Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Study on the Association of Homocysteine and C-Reactive Protein with Neurofunctional Changes in Patients with Acute Ischemic Stroke After Endovascular Stent Treatment
Authors Chen Q, Ling WT, Han DK
Received 10 January 2022
Accepted for publication 21 March 2022
Published 13 April 2022 Volume 2022:18 Pages 881—889
DOI https://doi.org/10.2147/NDT.S356331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Qiong Chen,1 Wen-Tong Ling,2 Deng-Ke Han3
1Teaching and Research Department, Foshan Fosun Chancheng Hospital, Foshan, 52800, People’s Republic of China; 2Department of Neurology, Zhongshan City People Hospital, Zhongshan, 528400, People’s Republic of China; 3Clinical Laboratory Medicine Center, Zhongshan City People Hospital, Zhongshan, 528400, People’s Republic of China
Correspondence: Wen-Tong Ling, Department of Neurology, Zhongshan City People Hospital, No. 2 Sunwen East Road, Zhongshan City, Guangdong, 528400, People’s Republic of China, Tel +86-18676163223, Email [email protected]
Objective: To examine the association of homocysteine (HCY) and C-reactive protein (CRP) with neurofunctional changes in patients with acute ischemic stroke (AIS) after stent treatment.
Methods: A total of 110 patients with AIS treated with stents were divided into a high HCY group (n = 59) and a normal HCY group (n = 51) based on the HCY level. Pearson correlation analysis and logistic linear regression analysis were used to analyze the related factors that affect the National Institutes of Health Stroke Scale (NIHSS) score changes after stent treatment.
Results: (1) The area under the receiver operating characteristic (ROC) curve for HCY was 0.995 (95% confidence interval [CI]: 0.984– 1.005, P = 0.000), and the best predictive value was 12.75 μmol/L (sensitivity 89.9%, specificity 98.0%). The area under the ROC curve for CRP was 0.665 (95% CI: 0.564– 0.767, P = 0.003), and the best predictive value was 9.7 mg/L; (2) comparison between the high HCY group and the normal HCY group showed statistical differences (P < 0.05) in HCY, CRP, and the NIHSS score at admission, the NIHSS score after treatment, gender, history of diabetes, and history of atrial fibrillation; (3) both HCY and CRP were proven to be correlated with the NIHSS score after treatment (0.188, P = 0.050) and (0.194, P = 0.042), respectively, using Pearson correlation analysis; (4) HCY, low-density lipoprotein, CRP, cystatin C, glucose, history of atrial fibrillation, history of diabetes, and the NIHSS score at admission as the risk factors.
Conclusion: High HCY and CRP levels are related to the neurofunctional changes in patients with AIS treated with stents and can be used as indicators to assess the risk of treating AIS with stents and as serum markers to predict prognoses.
Keywords: cerebrovascular stent, acute ischemic stroke, homocysteine, C-reactive protein, degree of neurological deficit
Introduction
Acute ischemic stroke (AIS) is the most common cerebrovascular disease, with its incidence increasing annually and mortality continuing to grow, seriously threatening people’s lives and health.1 Atherosclerotic plaque and stenosis are important causes of ischemic stroke. According to the Chinese Intracranial Atherosclerosis (ICAS) study in 2014, the incidence of ICAS in Chinese patients with ischemic stroke or transient ischemic attack (TIA) was 46.6%, and those patients who also had ICAS had more severe symptoms, longer hospital stays, and a higher recurrence of stroke, with the probability of recurrence rising with the increase in stenosis.2
Endovascular intervention is one treatment for ICAS, and even under the optimal conservative medical treatment, balloon dilation and/or stent implantation can be considered for patients with severe vascular stenosis that cannot be controlled or improved by drugs.3,4 Metal stents have strong support and can eliminate vascular retraction and effectively relieve vascular stenosis. However, there is a high restenosis rate after metal stent implantation.
Strict and full pre-surgical evaluation and screening are a strong guarantee of the safety of interventional therapy for ischemic cerebrovascular disease. The risk factors for cerebrovascular disease include traditional risk factors such as age, gender, smoking history, hypertension, diabetes, and dyslipidemia,5,6 and also C-reactive protein (CRP) and homocysteine (HCY). As an inflammatory marker, CRP can show the severity of cerebral infarction, is associated with the stability of atherosclerotic plaques, and plays a role in the secondary prevention and fatality rate prediction in patients with stroke.7,8 Currently considered an independent risk factor for AIS, HCY’s roles in atherosclerotic modification and plaque formation9–11 are associated with vascular endothelial injury and inflammation. Previous studies have reported the correlation between HCY and prognoses following coronary stent implantation.12,13 However, there are few studies on the impact of HCY and CRP in patients with AIS treated with cerebrovascular stent implantation. This study analyzes the correlation of HCY and CRP with neurofunctional changes after stent implantation in patients with AIS and discusses whether HCY and CRP can be used as indicators to assess the risk of treating AIS with endovascular stent implantation and as serum markers to predict prognoses.
