Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Subcentimeter Nodules with Diagnostic Hallmarks of Hepatocellular Carcinoma: Comparison of Pathological Features and Survival Outcomes with Nodules Measuring 1–2 cm
Authors Huang P, Ni X, Zhou C, Shi Z, Wu F , Xiao Y, Yang C, Zeng M
Received 10 December 2022
Accepted for publication 30 January 2023
Published 8 February 2023 Volume 2023:10 Pages 169—180
DOI https://doi.org/10.2147/JHC.S401027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Jörg Trojan
Peng Huang,1,2,* Xiaoyan Ni,1,2,* Changwu Zhou,1– 3 Zhang Shi,1,2 Fei Wu,1,2 Yuyao Xiao,1,2 Chun Yang,1,2 Mengsu Zeng1– 3
1Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of Cancer Center, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Shanghai Institute of Medical Imaging, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengsu Zeng, Shanghai Institute of Medical Imaging, Department of Radiology, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Xuhui District, Shanghai, 200032, People’s Republic of China, Tel +86 13501922963, Email [email protected] Chun Yang, Department of Radiology, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Xuhui District, Shanghai, 200032, People’s Republic of China, Tel +86 18702135336, Email [email protected]
Objective: To compare the pathologic diagnosis and survival of patients with subcentimeter and 1– 2 cm nodules that present with diagnostic hallmarks of hepatocellular carcinoma (HCC).
Methods: Diagnostic hallmarks of HCC were defined as hyperintensity on T2 weighted imaging, restricted diffusion, arterial phase hyperenhancement, washout on portal venous phase, and hypointensity on hepatobiliary phase. We retrospectively included 139 patients undergoing curative resection with single nodules ≤ 2 cm that present imaging features described above on gadoxetic acid-enhanced MRI. The final diagnosis was confirmed by histopathological assessment. Recurrence-free survival (RFS) was compared using Kaplan–Meier analysis with the Log-rank test. Factors associated with overall and early recurrence were identified using Cox regression analysis.
Results: Among 139 nodules (49 nodules < 1 cm), there was no significant difference in the percentage of HCC between subcentimeter and 1– 2 cm nodules (94.0% vs 94.4%, P > 0.999). Microvascular invasion (MVI) was less common in subcentimeter HCC (4.3% vs 17.6%, P = 0.032). There were 27 recurrences during a median follow-up time of 46.7 months. Patients with subcentimeter HCC achieved less recurrence, with a 5-year RFS rate of 87.3%. The MVI-positive patients had more early and overall recurrence. A tumor size < 1 cm was associated with lower overall recurrence (HR, 0.336; P = 0.047). No factors were independently associated with early recurrence.
Conclusion: Subcentimeter nodules with diagnostic hallmarks of HCC are highly associated with HCC diagnosis and achieve less tumor recurrence after resection. Early diagnosis and treatment of subcentimeter HCC may be more appropriate.
Keywords: carcinoma, hepatocellular, magnetic resonance imaging, gadolinium ethoxybenzyl DTPA
Introduction
Early diagnosis and treatment improve the prognosis of patients with hepatocellular carcinoma (HCC).1 In high-risk patients, HCC can be diagnosed noninvasively based on its typical diagnostic hallmarks without pathological confirmation.2 As gadoxetic-acid disodium, a liver-specific contrast agent, is widely used, subcentimeter HCC is not uncommonly encountered in clinical practice.3 Guidelines from the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of the Liver (EASL) only recommend a definitive diagnosis of HCC for lesions larger than 1 cm.4,5 However, the diagnosis algorithms in some Asian guidelines are not constrained by the diameter, and subcentimeter HCC can be diagnosed and treated as early as possible.6–8
The discrepancies among guidelines reflect two major hotspots of research related to subcentimeter HCC, that is, how to accurately diagnose it and whether it should be treated aggressively. Recent studies related to preoperative diagnosis found that the incidence of HCC among subcentimeter nodules presenting with typical five image features of HCC was as high as 80–90%.9,10 However, to our knowledge, no pathology study has been performed to investigate the pathological diagnosis of such nodules and few studies have focused on patients without a history of HCC. With regard to management strategy, there has been little agreement on how best to deal with these nodules. Although previous studies concluded that early treatment of subcentimeter HCC did not improve survival compared with those measuring 1–2 cm, these studies were flawed by insufficient sample sizes and different treatment modalities.11,12
In the present study, we aimed to investigate the pathological diagnosis of subcentimeter nodules with diagnostic hallmarks of HCC in treatment-naïve patients and to determine whether treatment of HCC in a subcentimeter level would improve survival outcomes.
