Back to Journals » Clinical Ophthalmology » Volume 9
Submacular predominantly hemorrhagic choroidal neovascularization: resolution of bleedings under anti-VEGF therapy
Authors Dimopoulos S, Leitritz M, Ziemssen F , Voykov B , Bartz-Schmidt KU, Gelisken F
Received 4 May 2015
Accepted for publication 11 June 2015
Published 24 August 2015 Volume 2015:9 Pages 1537—1541
DOI https://doi.org/10.2147/OPTH.S87919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Spyridon Dimopoulos, Martin Alexander Leitritz, Focke Ziemssen, Bogomil Voykov, Karl Ulrich Bartz-Schmidt, Faik Gelisken
Centre for Ophthalmology, Eberhard-Karls University, Tuebingen, Germany
Purpose: To report the visual and morphological outcomes following intravitreal bevacizumab in neovascular age-related macular degeneration (nAMD) with submacular, predominantly hemorrhagic, lesions.
Methods: Retrospective study of patients with a follow-up after 1 year. All eyes with submacular hemorrhages larger than 50% of the total lesion size and received only anti-VEGF (vascular endothelial growth factor) monotherapy (intravitreous administration of 1.25 mg bevacizumab, PRN). The primary endpoint was the change in hemorrhage size and time to resolution, in association with the mean best-corrected visual acuity (BCVA). The eyes were grouped based on the size of the hemorrhage: group A (≥1 to <4 disc area [DA]), group B (≥4 to <9 DA), and group C (≥9 DA).
Results: Forty-six consecutive eyes were included. The mean area of the hemorrhage was 6 DA at baseline. Eyes with smaller bleeding (group A) had better chances of stabilized or improved vision. Complete resolution of the hemorrhage was seen in 96% of the eyes within 1 year. The mean BCVA increased from 0.81 logarithm of the minimum angle of resolution (logMAR) (95% confidence interval [CI]: 0.70–0.92) (Snellen 20/125) at baseline to 0.75 logMAR (95% CI: 0.62–0.88) (20/125) after 1 year (P=0.11). BCVA improved (one or more ETDRS [Early Treatment of Diabetic Retinopathy Study] lines) in 57% of the eyes (13/23) in group A; 53% (8/15) in group B; and 38% (3/8) in group C.
Conclusion: Many of the eyes with hemorrhagic lesions showed stabilization or improvement of the mean BCVA after treatment within 1 year. Anti-VEGF treatment can be considered as a useful treatment option in eyes with hemorrhages secondary to nAMD.
Keywords: age-related macular degeneration, bevacizumab, submacular hemorrhage
Introduction
Neovascular age-related macular degeneration (nAMD) is the main cause of legal blindness in the Western world, among people >50 years of age.1 If left untreated, submacular hemorrhage caused by nAMD leads – besides the acute vision loss – to a macular scar with irreversible vision loss.2,3
The available treatment modalities for eyes with nAMD complicated by submacular predominantly hemorrhage, include macular surgery,4 intraocular injection of recombinant tissue plasminogen activator (with or without expanding gas), treatment by intravitreal drugs, and watchful waiting.5–11 When anti-VEGF (vascular endothelial growth factor) drugs were extensively studied in the Phase III trials,12–14 all eyes with corresponding hemorrhages (>50% of lesion or >1 disc area [DA]) were excluded. Although, the outcomes of anti-VEGF monotherapy for hemorrhagic choroidal neovascularization (CNV) have been previously reported, only a few large case series with longer follow-ups are available.15–20
In this retrospective study, we report on morphological and functional outcomes following intravitreal bevacizumab monotherapy.
Methods
The charts of all nAMD patients who received bevacizumab in a tertiary referral center (University Eye Hospital at the Centre for Ophthalmology, Tuebingen) from December 2006 to November 2009, were screened, using the electronic patient records.
Inclusion criteria were: an age ≥50 years, to evaluate only lesions of nAMD including a submacular predominantly hemorrhagic CNV; the area of the submacular hemorrhage had to be at least 50% of the total lesion; and the hemorrhage itself ≥1 DA. Fluorescein angiography was used to prove evidence of CNV. Only patients who received at least one intravitreous injection of 1.25 mg of bevacizumab (after obtaining informed consent and disinfection with povidone iodine) were included. It has to be considered that the as-needed (PRN) retreatment was used sparingly, based on a decrease of visual acuity and activity seen in time-domain ocular coherence tomography.