Materials and Methods
Subjects
A retrospective analysis was conducted on 110 patients with AIS treated with stent implantation by the Department of Neurology, Zhongshan City People’s Hospital, from January 2015 to January 2018, comprising 82 males and 28 females, with an average age of 62.67 ± 13.54 years. A receiver operating characteristic (ROC) curve was plotted for HCY to obtain the cut-off values, and patients were divided into the high HCY (HHCY) group (n = 59) and the normal HCY (NHCY) group (n = 51).
Inclusion and Exclusion Criteria
The indications to include patients treated with cerebrovascular stent implantation were: (1) an ICAS rate ≥ 70%, failing intensive drug treatment or with poor cerebral collateral circulation compensation and with hypoperfusion in the blood supply area of offending vessels; (2) age < 80; (3) offending vessel occlusion corresponding to neurological dysfunction as confirmed by digital subtraction angiography; (4) no significant intracranial infarction found following an examination by acute cranial computerized tomography (CT), and cerebral hemorrhage or other intracranial diseases excluded; (5) the treatment was within 6 hours of onset and 12 hours of basilar artery occlusion; and (6) informed consent forms agreed and signed by the patient’s family members.
The exclusion criteria were: (1) National Institutes of Health Stroke Scale (NIHSS) score ≥ 22; (2) bleeding tendency; (3) history of major surgery or trauma within two months; (4) dysfunction or failure of vital organs; and (5) systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg before treatment.
Vital signs were monitored after surgery, and cranial CT re-examinations were conducted 24 hours later to check for complications, such as cerebral hemorrhage. Contraindications: (1) over 80 years old or expected survival <2 years; (2) complicated with severe systemic disease or not suitable for, or intolerant to, dual antiplatelet therapy; (3) severe neurological dysfunction (modified Rankin Scale [mRS] score ≥3) before the current stroke or TIA; (4) occurrence of severe myocardial infarction within two weeks; (5) Moyamoya disease, active arteritis, or unexplained non-atherosclerotic stenosis; (6) international normalized ratio >1.5; (7) pregnant women; and (8) patients determined by neurology/neurosurgery physicians or neuro-interventional physicians as unsuitable for endovascular treatment.
A loading dose of antiplatelet drugs (300 mg aspirin and 300 mg clopidogrel) should be taken before emergency surgery, 100 mg aspirin and 75 mg clopidogrel should be taken daily for at least one month after surgery, and aspirin should be taken for the long term thereafter. The main endovascular treatments for ICAS include balloon angioplasty, balloon-expandable stent implantation, and self-expanding stent implantation. The suitable endovascular treatment methods should be selected according to the specific lesions and pathway characteristics of the patients. The vascular stent implantation types of patients in this study included self-expanding stent implantation in 61 cases and balloon-expandable stent implantation in 50 cases. The locations of the vessels implanted with stents included the internal carotid artery in 35 cases, the common carotid artery in 7 cases, the middle cerebral artery in 28 cases, the basilar artery in 9 cases, and the vertebral artery in 22 cases (Figure 1).
 |
Figure 1 Research flow chart. Abbreviations: AIS, acute ischemic stroke; HHCY, high homocysteine; NHCY, normal homocysteine. |
Outcome Measures
The HCY, CRP, total cholesterol, triglyceride level, creatinine, Cystatin C (CysC), uric acid, glucose, glycohemoglobin, blood pressure at admission, history of smoking, history of alcohol consumption, history of heart disease, history of atrial fibrillation, history of stroke, history of hypertension, and incidence of complications (bleeding, hyperperfusion, restenosis, and death) were collected from the patients. The NIHSS scores at admission and after treatment were evaluated. All surgical treatments were completed by the study neurologists. Neurofunctional scores were evaluated jointly by the residents of the Neurology Department or physicians with higher professional titles.
Statistical Method
The SPSS™ Statistics v13.0 statistical software was used. The cut-off values of HCY were calculated using the ROC curve, and the measurement data were expressed as 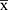 ± S. The t-test was used for group comparison, and the X2-test analysis was used for enumeration data comparison. Univariate Pearson product–moment correlation analysis and multivariate linear logistic regression analysis were conducted to analyze the relationships between the multiple risk factors and the neurofunctional improvement, with P < 0.05 considered as statistically significant.
± S. The t-test was used for group comparison, and the X2-test analysis was used for enumeration data comparison. Univariate Pearson product–moment correlation analysis and multivariate linear logistic regression analysis were conducted to analyze the relationships between the multiple risk factors and the neurofunctional improvement, with P < 0.05 considered as statistically significant.
Results
Results of ROC Curves for HCY and CRP
According to the analysis of the ROC curve for HCY, the area under the curve was 0.995 (95% CI: 0.984–1.005, P = 0.000), the cut-off value was 12.75 µmol/L, the sensitivity was 0.898, and the specificity was 0.980. Refer to Figure 2 for the ROC curve.