Materials and Methods
Patients
The Institutional Review Board of Zhongshan Hospital Fudan University approved this retrospective study (B2020-372R), which followed the Declaration of Helsinki’s ethical guidelines. Written informed consent was waived due to its retrospective nature. We stated that patient data was strictly confidential.
We registered consecutive treatment-naïve patients suspected of solitary hepatic solid lesions according to gadoxetate acid-enhanced MRI (EOB-MRI) reports from January 2012 to December 2020 by searching the electronic imaging database of the local institution. The inclusion criteria were as follows: (a) patients who had chronic hepatitis B virus (HBV) infection or liver cirrhosis of any etiology; (b) lesion size ≤2 cm on EOB-MRI; (c) patients who had no prior history of other malignancies; and (d) patients who received curative hepatectomy (R0 resection). This selection yielded 349 initial eligible patients with 349 hepatic lesions. Of those, we excluded 51 patients who: (a) had a history of antitumor treatment before surgery (n = 22); (b) whose interval between MR scan and surgery >1 month (n = 10); (c) who had inadequate MR image quality (n = 15); and (d) who were lost to follow-up or died in the perioperative period (n = 4).
A total of 159 nodules that did not show diagnostic hallmarks of HCC were excluded. Diagnostic hallmarks of HCC were defined as the combination of the following five imaging features that known to be suggestive of HCC: T2-weighted imaging (T2WI) mild-moderate hyperintensity, restricted diffusion, nonrim arterial hyperenhancement (nonrim APHE), nonperipheral washout only in portal venous phase (PVP), and hypointensity on hepatobiliary phase (HBP). Whether a nodule depicted diagnostic hallmarks of HCC was determined according to the consensus of two radiologists (with 14 and 16 years of experience) (Supplementary Table 1). Ultimately, 139 nodules from 139 patients were included in final analysis (Figure 1).
 |
Figure 1 Flow chart of patient inclusion and exclusion. |
Electronic medical records were used to collect clinical information and laboratory data for all patients, including patient demographics, etiology of chronic liver disease; serum levels of hepatitis B virus deoxyribonucleic acid load, albumin, total bilirubin, aspartate transaminase, alanine transaminase, platelets, prothrombin time, international normalized ratio, alpha-fetoprotein (AFP); Child-Pugh class; and Albumin-bilirubin grade.
Acquisition of EOB-MRI Images
All patients were scanned on a 1.5-T scanner (Magnetom Aera, Siemens Healthcare). Routine liver protocols consisted of transverse T2WI, T1-weighted imaging, in-phase and opposed-phase sequences, and diffusion-weighted imaging. Gadoxetic acid–enhanced T1-weighted imaging was performed at the arterial phase, PVP, transitional phase (TP), and HBP after the injection of gadoxetate disodium (Primovist, Bayer Pharma) at a dose of 0.025 mmol/kg. Detailed parameters of all the sequences are found in a published paper.13
Image Analysis
The MR images were retrieved from the picture archiving and communication system workstation (Centricity RA1000, General Electric). All images were deidentified and then presented randomly to two board-certified abdominal radiologists. They were blinded to the clinical data and histological results but were aware that the patients were at high risk for HCC. Two observers independently measured the maximum diameter of observations on HBP. The average diameter was applied to minimize measurement bias. The detailed definition of the aforementioned five MRI features is summarized in Supplementary Table 2.
Measurement of Outcomes
Clinical and radiologic follow-up was performed for all HCC patients after surgery, including contrast-enhanced CT or MRI at 1 month after resection and every 3–6 months thereafter, according to the institutional protocol. Decisions about imaging modality and exploring extrahepatic metastasis were made by our hepatology clinic. Recurrence was defined as intrahepatic or extrahepatic neoplasms detected by CT/MRI, positron emission tomography (PET)-CT or confirmed by histopathology. Early recurrence was defined as recurrence within the first 2 years after resection. The data were censored on December 31, 2021.