Exclusion criteria included: all causes of the hemorrhage other than AMD (such as myopia of more than 6 D); eyes with subfoveal scar; any previous treatment of nAMD (except for nutritional agents); and combination therapies, such as vitreomacular surgery, intravitreal recombinant tissue plasminogen activator with expansive gas or air injection, intravitreal steroids, or anti-VEGF therapy other than bevacizumab.
Patients had a routine ophthalmic examination, including best-corrected visual acuity (BCVA) using ETDRS (Early Treatment of Diabetic Retinopathy Study) charts at baseline and at follow-up examinations at 4-week intervals. All eyes had fluorescein angiography and color fundus photography at baseline. Fluorescein angiograms and color fundus photographs were used for determining the area of the CNV and hemorrhage by using the digital image analysis software of Visupac (Visupac 4.2.2, Carl Zeiss Meditec AG, Jena, Germany). Color fundus photography of the macula was performed every 3 months. Eyes were categorized into three groups, based on the size of the hemorrhage. Group A (small): ≤1 and <4 DA, group B (medium): ≥4 and <9 DA, and group C (large): ≥9 DA. Optical coherence tomography (OCT; Stratus OCT, Carl Zeiss Meditec AG) was performed if the subfoveal hemorrhage was thin enough, allowing the visualization of the retinal pigment epithelial band, and determining whether the fovea was free of the hemorrhage. Indocyanine green angiography was performed at baseline, or at the follow-up examination if the physician expected additional clinical information.
Bevacizumab administration (1.25 mg/0.05 mL) was performed under sterile conditions. The retreatment was administered at 4-week intervals at the physician’s discretion. Reinjections were offered in the event of lesion activity (indicated by increased/persistent intraretinal/subretinal fluid or new hemorrhage) or vision decrease of at least one ETDRS line.
For every patient, age, sex, cardiovascular risk factors like artery hypertension and diabetes, and the use of anticoagulant medication were recorded. The patients were informed about the experimental nature of the therapy as well as the alternative treatment options. The analysis of the data was performed after the approval from the institutional ethics committee. The study was conducted in accordance with the Declaration of Helsinki.
The Wilcoxon signed-rank test was used for data not normally distributed. Results are presented as mean including 95% CI (confidence interval). The statistical software package JMP 4.0 (SAS Institute Inc, Cary, NC, USA) was used for analysis. A P-value <0.05 was considered statistically significant. BCVA was converted to the logarithm of the minimum angle of resolution scale (logMAR).
Results
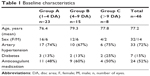
This retrospective study included 46 eyes of 46 consecutive patients (32 female, 14 male). Baseline characteristics of the patients are summarized in Table 1. The mean age of the patients was 77.2 years (standard deviation [SD]: 6.8 years; 95% CI: 75–79 years). Mean duration of the symptoms was 11.5±19 days (range: 1–45 days). Mean number of bevacizumab injections was 2.6 (95% CI: 2.4–3.0) within 12 months.
  | Table 1 Baseline characteristics |
The mean size of the hemorrhage was 6.0 DA (95% CI: 4.4–7.9 DA, range: 1.1–33.83 DA). The mean total lesion size was 7.5 DA (95% CI: 5.8–11.0 DA, range: 1.4–50.76 DA). The hemorrhage showed complete resolution in 96% (44/46) of the eyes within 1 year. The mean time of absorption for the hemorrhage was 21 weeks (95% CI: 16–27 weeks; range: 7–48 weeks).
Mean BCVA significantly increased from 0.81 logMAR (95% CI: 0.70–0.92) (20/129) at baseline to 0.68 logMAR (95% CI: 0.57–0.80) (20/96) at the 6-month examination (P=0.0034); however, the mean BCVA declined again to 0.75 logMAR (95% CI: 0.62–0.88) (20/112) by the 1-year point (P=0.11). In total, BCVA improved in 53% (24/46) of the eyes by at least one or more ETDRS line, remained unchanged in 9% (4/46), and worsened in 39% (18/46) (Table 2).
The improvement of BCVA correlated positively with the size of the hemorrhage, in favor of smaller hemorrhages (group A). The BCVA improved in 57% of eyes (13/23) in group A, but worsened in 35% (8/23). In group B and group C, improvement of the BCVA was found in 53% (8/15) and 38% (3/8) of eyes, respectively.
