Back to Journals » Journal of Experimental Pharmacology » Volume 14
Success of Intralesional Purified Protein Derivative Immunotherapy in the Treatment of Anogenital Warts: A Case Report
Authors Achdiat PA , Antariksa NC , Rowawi R, Suwarsa O , Hidayat YM , Dwiyana RF , Gunawan H , Hindritiani R
Received 2 November 2021
Accepted for publication 22 March 2022
Published 4 April 2022 Volume 2022:14 Pages 131—135
DOI https://doi.org/10.2147/JEP.S347241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
Pati Aji Achdiat,1 Narizka Civiadenta Antariksa,1 Rasmia Rowawi,1 Oki Suwarsa,1 Yudi Mulyana Hidayat,2 Reiva Farah Dwiyana,1 Hendra Gunawan,1 Reti Hindritiani1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran-Dr.Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 2Department of Obstetry and Gynecology, Faculty of Medicine, Universitas Padjadjaran-Dr.Hasan Sadikin Hospital, Bandung, West Java, Indonesia
Correspondence: Pati Aji Achdiat, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr.Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +6281322750101, Email [email protected]
Abstract: Anogenital warts (AGW) are among the most common sexually transmitted infections worldwide. The condition may be persistent, increase in size and number, and have a high recurrence rate. There are many therapeutic options of AGW, but none of them prevented recurrence, only yielded partial responses and have the propensity to cause scars. Immunotherapy by purified protein derivative (PPD) is one of the therapeutic options for AGW, which effectively reduces the number of lesions until complete clearance, with minimal side effects and less recurrence rate. This case report aims to demonstrate the effectiveness, safety, and low recurrence rate of intralesional PPD injection as an alternative therapy for AGW. We reported one case of AGW in an immunocompetent 30-year-old homosexual man who was given 3 doses of 0.2 mL PPD injected intralesionally. As a result, clinical improvement was observed starting from the 18th day, with some of the lesions decreasing in size, and on the 46th day, all of the lesions disappeared. There was no significant side effect. Within two years of follow-up, no recurrence was observed. Intralesional injection of PPD can stimulate the immune response against human papillomavirus (HPV) infection both on the injection site and distant from the injection site. Previous studies have shown promising results of intralesional PPD, with low recurrence in over six-month follow-up and no side effects. Intralesional injection of PPD can be considered as an alternative therapy due to its minimal side effects and its long-term low recurrence rate.
Keywords: anogenital warts, immunotherapy, intralesional purified protein derivative, long term observation
Introduction
Anogenital warts (AGW) are caused by Human papillomavirus (HPV) infection, which is transmitted through sexual contact, affecting the genital and anal areas.1,2 There are many therapeutic options for the treatment of AGW, but most of them are essentially painful, may leave scars, and cannot prevent recurrence.1–3 Despite its spontaneous resolution in 2 months to 2 years, AGW spreads and increases in size and number in many cases. Currently, a newer form of therapeutic approach known as immunotherapy has been used to treat AGW with promising results. This includes immunotherapy with intralesional injection of purified protein derivative (PPD), which works as an immunomodulator2 and stimulates the immune system against lesions not only on the site of injection but also on areas distant from the injection site. This treatment offers long-term immunity with minimal side effects.4 Purified protein derivative is a sterile protein extract from Mycobacterium tuberculosis. Purified protein derivative antigens are injected into the AGW lesion, which stimulate immune response not only against M. tuberculosis but also against HPV, resulting in warts healing.3,5 Recurrence is minimal in immunotherapy, compared to conventional therapy which has recurrence rate 20−30%.1,5 The longest reported observation duration of PPD treatment for AGW and cutaneous warts was eight months after treatment with no recurrence.6 This case report aims to demonstrate the effectiveness, safety, and recurrence rate in long-term observation up to two years after intralesional injection of PPD as a treatment for AGW.
