regarding the relevant aspects of SNEC.
Keywords: small cell neuroendocrine carcinoma, head and neck, multiple metastases, complete remission
Introduction
Neuroendocrine carcinomas are included among the heterogeneous group of the malignant small cell tumors, which also include carcinoid, atypical carcinoid, and small cell carcinoma. Small cell neuroendocrine carcinoma (SNEC) is a highly invasive neuroendocrine tumor that commonly originates in the pulmonary tissue, whereas extrapulmonary SNEC accounts for only 2.5%–5% of cases.1,2 Despite the extreme rarity of extrapulmonary SNEC, sinonasal, laryngeal, cervix, parotid, and even lower gingival tumors have been reported.3–5 Many of the reported cases involved high-grade carcinomas, a highly invasive form of cancer with distant metastasis.6 Some smaller series have reported that 14.5%–25% of patients present with distant metastatic disease.7,8 Patients with a locally advanced stage and metastatic disease often have a low median survival rate, according to previous reports.9
To date, reports of a patient with multiple metastases of SNEC have been rare. Additionally, such cases have not previously involved the head and neck. Therefore, clinical research is scarce, especially with regard to the point of treatment. In this report, we describe our experience with the successful achievement of a complete response (CR) of the primary tumor and cervical lymph nodes in a woman with extrapulmonary SNEC.
Case report
In May 2016, a 70-year-old woman with no history of smoking was referred to the hospital with a 2-month history of persistent throat pain which had been accompanied by disordered eating for 1 week. Her medical history included long-term hypertension and type 2 diabetes mellitus. At admission, a physical examination revealed a tender mass in the left tonsil (Figure 1) and a 1.1×1.3 cm lymph node at the left-side level II upon careful neck palpation. All other head and neck findings were normal. All laboratory data were within the normal limits.
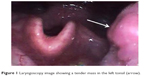  | Figure 1 Laryngoscopy image showing a tender mass in the left tonsil (arrow). |
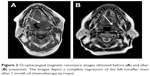
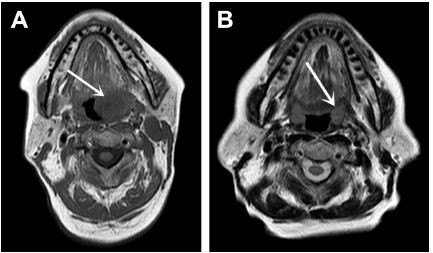
Magnetic resonance imaging (MRI) of the oropharyngeal region revealed a well-defined oval-shaped mass lesion measuring 2.5×2×1.2 cm on the left tonsil, as well as metastatic enlargement of the cervical lymph nodes (Figure 2A). A computed tomography (CT) scan of the chest revealed a polypoidal lesion in the upper lobe of the right lung and enlargement of the multiple mediastinal lymph nodes. Additionally, positron emission tomography (PET)-CT identified a heterogeneously enhanced and highly metabolic mass lesion on the left tonsil, as well as metastatic spread to the right lung, mediastinal lymph nodes, left cervical lymph node, and fourth lumbar (L4) vertebra body (Figure 3A).
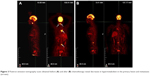  | Figure 3 Positron emission tomography scans obtained before (A) and after (B) chemotherapy reveal decreases in hypermetabolism in the primary lesion and metastases (arrows). |
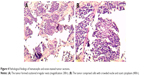
A biopsy specimen was then collected from the primary lesion under local anesthesia. Pathological findings of the lesion in the left tonsil confirmed a single tumor type. Histologically, the biopsy specimens of the primary lesion revealed small round-to-oval-shaped tumor cells scattered in irregular nests (Figure 4A). At high magnification, these malignant neoplasms were found to have crowded nuclei with hyperchromatism and scant cytoplasm (Figure 4B). The immunohistochemistry findings were as follows: the cells showed positivity for synaptophysin, CD56, and cytokeratin 8/18 and negativity for vimentin, S-100, CD10, and P63 (Figure 5), as well as a Ki-67 proliferation index of >50% (+).
