Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Subsection Laminectomy with Pedicle Screw Fixation to Treat Thoracic Ossification of Ligamentum Flavum: A Comparative Analysis with Lamina Osteotomy and the Replantation Technique
Authors Zhang J, Lei T, Yang L, Lin YS, Wang ZH, Cao JM
Received 24 October 2019
Accepted for publication 4 March 2020
Published 17 April 2020 Volume 2020:16 Pages 311—319
DOI https://doi.org/10.2147/TCRM.S235868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Jing-tao Zhang, Tao Lei, Liu Yang, Yong-Sheng Lin, Zhi-Hong Wang, Jun-Ming Cao
Department of Orthopedics, The Third Hospital of HeBei Medical University, Shijiazhuang, People’s Republic of China
Correspondence: Jun-Ming Cao
Department of Orthopedics, The Third Hospital of HeBei Medical University, 139 Ziqiang Road, Shijiazhuang 050051, People’s Republic of China
Tel +86-311-88602016
Fax +86-311-87023626
Email [email protected]
Background: There are many surgical procedures that can be used to relieve compression caused by thoracic ossification of the ligamentum flavum (TOLF). The present study aims to retrospectively observe the differences in subsection laminectomy with pedicle screw fixation (SLPF) and lamina osteotomy and replantation with miniplate fixation (LORF) in the treatment of continuous TOLF.
Patients and Methods: From March 2014 to October 2017, 61 patients with continuous TOLF underwent SLPF (group A) or LORF (group B). The surgical duration, intraoperative blood loss, change in thoracic kyphosis, and perioperative complications were analyzed. Neurological function was evaluated in accordance with the Japanese Orthopedic Association (JOA) score and the American Spinal Injury Association (ASIA) neurological grading.
Results: The surgical duration, intraoperative blood loss, and postoperative bed-rest duration in group A were significantly lower than those observed in group B (P < 0.05). Both groups demonstrated a significant improvement in JOA score and ASIA grade (P < 0.05). The neurological recovery rate was 69.8% ± 13.5% in group A and 68.5% ± 12.7% in group B (P > 0.05). There was also a significant improvement in ASIA grade at the final follow-up (P < 0.05). During follow-up, the Cobb angle was significantly increased in group B (P < 0.05), whereas no significant difference was observed in group A (P > 0.05). The occurrence rate of perioperative complications was 15.6% (5/32 patients) in group A and 37.9% (11/29 patients) in group B (P < 0.05).
Conclusion: Both SLPF and LORF significantly promote recovery of neurological function. SLPF has a shorter surgical duration, less intraoperative blood loss, and a lower complication rate. SLPF is more conducive to the correction of sagittal sequence and maintenance of thoracic stability.
Keywords: thoracic ossification of the ligamentum flavum, thoracic vertebra, posterior decompression, internal fixation, therapeutic effect analysis
Introduction
Thoracic ossification of the ligamentum flavum (TOLF) is one of the most common causes of thoracic spinal stenosis and accounts for approximately 61% of cases. TOLF can cause compression of corresponding spinal cord segments, numbness, and weakness in both lower limbs, abnormal gait, dyskinesia, sphincter dysfunction, and lower limb paraplegia.1,2 With the popularization of large-scale imaging equipment and a deeper understanding of the disease, the number of clinical reports into TOLF has increased.1–4 Epidemiological studies have shown that it is particularly prevalent in the Asian population.3,4 Conservative treatment is usually ineffective for TOLF, so surgical treatment is widely accepted as the best approach.5–9 Posterior spinal canal resection and decompression are among the most frequently used surgical techniques, including laminae delamination, 5 lamina fenestration technique,6 open-door or French-door laminectomy,7 en-bloc resection,8,9 and lamina osteotomy and replantation.10
Each technique has specific characteristics. As a classical operation, the delamination method of decompression is easy to perform, but the surgical forceps must invade the vertebral canal continuously during lamina removal. The decompression range of lamina fenestration techniques is small, and the effect of decompression is uncertain for patients with fused-type TOLF.11 In clinical practice, the applications of open-door laminectomy and French-door laminectomy are considerably fewer. With this technique, the laminae are opened at a certain angle, but the ligamentum flavum inside the lamina is not removed, which presents a risk for a long-term decompression effect. The decompression method of the en-bloc resection technique and lamina osteotomy and replantation with miniplate fixation (LORF) has the same procedure in decompression; however, with LORF, after the ossification of the medial lamina is removed, the lamina can be replanted in situ with the help of a mini titanium plate. In this way, the integrity of the spinal canal is maintained, and compression of the spinal cord by scar tissue is avoided. However, the surgical procedure is complex, and the incidence of dural injury is high after long-segment holistic excision.