Case Presentation
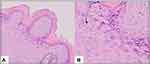
A 30-year-old homosexual male presented with multiple skin-colored papules on the anal area without any itch or pain. There was a history of repeated homosexual-anogenital exposures with the patient being the receptive partner. There was no history of other STIs symptoms. The lesion initially appeared six months prior to the patient’s visit as one skin-colored papule on the anal region, and two months later, the papules increased in size and multiplied. The patient was previously treated with 80% trichloroacetic acid (TCA) for two weeks, but there was no improvement. Physical examination showed multiple, nontender, moist, 0.1–0.8 cm in diameter, verrucous papules around the anal verge (Figure 1A). There was no genital, oral, or skin lesions. Hair, nails, palms, and soles were normal. No lymphadenopathy was found. Acetowhite test showed a positive result. Tuberculin skin test revealed a 15 mm diameter of induration. Chest radiography was within normal limits. Blood serum examination for venereal disease research laboratory (VDRL), Treponema pallidum hemagglutination assay (TPHA), and human immunodeficiency virus (HIV) were non-reactive. Based on the patient’s history and physical examination, a diagnosis of anogenital warts was established. The patient was then treated with three doses of 0.2 mL of PPD-2TU (tuberculin unit) per week for three sessions, injected intralesionally. The warts injected in one lesion in each treatment session. The patient initially reported pain on the site of injection, which diminished in less than two hours without administration of analgesics. There were no signs of swelling, nodules, blisters, or abscesses on the injection site, nor flu-like symptoms. Some of the AGW lesions decreased in size on the 18th day after initial injection (Figure 1B), and on the 46th day after the initial injection, all of the lesions disappeared (Figure 1C) without leaving scars. Within two years of observation, there was no evidence of recurrence. The histopathologic result from the lesion before starting injection was released showed hyperkeratosis with koilocytes, which consistent and confirmed the final diagnosis as anogenital warts (Figure 2A and B).
Discussion
Anogenital warts are among the most common clinical manifestations of HPV infection in the genital area, which mainly occurred in developed countries, and has strong evidence for an association with anogenital cancers.2 Highest rate of AGW are occurred among women aged 20−24 years and men aged 25−29 years.7 HPV seroprevalence is approximately 2−6 times higher among men who have sex with men (MSM) than heterosexual men and has been associated with 80−85% of anal cancers.8 Anal warts are more common in MSM with history of condomless anogenital contact or other sexual behavior with anal penetration,1 with incidence 10 times higher among HIV patient.2 There are several other risk factors for HPV infection, including genital contact, early coitarche, multiple sex partners, and history of sexually transmitted infections.9 Genital HPV can be transmitted via sexual and non-sexual route.4 Transmission rates of HPV between sexual partners are high and can occur without any visible warts.1
The clinical form of AGW have a highly variable contour appearance as papules or verrucous which may be flat or pedunculated.10 The lesion may vary in color and appearance, ranging from white to pink, purple, red, or skin-colored.1,10 The diagnosis of AGW is usually established based on the characteristic of skin lesions.7 However, in some cases, when the lesions do not respond to standard therapy or become worsened during therapy, or are suspected as malignancy, a biopsy may be indicated.1,2,7
The primary treatment options to date for AGW is to remove the warts, rather than eliminate the underlying infection,10 which makes recurrence a common problem.3,6 However, the choice of AGW therapy can be considered based on the number, size, location, morphological lesions, patient preference, convenience, physician experience, cost, and side effects.11 There are a variety of treatment options for AGW including surgical procedures such as electrocautery, excision surgery, cryotherapy, and laser, and non-surgical treatment such as podophyllin, podophyllotoxins, trichloroacetic acid, and immunotherapy.12 Treatment with electrocautery, excision surgery, cryotherapy, trichloroacetic acid may be undertaken in the event of anal warts.13 However, in various studies, pain, burning, crust, ulceration, infection, permanent scarring have all been reported as side effects of their therapy.10,11,13 In this case, the patient had been given trichloroacetic acid therapy after twice therapy, but it showed no change and the patient refuse to repeat the trichloroacetic acid therapy. The choice of therapy using immunotherapy is known to have a lower risk of causing scar tissue, with a relatively rare recurrence rate.14
Immunotherapy is a biological therapy that can stimulate the immune system against infection, cancer, and other diseases. Several immunotherapy agents which previously have been used for the treatment of AGW including cimetidine, imiquimod, interferons, Candida albicans antigens, measles, mumps, rubella (MMR), intralesional vitamin D, Bacillus-Calmette-Guerin (BCG), and PPD,3–5 which works by stimulating the immunologic response against warts proliferation caused by HPV infection, resulting in modulating HPV-induced proliferation.6 Thappa et al15 suggested the indications for immunotherapy as AGW treatment including recalcitrant warts, extensive lesion, lesion in areas that are difficult to treat, and recurrent lesion.