Discussion
In this retrospective study of 46 eyes with submacular hemorrhage, a fast resolution of the submacular hemorrhages accompanied by a moderate visual improvement, from 0.81 logMAR (20/125) at baseline to 0.75 logMAR (20/125) after 1 year. BCVA improved (one or more ETDRS lines) in 57%, 53%, and 38% of eyes with small-, medium-, and large-sized submacular hemorrhages, respectively. In almost all eyes (96%), the submacular hemorrhage showed complete reabsorption within 1 year.
The study has major limitations. The analysis was based solely on the size of CNV-induced hemorrhages, therefore not allowing potential confounding factors such as the location of CNV, the retinal thickness at baseline, and the coexistence of negative prognostic predictors to be addressed.21,22 While the treatment took place at the beginning of anti-VEGF treatment, the number of injections was quite low, in relation to modern PRN treatment algorithms (mean: 2.6). The probable undertreatment might explain the discrepancy between the visual prognosis data of other, more recent studies.21 The retrospective design and the lack of a control group have to be considered. On the other hand, the strengths of this study include the available follow-up of the treatment under real-world conditions for 1 year, and the inclusion criteria that restricted the study to treatment-naïve eyes.
Nevertheless, the observations can provide some information about the prognosis (in spite of the likely undertreatment). No recurrences of hemorrhages or vitreous hemorrhages were seen in this cohort, contributing to a high rate of blood-free lesions after 1 year. Newer reports have proven that the presence of blood leads to damage of the outer retina, apparent in the interruption of the ellipsoid zone.23,24 When discussing treatment alternatives to anti-VEGF drugs, the location of the hemorrhage in relation to the pigment epithelium might be detrimental, as massive bleedings under the pigment epithelium are more closed to surgical interventions.4
The natural course of submacular hemorrhage in AMD is poor.2,3 In a retrospective study, 60 eyes in Italy and France had a decrease of the visual acuity from 20/240 to 20/1250 after a follow-up of 16 months, 80% of all eyes lost vision.25 Another case series reported a median decrease in vision of four ETDRS lines in three years.2 In the Subretinal Surgery Trial (SST), the only prospective randomized study in these patients, the observation group with a hemorrhage showed a median baseline BCVA of 20/200, and thereafter, 20/500, 20/500, and 20/640 after 1, 2, and 3 years, respectively.4 Median loss of vision was 3.2 ETDRS lines in 3 years in eyes without treatment.
All retrospective case series to date reported stabilization and/or improvement of the visual acuity in eyes with subretinal hemorrhage by using the anti-VEGF therapy.15–20,23,24 In 21 eyes with large-sized submacular hemorrhage, stabilization of the visual acuity and decrease in hemorrhage was found after only 4 months. Twelve eyes had a follow-up of 12 months or longer with a mean visual acuity of 0.28 (20/40).16 Using bevacizumab, ranibizumab, or both, ten eyes had an improvement of the median BCVA from 20/400 at baseline to 20/125 after 1 year.
In a large retrospective case series, eyes with subretinal bleedings secondary to typical AMD and polypoidal choroidal vasculopathy after anti-VEGF therapy were analyzed.19 In 91 eyes, the mean BCVA improved from 20/479 at baseline to 20/182 at 6 months. Factors such as longer duration of symptoms, greater extent of hemorrhage, and greater central foveal thickness were associated with a poor functional outcome.20 In a small prospective 1-year study, ranibizumab therapy was given to seven patients, having hemorrhagic lesions secondary to AMD. A median gain of 1.5 ETDRS lines of visual acuity was found, and no systemic or local side effects were noted.17 Another prospective, nonrandomized study analyzed 23 eyes with thin hemorrhages secondary to occult CNV for 12 months after intravitreal ranibizumab treatment. The mean BCVA improved significantly (from 0.82 [20/125] ± 0.22 to 0.68 [20/100] ± 0.41) and progressive resolution of the macular bleeding was noted in 22 of 23 eyes.18
Meanwhile, Altaweel et al (the CATT research group) presented a large analysis of patients with hemorrhagic lesions.21 They described a lower baseline BCVA, similar to our small cohort, but warn against a considerable growth of the CNV lesions during the first 2 years. This underlines the role of blood for the enforcement of subretinal fibrosis. Based on the functional development, the CATT scientists concluded that CNV with extensive bleeding can be managed similarly to those “with less or no blood”.21
Several clinical features may influence the outcome of eyes with subretinal hemorrhages, such as duration of the symptoms, follow-up period, previous treatments, and the presence of submacular fibrosis, size, thickness, and location of the hemorrhage.25 There are promising data and a growing body of evidence showing that post-injection rates of hemorrhages are lower than the incidence of spontaneous bleeding.26 The results of this study demonstrate beneficial effects of anti-VEGF therapy in eyes with hemorrhagic lesions. The benefit of the treatment was most pronounced in eyes with small- and medium-sized submacular hemorrhages.