To solve the aforementioned difficulties, we aimed to improve en-bloc resection. The lamina was excised using a segmental method, and a pedicle screw was implanted to fix the decompression area. Then, a subsection laminectomy with pedicle screw fixation (SLPF) was performed. To verify the efficacy of SLPF, patients who had undergone SLPF and LORF were compared, and the differences between the two groups were assessed.
Patients and Methods
General Information
Clinical data from 72 patients with continuous TOLF who underwent thoracic posterior spinal decompression from March 2014 to October 2017 were retrospectively analyzed. Sixty-one patients participated in a complete clinical follow-up (>12 months). Patients were divided into group A and group B according to the surgical method they had undergone. There were a total of 32 patients in group A (SLPF group): 15 males and 17 females, with an average age of 56.2 ± 13.9 years and an age range of 40–70 years. The average disease course was 17.5 ± 4.6 months with a range of 10–26 months. There were a total of 29 patients in group B (LORF group): 12 males and 17 females, with an average age of 55.6 ± 13.2 years and an age range of 41–69 years. The average disease course was 16.9 ± 4.3 months with a range of 9–27 months. Patients’ data are shown in Table 1, including age, sex ratio, disease course, incidence of diabetes mellitus, body mass index, intramedullary increased signal intensity (ISI), and number of OLF levels. This study was approved by the ethics committee of the third hospital of Hebei Medical University. Patient consent for review of medical records was not required because all data were de-identified. All protocols were conducted in accordance with the research principles outlined in the Declaration of Helsinki.
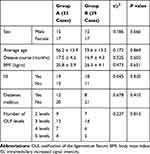 |
Table 1 A Comparison of the Preoperative General Condition Between the Two Groups |
Inclusion and Exclusion Criteria
Inclusion criteria include the following: (1) symptoms, signs, and imaging data consistent with thoracic spinal stenosis; (2) a follow-up period of >12 months and completion of imaging data both prior to and after surgery; (3) continuous TOLF for ≥2 levels; (4) normal cardiopulmonary, hepatic, and kidney function and blood sugar and blood pressure within the normal range after surgery; and (5) an informed understanding of the study and willingness to participate.
Exclusion criteria include the following: (1) accompanying cervical and lumbar spinal stenosis; (2) TOLF combined with other thoracic vertebral diseases (e.g., TOLF combined with thoracic disk herniation or OPLL); and (3) accompanying thoracic vertebral fracture, infection, tumor, or abnormal coagulation.
Imaging Data
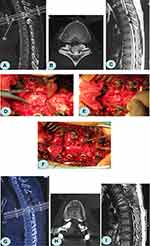
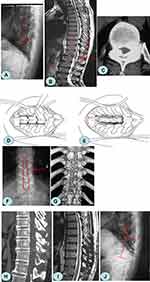
All patients were examined by X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) before and after surgery. Lateral X-ray generally determined the location of the TOLF lesion, which was revealed by the presence of a high-density shadow from the lamina to the nerve root canal. CT was used to observe the degree of ossification, morphology, the affected area of the ligamentum flavum (Figure 1A and B), and the decompression effect (Figure 1G and H). MRI showed that the low-signal tissue invaded the vertebral canal from back to front, and the thoracic spinal cord was compressed and thinned (Figure 1C). At the same time, ISI could be observed on T2-weighted MRI, and the patency of the spinal canal could also be observed (Figure 1I).
Surgical Techniques
All surgeries were performed by the same senior surgeon (Dr. Cao). The operation was performed under the monitoring of somatosensory-evoked potentials. After induction of general anesthesia, patients assumed a prone position, and a posterior median incision was made with the lesion at its center. Bilateral paravertebral muscles were dissected to expose the lamina, articular processes, and bilateral transverse processes.