Several studies have reported that PPD is effective and safe in treating recalcitrant warts (multiple warts, extensive lesion, palmoplantar, periungual, and genital types) via the intralesional route.3–5,14 Intralesional immunotherapy such as PPD injection has been used to stimulate the immune response against HPV infection on the site of injection and distant lesions, eradicating all warts.17 PPD stimulates a strong systemic immune response to produce proinflammatory signals such as the release of cytokines Interferon gamma (IFN-δ) and interleukin (IL)-12, a T-helper 1 pattern cytokines, activates cytotoxic T cells, and natural killer (NK) cells against HPV infection.16,17 Puri et al16 evaluated the efficacy of PPD in the treatment of recalcitrant warts in 25 patients, including 2 AGW patient. The patients were given three doses of 0.25 mL intralesional PPD every three weeks. The study reported complete response in 18 patients (72%), partial response in 4 patients (16%), and no response in 3 patients (12%). Another study by Kus et al18 reported PPD efficacy on 13 patients with history of warts for at least two years, unresponsive to at least one conventional treatment of AGW, and positive tuberculin skin test with induration of ≥ 0.5 cm in 48 hours. The PPD dose is determined according to the size of the induration which is 0.3 mL (0.5−0.9 cm), 0.2 mL (1−1.5 cm), and 0.1 mL (> 1.5 cm). The study reported complete clearance in 2 patients, partial response in 5 patients, minimal response in 4 patients, and no response in 2 patients. Other studies also suggested time intervals for administering PPD in the treatment of AGW, which can be given weekly.15
Various studies have been conducted to compare the effectiveness of PPD with other agents as immunotherapy for warts. A study by Lahti and Hannuksela who used topical tuberculin or PPD jelly in treatment of warts in 14 patients, 8 patients (57%) showed complete clearance in 3−4 months.4 A study comparing the efficacy of intralesional BCG and PPD for the clearance of warts showed the BCG group had complete resolution in 30.8% of the patients, while the PPD group showed complete resolution in 35.3% of the patients. The study showed PPD may be slightly more effective than BCG.5 Another study comparing the efficacy of intralesional vitamin D3 and PPD showed complete clearance 72.5% of subjects in the vitamin D3 group, while PPD group showed complete clearance 80% of subjects. Both intralesional vitamin D3 and intralesional PPD immunotherapy showed significant results in treating viral warts, but PPD was found to be superior to vitamin D3.3 A study by Shaheen et al17 compare the efficacy of intralesional MMR and PPD in treating multiple warts and showed complete response in 80% of subjects in the MMR group and 60% of subjects in PPD group.17 In this case, we used 0.2 mL intralesional PPD injections immunotherapy as a treatment for AGW with a weekly interval for three sessions after the lesion failed to respond to TCA. The treatment response was firstly noticed on the 18th day after initial treatment, while complete clearance (100%) was achieved after the third injection, 46th day after the first injection.