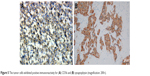  | Figure 5 The tumor cells exhibited positive immunoreactivity for (A) CD56 and (B) synaptophysin (magnification: 200×). |
From the medical history, clinicopathologic data, and PET-CT findings, the abnormalities in the lymph nodes and L4 vertebral body were considered to be metastatic carcinomas. Accordingly, a final diagnosis of SNEC with multiple metastases was confirmed. The patient refused surgery and radiotherapy and was administered 6 cycles of adjuvant chemotherapy comprising 60 mg/m2 etoposide on days 1–3 and 50 mg/m2 cisplatin on day 1 at 21-day intervals. MRI of the oropharyngeal region verified an excellent tumor response after 1 month of treatment (Figure 2B). PET-CT revealed significant decreases in the standardized uptake value (SUV) of the fluorodeoxyglucose tracer (Figure 3B) in the right lung and L4 vertebra body, as well as a decrease in the SUV of the primary lesion from 8.7 to 1.9. Surprisingly, the patient has been followed regularly for 1 year and has achieved a CR of the tonsil and cervical lymph node lesions.
Discussion
As noted above, primary SNEC is an unusual type of neuroendocrine tumor, particularly in the head and neck, and is rarely described in the literature. SNEC accounts for only 11%–16% of all extrapulmonary small cell carcinomas,2 and oropharyngeal tumors account for only 12.3% of all cancers in the head and neck.6 SNEC often presents in older patients (age: 50–66 years) without sex bias; however, the majority of cases in the tonsil involve male patients, with a male to female ratio of 1.75:1.10 Therefore, our current case is not consistent with the existing pattern.
The clinical symptoms of SNEC vary, although patients commonly experience pain, dysphagia, and obstructive sleep apnea syndrome. In the present case, the patient only experienced throat pain without specific clinical characteristics and was misdiagnosed with a peritonsillar abscess. Given these nonspecific symptoms and broad clinical spectrum of manifestation, the diagnosis of SNEC mainly depends on a histologic and immunohistochemical examination.11,12 Here, the expression of neuroendocrine markers such as synaptophysin, CD56, and chromogranin A will facilitate a diagnosis.13 In the present case, the tumor met the necessary histological criteria and exhibited high levels of synaptophysin, CD56, and Ki-67 upon immunohistochemical analysis.
Previously, SNEC has been observed to follow a short course and tend to metastasize at an early stage, in contrast to other head and neck cancers.14 To the best of our knowledge, only 15 cases of SNEC of the tonsil have been reported in the English literature, of which 9 had metastasized from primary sites.6,10 Accordingly, few data are available concerning primary tumors of the head and neck with multiple metastases.
Image detection plays an important role in the diagnosis of head and neck lesions, particularly in the exclusion of regional invasion or distant metastasis. PET-CT is highly valuable for diagnosis and treatment planning because it can confirm the presence of both the primary tumor and metastatic lesions based on tracer uptake.15 In this case, PET-CT confirmed a metabolically active mass on the left tonsil, as well as extensive metastases affecting the right lung, mediastinal lymph nodes, left cervical lymph node, and L4 vertebral body. Last but not least, PET-CT can be used as the primary means of evaluating the effects of treatment.16,17
Kao et al reported a 5-year relative survival of only 20.8% among SNEC patients.18 The rarity of SNEC of the oropharyngeal region and relatively worse prognosis when compared to other cancers affecting this region have hindered the identification of treatments that effectively improve survival. Currently, SNEC is mainly treated via surgical excision,6 although other treatments may be required depending on the tumor size, location, and systemic condition of the patient. In the present case, treatment planning is based on the poor general condition and preferences of the patient and the locations of primary and metastatic lesions. Previously, Johnson et al concluded that etoposide is among the most active agents against small cell lung cancer,19 and another study found a new therapeutic option for lung neuroendocrine tumors.20 In the present case, the patient received 6 courses of etoposide-cisplatin chemotherapy. Following this regimen, she has achieved a CR for more than 1 year and experiences an excellent quality of life.
Conclusion
SNECs of the tonsil are rare and have not previously been reported with multiple metastases. Our findings suggest that an etoposide-based multiagent chemotherapy regimen could be an effective therapeutic strategy for unresectable SNEC in the head and neck region.