Disclosure
Focke Ziemssen received consulting fees from Alimera, Allergan, Bayer HealthCare, Novartis, and speaker fees from Alcon, Alimera, Allergan, Bayer HealthCare, Heidelberg Engineering, and Novartis, although this study is not directly related to the corresponding licensed drugs. The authors report no other conflicts of interest in this work.
References
Bressler NM. Age related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1901. | ||
Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16(3):183–189. | ||
Bennet S, Folk J, Blodi C, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109(1):33–37. | ||
Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovasular lesions of age-related macular degeneration: ophthalmic findings: SST report no 13. Ophthalmology. 2004;111(11):1993–2006. | ||
Matt G, Sacu S, Stifter E, Prünte C, Schmidt-Erfurth U. [Combination of intravitreal rTPA, gas and ranibizumab for extensive subfoveal hemorrhages secondary to neovascular age-related macular degeneration]. Klin Monbl Augenheilkd. 2010;227(3):221–225. German. | ||
Haupert CL, McCuen BW, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131(2):208–215. | ||
Hillenkamp J, Surguch V, Framme C, Gabel VP, Sachs HG. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010;248(1):5–11. | ||
Arias L, Monés J. Transconjunctival sutureless vitrectomy with tissue plasminogen activator, gas and intravitreal bevacizumab in the management of predominantly hemorrhagic age related macular degeneration. Clin Ophthalmol. 2010;4:67–72. | ||
Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG. Combined treatment of acute subretinal hemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008;86(5):490–494. | ||
Treumer F, Roider J, Hillenkamp J. Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovasular AMD with submacular hemorrhage. Br J Ophthalmol. 2012;96(5):708–713. | ||
Sacu S, Stifter E, Vécsei-Marlovits PV, et al. Management of extensive subfoveal hemorrhage secondary to neovascular age-related macular degeneration. Eye (Lond). 2009;23(6):1404–1410. | ||
Brown DM, Kaiser PK, Michels M, et al. ANCHOR study group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA study group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. | ||
CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovasular age-related macular degeneration. N Engl J Med. 2011;19;364(20):1897–1908. | ||
Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovasular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol. 2007;144(6):886–892. | ||
Shienbaum G, Garcia Filho CA, Flynn HW Jr, Nunes RP, Smiddy WE, Rosenfeld PJ. Management of submacular hemorrhage secondary to neovasular age-related macular degeneration with anti-Vascular endothelial growth factor monotherapy. Am J Ophthalmol. 2013;155(6):1009–1013. | ||
Chang MA, Do DV, Bressler SB, Cassard SD, Gower EW, Bressler NM. Prospective one-year study of ranibizumab for predominantly hemorrhagic choroidal neovascular lesions in age-related macular degeneration. Retina. 2010;30(8):1171–1176. | ||
Iacono P, Parodi MB, Introini U, La Spina C, Varano M, Bandello F. Intravitreal ranibizumab for choroidal neovascularization with large submacular hemorrhage in age-related macular degeneration. Retina. 2014;34(2):281–287. | ||
Kim JH, Chang YS, Kim JW, Kim CG, Yoo SJ, Cho HJ. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology. 2014;121(4):926–935. | ||
McKibbin M, Papastefanou V, Matthews B, Cook H, Downey L. Ranibizumab monotherapy for sub-foveal hemorrhage secondary to choroidal neovascularisation in age-related macular degeneration. Eye (Lond). 2010;24(6):994–998. | ||
Altaweel MM, Daniel E, Martin DF, et al. Outcomes of eyes with lesions composed of >50% blood in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Ophthalmology. 2015;122(2):391–398. | ||
Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–129. | ||
Cho HJ, Koh KM, Kim JH, et al. Intravitreal ranibizumab injections with and without pneumatic displacement for treating submacular hemorrhage secondary to neovascular age-related macular degeneration. Retina. 2015;35(2):205–212. | ||
Chang W, Garg SJ, Maturi R, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol. 2014;157(6):1250–1257. | ||
Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213(2):97–102. | ||
Kauffmann Y, Isaico R, Lefebvre A, Bron AM, Creuzot-Garcher C. [Relationship between intravitreal anti-VEGF therapy and subretinal hemorrhage in patients with exudative age-related macular degeneration]. J Fr Ophtalmol. 2014;37(3):195–201. French. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.