Histopathologic Data
Two pathologists specializing in liver pathology (both with more than 10 years of experience) rereviewed the specimen slides of all patients. The final histopathologic diagnosis was remade by consensus based on the 2019 WHO classification.14 The following features were also recorded: liver fibrosis stage, hepatitis activity grade, tumor differentiation according to the Edmonson-Steiner grade, capsule formation, microvascular invasion (MVI), satellite foci, serosal invasion, and the expression of cytokeratin 19 (CK19) and Ki-67. MVI was defined as the presence of tumor in the portal vein, hepatic vein, or in a large capsular vessel of the surrounding hepatic tissue lined with endothelium that was visible only on microscopy.15 CK19-positive expression was defined as membranous or cytoplasmic immunoreactivity present in ≥5% of tumor cells, while Ki-67-positive expression referred to ≥10% of positive tumor cells.16,17
Statistical Analysis
Comparison of the frequencies of categoric variables between subcentimeter nodules and those measuring 1–2 cm was performed by Fisher’s exact test. The Mann–Whitney U-test was performed for continuous variables. The recurrence-free survival (RFS) and overall survival (OS) rates were calculated by the Kaplan–Meier method, and the differences were compared using the log–rank test. Univariable and multivariable analyses were carried out to identify prognostic factors for early recurrence and overall recurrence using the Cox proportional hazards regression model. Factors with a P value <0.1 in univariable analysis were candidates for the stepwise multivariable analysis. Hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. Differences with a P value of <0.05 were considered statistically significant. The kappa statistic (κ) was calculated as a measure of agreement between observers for image analysis, with values of 0.41–0.60 indicating moderate agreement, 0.61–0.80 indicating substantial agreement, and >0.81 indicating excellent agreement. Data were analyzed using SPSS Statistics version 24 (IBM Corp.).
Results
Patient and Nodule Characteristics
The final study cohort included 139 patients (109 male; median age, 52 years, interquartile range, 45–57 years) with solitary hepatic lesions who underwent curative hepatectomy. Cirrhosis was demonstrated in the majority of patients (stage F0-F2: 19.4%; stage F3: 13.7%; stage F4: 66.9%). Among the 135 patients (97.1%) with HBV infections, 78 (57.8%) were taking an oral antiviral agent. The median preoperative AFP serum level was 26.1 ng/mL (interquartile range, 4.2–153.0 ng/mL). There were 16 (11.5%) patients with AFP serum levels exceeding 400 ng/mL (Table 1). The median time interval between EOB-MRI examination and surgery was 14 days (interquartile range, 8–20 days).
 |
Table 1 Baseline Characteristics of Patients with Lesions Presenting Diagnostic Hallmarks of HCC |
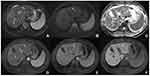
A total of 131 nodules (94.2%) were pathologically confirmed as HCC (Figure 2). The histological types of other nodules that also exhibited diagnostic hallmarks of HCC included angiomyolipoma (3 of 139 patients, 2.2%), combined hepatocellular carcinoma-cholangiocarcinoma (3 of 139 patients, 2.2%), intrahepatic cholangiocarcinoma (1 of 139 patients, 0.7%), and bile duct adenoma (1 of 139 patients, 0.7%). The mean size of all 139 nodules was 12.9 ± 3.6 mm, and 35.3% (49/139) were smaller than 1 cm. There was no significant difference in the proportion of HCC between subcentimeter nodules and those with a diameter of 1–2 cm (94.0% vs 94.4%, P > 0.999) (Table 2). The proportion of malignancy in subcentimeter nodules with diagnostic hallmarks of HCC was 95.9% (47/49).
 |
Table 2 Histological Type of Nodules with Typical Imaging Features of HCC |
Pathological Characteristics of HCCs Up to 2 cm
At pathologic analysis, the majority of HCCs (74.0%, 97/131) were located in the right lobe. Edmondson-Steiner grade I or II was observed in 100 (76.4%) HCCs. MVI was less frequently observed in <1 cm HCC than in 1–2 cm HCC (2 of 46 [4.3%] vs 15 of 85 [17.6%]; P = 0.032). However, there was no significant difference in the incidence of intact capsules, macrovascular invasion, serosal invasion, Ki-67 positivity, or CK-19 positivity between subcentimeter HCCs and 1–2 cm HCCs (P > 0.05 for all) (Table 3).