Acknowledgments
Our study received approval from the ethics committee of the Affiliated Xuzhou Hospital, College of Medicine, Southeast University. The patient and family members also provided written informed consent to publish the case report details. This study was supported by Jiangsu Province Medical Youth Talent Project (QNRC2016390) and Xuzhou Medical Creative Team Program (XWCX201604).
Disclosure
The authors report no conflicts of interest in this work.
References
Remick SC, Ruckdeschel JC. Extrapulmonary and pulmonary small-cell carcinoma: tumor biology, therapy, and outcome. Med Pediatr Oncol. 1992;20(2):89–99. | ||
Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol. 2007;34(1):3–14. | ||
Chai L, Ying HF, Wu TT, et al. Clinical features and hypoxic marker expression of primary sinonasal and laryngeal small-cell neuroendocrine carcinoma: a small case series. World J Surg Oncol. 2014;12:199. | ||
Ganesan R, Hirschowitz L, Dawson P, et al. Neuroendocrine carcinoma of the cervix: review of a series of cases and correlation with outcome. Int J Surg Pathol. 2016;24(6):490–496. | ||
Zeng M, Yang SD, Zhang JL, Chen XM. Primary small cell neuroendocrine carcinoma of the oral cavity: a case report and review of the literature. Oncol Lett. 2015;10(2):887–890. | ||
Pointer KB, Ko HC, Brower JV, et al. Small cell carcinoma of the head and neck: an analysis of the National Cancer Database. Oral Oncol. 2017;69:92–98. | ||
Aguilar EA 3rd, Robbins KT, Stephens J, Dimery IW, Batsakis JG. Primary oat cell carcinoma of the larynx. Am J Clin Oncol. 1987;10(1):26–32. | ||
Hatoum GF, Patton B, Takita C, et al. Small cell carcinoma of the head and neck: the university of Miami experience. Int J Radiat Oncol Biol Phys. 2009;74(2):477–481. | ||
Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. | ||
Wang HY, Zou J, Zhou GY, Yan JQ, Liu SX. Primary small cell neuroendocrine carcinoma of the tonsil: a case report and review of the literature. Int J Clin Exp Pathol. 2014;7(5):2678–2682. | ||
Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28(3):213–227. | ||
Xu B, Chetty R, Perez-Ordoñez B. Neuroendocrine neoplasms of the head and neck: some suggestions for the new WHO classification of head and neck tumors. Head Neck Pathol. 2014;8(1):24–32. | ||
Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85(2):74. | ||
Duan YF, Tan Y, Yuan B, Zhu F. Spontaneous rupture of hepatic metastasis from small cell neuroendocrine carcinoma of maxillary sinus. World J Surg Oncol. 2014;12:126. | ||
Chen XH, Bao YY, Zhou SH, Wang QY, Zhao K. Palatine tonsillar metastasis of small-cell neuroendocrine carcinoma from the lung detected by FDG-PET/CT after tonsillectomy: a case report. Iran J Radiol. 2013;10(3):148–151. | ||
Schouten CS, de Graaf P, Alberts FM, et al. Response evaluation after chemoradiotherapy for advanced nodal disease in head and neck cancer using diffusion-weighted MRI and 18F-FDG-PET-CT. Oral Oncol. 2015;51(5):541–547. | ||
Johnson JT, Branstetter BF 4th. PET/CT in head and neck oncology: state-of-the-art 2013. Laryngoscope. 2014;124(4):913–915. | ||
Kao HL, Chang WC, Li WY, Chia-Heng Li A, Fen-Yau Li A. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36(2):185–192. | ||
Johnson DH, Hainsworth JD, Hande KR, Greco FA. Current status of etoposide in the management of small cell lung cancer. Cancer. 1991;67(1 Suppl):231–244. | ||
Malapelle U, Morra F, Ilardi G, et al. USP7 inhibitors, downregulating CCDC6, sensitize lung neuroendocrine cancer cells to PARP-inhibitor drugs. Lung Cancer. 2017;107:41–49. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.