Group A
A pedicle screw was inserted into the lesion segment (Weigao Orthopedic Device Co Ltd., Weihai City, China). The supraspinous and interspinous ligaments at the head and tail of the decompression area were dissected transversely, and the spinous process, and attached ligament were removed. A high-speed grinding drill (3 mm in diameter) (Stryker corporation, Michigan, USA) was used to bilaterally groove the inner cortex along the lamina and transverse process, and bone-biting forceps were used to transversely separate the tissue from the middle of the upper and lower lamina to the inner cortex. A towel clamp was used to clamp the root of the spinous process, which was then lifted, and the residual medial cortex of the lamina was removed using ultrathin laminectomy forceps. A bone pry was inserted into the marginal gap of the free lamina. The tail was pressed to retract the lamina (Figure 1D). The adhesion between the ossified ligamentum flavum and the dura mater was separated using a nerve dissector, and a sharp knife was used if necessary. Finally, the lamina and ligamentum flavum were excised and removed (Figure 1E). The laminae were removed from the top to the bottom by segmental excision (Figure 1F). After decompression, the lamina was pruned into bone granules and implanted into the bilateral transverse processes. A titanium rod of a suitable length was pre-bent and placed into the U-groove of the pedicle screw, and the screw was tightened and fixed.
Group B
Bilateral grooves were made along the medial portion of the facet joints. The outer cortex was removed using a high-speed grinding drill, and the inner cortex of the lamina was polished to paper thickness. The supraspinal ligament, interspinous ligament, and ligamentum flavum adjacent to the decompression area were dissected and removed. A towel clamp was used to clamp the root of the spinous process, which was lifted up, and the residual medial cortex of the lamina was removed using ultrathin laminectomy forceps. The whole free lamina was lifted slowly. The adhesion between the ossified ligamentum flavum and the dura mater was separated using a nerve dissector, and a sharp knife was used if necessary. The ossified ligamentum flavum of the medial lamina was removed with bone-biting forceps. An appropriate mini titanium plate length was selected, and screws were fixed onto the base of the spinous and transverse processes. The bone defect at the outer edge of the lamina was covered with allogenic bone strips (Figure 2).10
Evaluation of the Curative Effect
The modified Japanese Orthopedic Association (JOA) 11-point scoring system was used to evaluate the spinal cord condition of patients both before and after surgery, which excluded the sections associated with upper extremity function.12 The neurological recovery rate was calculated as follows: postoperative JOA score − preoperative JOA score)/(11 − preoperative JOA score) × 100%. The American Spinal Injury Association (ASIA) neurological grading10 was used to determine the recovery of neurological function. Grade A indicated that nerve damage was the most serious complication, and grade E indicated the lightest damage. Determination of local kyphosis was achieved by measuring the Cobb angle between the upper endplate of the last vertebral body and the lower endplate of the next vertebral body at the lesion level.2
Statistical Analysis
SPSS 20.0 statistical software was used to analyze the data. A variance analysis of repeated measurements or a paired t-test was used to compare the data. An χ2 test was used to compare the count data. A Kruskal–Wallis rank-sum test was used to analyze rank data. Data were expressed as mean ± standard deviation. Two-sided P-values of <0.05 were considered statistically significant.
Results
In group A, the surgical duration was 205.6 ± 38.7 min, intraoperative blood loss was 478.1 ± 125.3 mL, and postoperative bed-rest time was 3.7 ± 0.6 days. In group B, the surgical duration was 228.5 ± 43.2 min, intraoperative blood loss was 564.3 ± 171.9 mL, and postoperative bed-rest time was 5.3 ± 1.1 days. There was a significant difference between the two groups (P < 0.05) (Table 2).
 |
Table 2 Comparison of Intraoperative Data and Complications Between the Two Groups |
Three months after surgery and final follow-up, the JOA score had significantly improved in both groups (P < 0.05), and there was no significant difference at different time points (P > 0.05). The recovery rate of neurological function was 69.8% ± 13.5% in group A and 68.5% ± 12.7% in group B; there was no significant difference between the two groups (P > 0.05). According to the ASIA neurological grading at an average follow-up duration of 17.4 months, both groups demonstrated a significant improvement in ASIA grade (P < 0.05). There was no significant difference at the final follow-up between the two groups (Z = 0.531, P = 0.595) (Tables 3 and 4).
 |
Table 3 Changes in JOA Score and Cobb Angle Before and After Surgery in the Two Groups |
 |
Table 4 Comparison of ASIA Grading Before and After Surgery in the Two Groups (n) |
The local Cobb angle in group A was significantly corrected after surgery (F = 3.941, P = 0.023), whereas the local Cobb angle was significantly increased during follow-up in group B (F = 0.547, P = 0.033). There was a significant difference between the two groups at different time points after surgery (P < 0.05) (Table 3).