 |
Table 3 Comparison of Pathological Variables Between HCC of Different Sizes |
Analysis of Survival in Patients with Solitary HCCs Up to 2 cm
During the median follow-up period of 46.7 months (interquartile range, 28.9–67.3 months), 27 recurrences were observed, including 15 early recurrences (55.6%). The estimated RFS rates were 93.9%, 84.3%, and 72.9% at 1, 3, and 5 years, respectively (Figure 3A). Twenty-five patients suffered intrahepatic recurrence, and two patients had both extrahepatic and intrahepatic recurrences. Among these patients, 18 (66.7%) received a second surgical resection, 4 (14.8%) received radiofrequency ablation, 3 (11.1%) received transarterial chemoembolization, 1 (3.7%) received systemic therapy, and 1 (3.7%) received stereotactic radiotherapy. Eight patients died during long-term follow-up, and 7 deaths were due to tumor progression. The estimated OS rates were 99.2%, 96.6%, and 95.4% at 1, 3, and 5 years, respectively (Figure 3B).
Variables associated with overall tumor recurrence in univariable analysis were MVI (HR, 2.418; P = 0.058) and tumor size <1 cm (46 of 131 HCCs) (HR, 0.306; P = 0.029). Multivariable analysis showed that only tumor size <1 cm was associated with low recurrence (HR, 0.336; P = 0.047). In addition, Kaplan‒Meier survival analysis demonstrated that tumor size <1 cm was associated with both lower overall and early tumor recurrence (P = 0.021 and 0.048, respectively) (Figure 3C and D). However, this association for early recurrence was not confirmed with multivariable Cox regression analysis (HR, 0.305; P = 0.123). The estimated RFS rates at 1, 3, and 5 years were 97.8%, 92.4%, and 87.3% for subcentimeter HCCs and 91.8%, 80.1% and 65.7% for 1–2 cm HCCs, respectively. MVI was associated with both overall and early tumor recurrence (P = 0.049 and 0.014, respectively) in the Kaplan‒Meier survival analysis (Figure 4). However, MVI was not significant in multivariable Cox regression analysis for either overall (HR, 1.960, P = 0.152) or early recurrence (HR, 2.709, P = 0.070). In addition, none of the baseline clinical covariates, including serum AFP level, ALBI grade, and cirrhosis, showed an association with risk for overall and early recurrence in Cox regression analysis (Table 4).
 |
Table 4 Univariable and Multivariable Analysis for Overall and Early Recurrence in HCC Cohort |
 |
Figure 4 Kaplan–Meier curves of overall recurrence (A) and early recurrence (B) showing a comparison between patients with and without microvascular invasion. |
Discussion
The present study reconfirms that subcentimeter nodules with typical imaging features of HCC are strongly correlated with HCC diagnosis and newly shows that patients with subcentimeter-sized HCC may have a lower recurrence rate after surgical resection. Our results may help in establishing appropriate management of suspicious subcentimeter HCC in treatment-naïve patients.
Several studies evaluated the imaging features of subcentimeter HCCs and proposed possible diagnostic algorithms.18–20 According to a study of subcentimeter hypervascular nodules detected on EOB-MRI, 52.8% of HCC demonstrated hyperintensity on T2WI and diffusion-weighted imaging, washout on PVP or TP, and hypointensity on HBP.18 In patients with a prior history of HCC, Song et al10 reported that the majority of such nodules (89.9%) would progress to overt HCC in 12 months. In patients without a history of HCC, 81.3% (13/16) of such subcentimeter nodules were eventually diagnosed as overt HCC.9 However, washout appearance was evaluated on either PVP or TP in these studies, which merits closer inspection. In addition to the de-enhancement of the tumor, TP hypointensity may also reflect the early uptake of gadoxetic acid by the surrounding liver parenchyma.21 Therefore, this may more or less lead to a decrease in diagnostic specificity.22 To avoid this controversy, a stricter definition of washout appearance (nonperipheral hypointensity only in PVP) was utilized in our study, which is in line with the AASLD and EASL guidelines.4,5 We believe that the higher percentage of subcentimeter HCC (94.0%), compared with previous studies, may be partially attributed to the strict criteria of washout appearance used in our study. These findings may suggest that among high-risk treatment-naïve patients, a diagnosis of HCC could be made with confidence if subcentimeter nodules present with all the diagnostic hallmarks of HCC, including washout only in PVP.