Furthermore, the main advantages of immunotherapy are lower risk of scarring and lower recurrence rate.14 In several studies, common side effects after administration of PPD include rashes, swelling, pain or discomfort in the area of injection, and fever.14,15 A severe side effect that could be found includes the development of painful nodules, which could turn into blisters or abscesses on the site of injection, but the incidence was rare.3 Previous studies on anogenital and common warts showed no recurrence after eight months posttreatment,6,15 In our study, the side effect was mild pain at the site of PPD injection, which disappeared after a few hours and there was no recurrence within two years of observation.
Conclusion
Overall, PPD is an effective, safe, and well-tolerated mode of treatment for AGW with minimal side effects. It also offers long-term protection against HPV infection, although further studies are needed to confirm this finding.
Ethic Statement
This study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practices, local regulatory requirements, and was approved by the Medical Ethics Committee of Hasan Sadikin General Hospital Bandung (approval number: LB.02.01/X.6.5/234/2021).
Consent Statement
The patient signed informed consent forms. He also signed forms giving consent for the use of case details and images for publication and for scientific purposes.
Acknowledgments
The authors would like to thank all staff of the Dermatology and Venereology Department, Faculty of Medicine Universitas Padjadjaran – Hasan Sadikin General Hospital Bandung, West Java Indonesia.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Gilson R, Nugent D, Werner RN, Ballesteros J, Ross J. European guideline for the management of anogenital warts; 2019.
2. Golusin Z. Genital warts: new approaches to the treatment. Serb J Dermatol Venerol. 2009;1(3):107–114. doi:10.2478/v10249-011-0010-3
3. Singh SK, Mohan A, Gupta AK, Pandey AK. A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts. Int J Res. 2018;4(2):197.
4. Gupta K, Jaiswal A, Sharma RP, Bedi G. Immunotherapy with PPD in treatment of warts: an open labelled study from western Uttar Pradesh. Indian J Clin Exp Dermatol. 2019;5(1):41–45. doi:10.18231/2581-4729.2019.0010
5. Rajashekar TS, Amulya R, Sathish S, Kumar S. Comparative study of intralesional BCG and PPD in the treatment of multiple cutaneous warts. Indian J Clin Exp Dermatol. 2018;4(1):1–6.
6. Eassa BI, Abou‐Bakr AA, El‐Khalawany MA. Intradermal injection of PPD as a novel approach of immunotherapy in anogenital warts in pregnant women. Dermatol Ther. 2011;24(1):137–143. doi:10.1111/j.1529-8019.2010.01388.x
7. Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infec Dis. 2015;61(8):849–855. doi:10.1093/cid/civ813
8. Blas MM, Brown B, Menacho L, Alva IE, Silva-Santisteban A, Carcamo C. HPV prevalence in multiple anatomical sites among men who have sex with men in Peru. PLoS One. 2015;10:1–9. doi:10.1371/journal.pone.0139524
9. Juckett G, Hartman AH. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;10:1209.
10. Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5(6):25.
11. Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician. 2004;70(12):2335–2342.
12. Jeo W, Sugiharto B, Kekalih A. Perianal condyloma acuminata: factors that contribute to the recurrence. Am J Surg. 2018;2(3):31–33.
13. Lopaschuk CC. New approach to managing genital warts. Can Fam Physician. 2013;59(7):731–736.
14. Puri N. A study on the efficacy of immunotherapy with purified protein derivative for the treatment of recalcitrant warts. Nepal J Dermatol. 2017;15(1):12–16.
15. Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian J Dermatol. 2016;7(5):364. doi:10.4103/2229-5178.190487
16. Shaheen MA, Salem SA, Fouad DA, El‐Fatah AA. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: a comparative clinical and immunological study. Dermatol Ther. 2015;28(4):194–200. doi:10.1111/dth.12230
17. Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19(4):515–516. doi:10.1111/j.1468-3083.2004.01176.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.