After surgery, there were three cases of cerebrospinal fluid leakage, one case of wound infection, and one case of lower extremity venous thrombosis in group A. The complication incidence was 15.6% (5/32 patients). There were five cases of cerebrospinal fluid leakage, one case of hypostatic pneumonia, one case of lower extremity venous thrombosis, one case of neurological deterioration, one case of epidural hematoma, and two cases of internal fixation loosening in group B. The complication incidence was 37.9% (11/29 patients). There was a significant difference between the two groups (P < 0.05) (Table 2).
Discussion
TOLF usually begins with the ligamentum flavum at the zygapophysial joints and gradually forms a nodular ossification process that projects into the spinal canal and extends to the ligamentum flavum at the central portion of the spinal canal. Sometimes bilateral ossified nodules fuse with each other and occupy space in the vertebral canal from the posterior side, which leads to osteogenic spinal stenosis. After spinal cord compression, various degrees of neurological impairment can occur.3,7 Guo et al4 conducted cross-sectional screening of 1736 volunteers using MRI, which revealed that 3.8% (66/1736) of subjects suffered from OLF. Multi-segmental OLF accounted for 31.8% (21/66) of cases, whereas continuous multi-segmental OLF accounted for 16.7% (11/66) of cases. Anatomically, the upper portion of the ligamentum flavum attaches to the anterior portion of the lower two thirds of the upper lamina, and the lower portion of the ligamentum flavum attaches to the posterior portion and the upper edge of the lower one third of the lower lamina. One third of the medial surface of each lamina is classed as a “blank area” without ligamentum flavum attachment.3 Even if ossification of the ligamentum flavum is severe, it is remain a normal lamina structure in this area, it is referred to as the so-called first safe area for surgical decompression. The ossification is not serious if it resides in the extension between the bilateral laminae and the transverse transition and is thus called “the second safe area” for surgical decompression.
Wang et al13 defined the two adjacent segments of the lamina and facet joints of each ossified ligamentum flavum as “decompression segments,” and the range of decompression includes ossified ligamentum flavum, adjacent head lamina, and the upper half of the tail lamina. Multi-segment TOLF can be carried out from top to bottom according to the “decompression segment”. Subsection laminectomy with SLPF is under the guidance of this concept. The lamina was dissected transversely in the first safe area and longitudinally in the second safe area, allowing the lamina to reside in a free state. LORF is also a widely used surgical method in the clinic. It is mainly used to treat benign tumors of the spinal canal.14 After improvement, LORF has been applied to the treatment of TOLF.10,15 First, bilateral grooves are made in the second safe area, then the head and tail laminae and adjacent structures are dissected and removed. After the ossified ligament tissue was removed, in situ replantation was performed. One question that this study aimed to address was whether there were any differences between the two methods in terms of their clinical efficacy and imaging performance.
In this study, group B had a larger number of surgical steps, and its surgical duration and volume of intraoperative blood loss were significantly greater than those observed in group A. To assess recovery of neurological function, the JOA scores significantly improved after surgery in the two groups, and there was no significant difference between the two groups at different time points. The recovery rate in group A was 69.8% ± 13.5% and in group B was 68.5% ± 12.7%; no statistical significance was observed. According to the ASIA scale, at an average follow-up duration of 17.4 months, both groups demonstrated a significant improvement in ASIA grade, and there was no significant difference between the two groups at the last follow-up. This observation indicated that both SLPF and LORF can completely relieve spinal cord compression and significantly promote recovery of motor and sensory function in the lower limbs. However, after several years of clinical observation, spinal surgeons found that LORF presented a higher surgical risk and incidence of complications.5,10