In our study, a tumor size <1 cm was an independent factor associated with lower overall recurrence and patients with subcentimeter HCC had a lower rate of both overall and early recurrence. To our knowledge, few studies have discussed the survival outcomes of patients with subcentimeter HCC. A study of 618 patients with different-sized HCC undergoing resection found a prolonged OS of 28.5 months for patients with subcentimeter HCC compared with those with HCC measuring 1–2 cm.23 In addition, tumor size ≥1.5 cm was found to be a significant factor of recurrence after resection of HCC up to 2 cm.24 However, being inconsistent with our result, Sun et al12 reported a similar RFS and OS between patients with subcentimeter HCC and 1–2 cm HCC undergoing resection or ablation. These discrepancies may be explained by the insufficient sample size of patient with subcentimeter HCC undergoing resection (only 13 cases) and the length of follow-up (median: 24.3 months) of their study. In addition, intrahepatic recurrence may represent minor metastasis or multicentric occurrence that was undetected before curative treatment. EOB-MRI showed higher sensitivity in detecting hepatic lesions in HBP images than dynamic CT and MRI, which could detect additional HCC nodules that have been missed on dynamic CT and therefore reduce the tumor recurrence.3,25 The utilization of EOB-MRI minimizes incorrect preoperative HCC identification and tumor staging, thus allowing for accurate assessment of recurrence. Another study reported the RFS in patients with subcentimeter HCC did not differ significantly between immediate treatment (including chemoembolization and ablation) and watchful waiting until it progresses to overt HCC.11 However, the survival was counted from the date of diagnosis in their study, which may result in a lead time bias that artificially inflates patient survival, even though the treatment is ineffective.26 We believe that our results, which are, of course, inevitably influenced by the intrinsic bias of the study design, are still meaningful, given the current lack of reliable data obtained from well-designed studies.
MVI is a direct pathological feature of intrahepatic metastases and a risk factor for tumor recurrence.27 It was present in 13.0% of HCC (only 4.3% for subcentimeter HCC) in our study, which is consistent with previous studies that reported incidence rates ranging from 12.4% to 33.1% for very early-stage HCC.28–30 MVI-positive patients had higher overall and early recurrence rates in our study. This finding was in accord with a recent propensity score matching analysis indicating that solitary HCC ≤ 2 cm with MVI had a poor prognosis.28 Unexpectedly, MVI was not an independent risk factor for either overall or early recurrence in multivariable analysis. The low present rate of MVI (only 13.0%) in included patients may explain the lack of significance in multivariable analysis. However, we cannot omit the impact of MVI on the prognosis of patients. Due to the lack of malignant biological behaviors and better survival outcomes after resection, it can be assumed that subcentimeter HCC should be treated immediately rather than adopting a watchful-waiting management strategy when the diagnosis is made with high confidence.
There are several limitations to this study. First, this is a retrospective and single-center study. Inevitably, some selection bias and influencing factors are involved. To make our results as objective as possible, we performed multivariable analysis regarding survival outcome. However, multicenter large sample-sized prospective studies are needed to verify our results. Second, given that the patients in the present study had very small HCCs, more than 5 years of follow-up would be required to reach conclusions about OS to be drawn. However, the recurrence after resection was a robust predictor associated with worse long-term survival.31 Last, our study may be limited in its general applicability. The predominant subjects included in this study had HBV-associated chronic liver disease with good liver functional reserve. Therefore, outcomes may vary in patients with different demographic characteristics.
In conclusion, subcentimeter nodules with all five diagnostic hallmarks of HCC are strongly correlated with HCC diagnosis. Because the better survival outcomes of patients with subcentimeter HCC, it may be more considerable to initiate treatment rather withhold treatment until it reach ≥1cm.