According to Chen et al,5 during the overall lifting process of long-segment laminae, three patients had a dural sac laceration with an incidence rate of 18.8% (3/16 patients). Moreover, one patient presented with lower limb paralysis after surgery. In a study by Nie et al,10 the incidence of dural injury was greater (22.2%, 4/18 patients). One patient had lower limb paralysis after surgery. In this study, the incidence of dural tear was 9.4% (3/32 patients) in group A, and the total incidence of complications was 15.6% (5/32 patients). In group B, these figures were 17.2% (5/29 patients) and 37.9% (11/29 patients), respectively. Moreover, one case of neurological deterioration and one case of epidural hematoma were observed. We believe that, although lamina replantation can maintain the relative integrity of the thoracic spinal canal, the near-closed spinal canal is not conducive to the drainage of blood, and there is a risk that hematoma will compress the spinal cord. In addition, “seesaw” easily occurs when long-segment laminae are lifted, which cannot be completely avoided even with use of towel pliers. The upper thoracic spinal cord has a limited blood supply and a small vertebral canal buffer space, so mild compression or stimulation may cause spinal cord injury during surgery.11,16,17 Intraoperative monitoring of somatosensory-evoked potentials can reflect the status of the spinal cord in time and increase the safety of surgery.5,10,18,19
Because the stability of the thoracic vertebrae is greater than the stability of the cervical vertebrae and lumbar vertebrae, there is much controversy about whether internal fixation should be implemented after laminectomy. Aizawa et al20 found that even after laminectomy was performed and more than half of facet joints were left intact, the local Cobb angle in the decompression area increased by 3.8°, and women who underwent multilevel laminectomy (≥3 level) had a higher risk of kyphosis. Chen et al5 reported that the local Cobb angle increased by an average of 4° after laminectomy. With the aggravation of kyphosis, intraspinal pressure increases significantly.21 Kawahara et al22 and Uei et al23 believe that a stable spinal sequence plays an important role in promoting the recovery of nerve function. Effective internal fixation can maintain the stability of the spine and avoid an increase in spinal cord tension and pressure caused by aggravation of local kyphosis.23,24 In this study, pedicle screw fixation was adopted in group A, and miniplate and monocortical screw fixation was adopted in group B. With the help of pressure correction using the nail rod, the local Cobb angle in group A was significantly lower than the Cobb angle before surgery, whereas the local Cobb angle was significantly increased during follow-up in group B (P < 0.05). The pedicle screw has high biomechanical strength, and three-column fixation can be achieved, whereas the monocortical screw has weak fixation strength and has an anchoring effect rather than an orthopedic effect. Therefore, the postoperative bed-rest duration in group B was longer, and screw loosening and local Cobb angle enlargement were observed during follow-up.
Conclusions
As effective surgical methods for TOLF, SLPF, and LORF can fully decompress the spinal cord and promote recovery of neurological function. However, SLPF has a shorter surgical duration, a lower volume of intraoperative blood loss, and fewer perioperative complications when compared with LORF. Implantation of a pedicle screw is favorable for sagittal sequence correction in the decompression area and for maintenance of stability.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Takenaka S, Kaito T, Hosono N, et al. Neurological manifestations of thoracic myelopathy. Arch Orthop Trauma Surg. 2014;134:903–912. doi:10.1007/s00402-014-2000-1
2. He S, Hussain N, Li S, Hou T. Clinical and prognostic analysis of ossified ligamentum flavum in a Chinese population. J Neurosurg Spine. 2005;3(5):348–354. doi:10.3171/spi.2005.3.5.0348
3. Mori K, Kasahara T, Mimura T, et al. Prevalence, distribution, and morphology of thoracic ossification of the yellow ligament in Japanese: results of CT-based cross-sectional study. Spine. 2013;38(19):E1216–E1222. doi:10.1097/BRS.0b013e31829e018b
4. Guo JJ, Luk KDK, Karppinen J, Yang H, Cheung KMC. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine. 2010;35(1):51–56. doi:10.1097/BRS.0b013e3181b3f779
5. Chen XQ, Yang HL, Wang GL, et al. Surgery for thoracic myelopathy caused by ossification of the ligamentum flavum. J Clin Neurosci. 2009;16:1316–1320. doi:10.1016/j.jocn.2008.12.025
6. Eun SS, Kumar R, Choi WG, Cho HR, Lee SH. Lamina fenestration technique for treatment of thoracic ossified ligamentum flavum: 2-year follow-up result. J Neurol Surg a Cent Eur Neurosurg. 2017;78:286–290. doi:10.1055/s-0036-1586253
7. Wang T, Du C, Zheng X, Sun Y, Liu X, Kou J. Surgical strategies for thoracic myelopathy due to ossification of ligamentum flavum: a technical note based on radiological type. Turk Neurosurg. 2018;28:616–624.
8. Yang Z, Xue Y, Dai Q, et al. Upper facet joint en bloc resection for the treatment of thoracic myelopathy caused by ossification of the ligamentum flavum. J Neurosurg Spine. 2013;19:81–89. doi:10.3171/2013.4.SPINE12345
9. Liu X, Li T, Shi L, et al. Application of the piezosurgery in en bloc laminectomy for the treatment of multilevel thoracic ossification of ligamentum flavum. World Neurosurg. 2019;126:541–546. doi:10.1016/j.wneu.2019.03.200
10. Nie ZH, Liu FJ, Shen Y, Ding WY, Wang LF. Lamina osteotomy and replantation with miniplate fixation for thoracic myelopathy due to ossification of the ligamentum flavum. Orthopedics. 2013;36:e353–e359. doi:10.3928/01477447-20130222-26
11. Yu S, Wu D, Li F, Hou T. Surgical results and prognostic factors for thoracic myelopathy caused by ossification of ligamentum flavum: posterior surgery by laminectomy. Acta Neurochir (Wien). 2013;155(7):1169–1177. doi:10.1007/s00701-013-1694-0
12. Wang H, Wei F, Long H, et al. Surgical outcome of thoracic myelopathy caused by ossification of ligamentum flavum. J Clin Neurosci. 2017;45:83–88. doi:10.1016/j.jocn.2017.07.008
13. Wang QD, Mei W, Zhang ZH, et al. Zoning laminectomy for the treatment of ossification of thoracic ligamentum flavum. Chin J Orthop. 2018;38:778–786.
14. Menku A, Koc RK, Oktem IS, Tucer B, Kurtsoy A. Laminoplasty with miniplates for posterior approach in thoracic and lumbar intraspinal surgery. Turk Neurosurg. 2010;20:27–32.
15. Jia LS, Chen XS, Zhou SY, Shao J, Zhu W. En bloc resection of lamina and ossified ligamentum flavum in the treatment of thoracic ossification of the ligamentum flavum. Neurosurgery. 2010;66:1181–1186. doi:10.1227/01.NEU.0000369516.17394.B0
16. Li KK, Chung OM, Chang YP, So YC. Myelopathy caused by ossification of ligamentum flavum. Spine. 2002;27:E308–E312. doi:10.1097/00007632-200206150-00026
17. Yonemoto N, Ogihara S, Kobayashi Y, Sawano M, Matsuda M, Saita K. Two-staged circumferential decompression and fusion surgery for upper thoracic myelopathy caused by concurrent beak-type ossification of the posterior longitudinal ligament and ligamentum flavum at T1-T2 level: a case report. World Neurosurg. 2019;122:144–149. doi:10.1016/j.wneu.2018.10.142
18. Higgs M, Hackworth RJ, John K, Riffenburgh R, Tomlin J, Wamsley B. The intraoperative effect of methadone on somatosensory evoked potentials. J Neurosurg Anesthesiol. 2017;29:168–174. doi:10.1097/ANA.0000000000000265
19. Jorge A, Zhou J, Dixon EC, Hamilton KD, Balzer J, Thirumala P. Area under the curve of somatosensory evoked potentials detects spinal cord injury. J Clin Neurophysiol. 2019;36(2):155–160. doi:10.1097/WNP.0000000000000563
20. Aizawa T, Sato T, Ozawa H, et al. Sagittal alignment changes after thoracic laminectomy in adults. J Neurosurg Spine. 2008;8(6):510–516. doi:10.3171/SPI/2008/8/6/510
21. Farley CW, Curt BA, Pettigrew DB, Holtz JR, Dollin N, Kuntz C. Spinal cord intramedullary pressure in thoracic kyphotic deformity: a cadaveric study. Spine. 2012;37(4):E224–E230. doi:10.1097/BRS.0b013e31822dd69b
22. Kawahara N, Tomita K, Murakami H, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2008;33(1):39–46. doi:10.1097/BRS.0b013e31815e3911
23. Uei H, Tokuhashi Y, Oshima M, Maseda M, Nakahashi M, Nakayama E. Efficacy of posterior decompression and fixation based on ossification-kyphosis angle criteria for multilevel ossification of the posterior longitudinal ligament in the thoracic spine. J Neurosurg Spine. 2018;29(2):150–156. doi:10.3171/2017.12.SPINE17549
24. Wang H, Ding W. Postoperative paraplegia in patient with thoracic ossification of ligamentum flavum and thoracolumbar kyphosis derived from wedged vertebrae. World Neurosurg. 2018;119:321–324. doi:10.1016/j.wneu.2018.08.077
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.