Abbreviations
EOB-MRI, gadoxetic acid-enhanced MRI; HCC, hepatocellular carcinoma; APHE, arterial phase hyperenhancement; PVP, portal venous phase; HBP, hepatobiliary phase; RFS, recurrence-free survival.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Clinical Research Plan of SHDC (grant number SHDC2020CR1029B), the National Natural Science Foundation of China (grant number 82171897), the Shanghai Municipal Key Clinical Specialty (grant number shslczdzk03202), and the Shanghai Municipal Health Commission (grant number 202240152).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
2. Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25(3):245–263. doi:10.3350/cmh.2018.0090
3. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635–654. doi:10.1148/radiol.14132361
4. European Association for the Study of the Liver. Electronic address EEE, European Association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
5. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
6. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
7. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
8. Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi:10.1159/000514174
9. Park CJ, An C, Park S, Choi JY, Kim MJ. Management of subcentimetre arterially enhancing and hepatobiliary hypointense lesions on gadoxetic acid-enhanced MRI in patients at risk for HCC. Eur Radiol. 2018;28(4):1476–1484. doi:10.1007/s00330-017-5088-1
10. Song KD, Kim SH, Lim HK, Jung SH, Sohn I, Kim HS. Subcentimeter hypervascular nodule with typical imaging findings of hepatocellular carcinoma in patients with history of hepatocellular carcinoma: natural course on serial gadoxetic acid-enhanced MRI and diffusion-weighted imaging. Eur Radiol. 2015;25(9):2789–2796. doi:10.1007/s00330-015-3680-9
11. Woo JH, Song KD, Kim SH. Subcentimeter hypervascular nodules with typical imaging findings of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: outcomes of early treatment and watchful waiting. Eur Radiol. 2017;27(10):4406–4414. doi:10.1007/s00330-017-4818-8
12. Sun X, Hu D, Zhang Y, et al. Can immediately treating subcentimeter hepatocellular carcinoma improve the survival of patients? J Hepatocell Carcinoma. 2020;7:377–384. doi:10.2147/JHC.S287641
13. Huang P, Zhou C, Wu F, et al. An improved diagnostic algorithm for subcentimeter hepatocellular carcinoma on gadoxetic acid-enhanced MRI. Eur Radiol. 2022. doi:10.1007/s00330-022-09282-5
14. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi:10.1111/his.13975
15. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi:10.1053/j.gastro.2009.06.003
16. Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49(2):138–151. doi:10.1111/j.1365-2559.2006.02468.x
17. Wu H, Han X, Wang Z, et al. Prediction of the Ki-67 marker index in hepatocellular carcinoma based on CT radiomics features. Phys Med Biol. 2020;65(23):235048. doi:10.1088/1361-6560/abac9c
18. Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196(6):W758–W765. doi:10.2214/AJR.10.4394
19. Hwang SH, Hong SB, Park S, et al. Subcentimeter hepatocellular carcinoma in treatment-naive patients: noninvasive diagnostic criteria and tumor staging on gadoxetic acid-enhanced MRI. Eur Radiol. 2021;31(4):2321–2331. doi:10.1007/s00330-020-07329-z
20. Yu MH, Kim JH, Yoon JH, et al. Small (</=1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271(3):748–760. doi:10.1148/radiol.14131996
21. Kitao A, Matsui O, Yoneda N, et al. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol. 2020;30(6):3438–3447. doi:10.1007/s00330-020-06687-y
22. Joo I, Lee JM, Lee DH, Jeon JH, Han JK. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol. 2019;29(4):1724–1732. doi:10.1007/s00330-018-5727-1
23. Lu XY, Xi T, Lau WY, et al. Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol. 2011;137(4):567–575. doi:10.1007/s00432-010-0909-5
24. Yamashita Y, Tsuijita E, Takeishi K, et al. Predictors for microinvasion of small hepatocellular carcinoma</= 2 cm. Ann Surg Oncol. 2012;19(6):2027–2034. doi:10.1245/s10434-011-2195-0
25. Kim HD, Lim YS, Han S, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148(7):1371–1382. doi:10.1053/j.gastro.2015.02.051
26. Lee MW, Lim HK. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: current status and future. World J Gastroenterol. 2018;24(46):5215–5222. doi:10.3748/wjg.v24.i46.5215
27. Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(2):293–303. doi:10.1007/s00432-016-2286-1
28. Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49(3):344–354. doi:10.1111/hepr.13241
29. Huang C, Zhu XD, Ji Y, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer. 2017;17(1):58. doi:10.1186/s12885-017-3050-x
30. Shindoh J, Andreou A, Aloia TA, et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: reappraisal of the staging system for solitary tumors. Ann Surg Oncol. 2013;20(4):1223–1229. doi:10.1245/s10434-012-2739-y
31. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi:10.1097/SLA.0000000000000710
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.