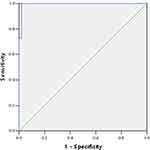 |
Figure 2 Homocysteine amino acid ROC curve. |
According to the analysis of the ROC curve for CRP, the area under the curve was 0.665 (95% CI: 0.564–0.767, P = 0.003), the cut-off value was 9.7 mg/L, the sensitivity was 0.610, and the specificity was 0.627. Refer to Figure 3 for the ROC curve.
 |
Figure 3 C-reactive protein ROC curve. |
Comparison of Clinical Data of Patients with AIS in Different HCY Groups
Refer to Table 1 for the comparison results of clinical data of patients with AIS treated with stents in different HCY groups. The HCY, CRP, gender, history of atrial fibrillation, history of diabetes, NIHSS score at admission, and NIHSS score after treatment were higher in the HHCY group than in the NHCY group (P < 0.05). The complications in the HHCY group included one case of bleeding and five cases of restenosis, while the NHCY group had one complication, a case of restenosis.
 |
Table 1 Comparison of Clinical Data Between the Two Groups ( |
Correlation of HCY and CRP with NIHSS
Refer to Table 2 for the results of Pearson correlation analysis of HCY and CRP with the NIHSS score at admission and the NIHSS score and complications after treatment. It was concluded that HCY and CRP were correlated with the NIHSS score after treatment (P < 0.05).
 |
Table 2 Pearson Correlation Analysis Between HCY/CRP and NIHSS/Complications |
Logistic Linear Regression Analysis
Refer to Table 3 for the results of relationships between multiple risk factors and neurofunctional improvement after treatment based on the multivariate logistic linear regression analysis with the NIHSS score as the dependent variable. It was concluded that the NIHSS score at admission, low-density lipoprotein (LDL), CRP, CysC, diabetes mellitus (DM), glucose, and HCY were the factors related to the NIHSS score changes after the cerebrovascular stent implantation (P < 0.05).
 |
Table 3 Results of Multivariate Logistic Linear Regression Analysis |
Discussion
This study analyzed the correlation of HCY and CRP with neurofunctional changes in patients with AIS after stent implantation and obtained the result that the HCY, CRP, gender, history of atrial fibrillation, history of diabetes, NIHSS score at admission, and NIHSS score after treatment were significantly higher in the HHCY group than in the NHCY group (P < 0.05). The complications in the HHCY group included one case of bleeding and five cases of restenosis, while the complication in the NHCY group was one case of restenosis. According to the Pearson correlation analysis of HCY and CRP with the NIHSS score at admission and the NIHSS score and complications after treatment, CRP was correlated with the NIHSS score after treatment (P < 0.05). According to the multivariate logistic linear regression analysis, the NIHSS score at admission, LDL, CRP, CysC, DM, glucose, and HCY were the factors related to the NIHSS score changes after the cerebrovascular stent implantation (P < 0.05).
The most sensitive inflammatory protein is CRP, with low content in normal serum, and it has a relationship with thrombosis. It can reduce the expression of nitric oxide synthase by inducing the production of monocyte chemoattractant protein to cause vascular endothelial dysfunction, and it may increase the expression and bioactivity of plasma thrombin activating and inhibiting factors in vascular endothelial cells to cause thrombosis.7,14 According to a study, the CRP level could predict the future occurrence of ischemic stroke and TIA in elderly patients.15 The CRP level was negatively correlated with the decrease in the NIHSS score within 24 hours of intravenous thrombolytic therapy, with a one-point decrease in the NIHSS score within 24 hours for every 12.5% mg/L increase in CRP. Therefore, it was considered that an increase in the CRP level after thrombolysis could predict an improvement in neurofunction and serve as a marker for predicting prognoses.16 This study obtained the result that the CRP level was significantly higher in the HHCY group than in the NHCY group, was unrelated to the NIHSS score at admission and the occurrence of complications, and was positively correlated with the NIHSS score after stent implantation treatment. It was concluded that CRP (cut-off value: 9.7 mg/L) could predict neurofunctional improvement after stent treatment for stroke.
Hyperhomocysteinemia is a risk factor for atherosclerosis. The active oxidizing substances produced by HCY can cause lipid peroxidation, platelet and leukocyte activation, and prothrombotic factor increasing, leading to vascular inflammation and thrombosis. Endothelial dysfunction can be caused when HCY releases nitrogen oxides and stimulates the overgrowth of the vascular smooth muscle; its wall shear stress causes vasodilation and leads to the aggregation and deposit of LDL. Being a trigger for vascular plaque disaggregation, this affects the coagulation and fibrinolytic system, and promotes atherogenesis formation and development, eventually resulting in cerebrovascular events.11
Additionally, HCY can predict the occurrence of AIS17 and is important for the severity assessment, clinical diagnosis, and treatment of patients with acute cerebral infarction.18,19 A high HCY (cut-off value: 19.95 µmol/L) level can predict poor prognoses of recombinant tissue plasminogen activator (rt-PA) intravenous thrombolytic therapy and spontaneous intracerebral hemorrhage in patients with AIS. In the relationship between serum HCY and the efficacy of rt-PA intravenous thrombolytic therapy for cerebral infarction in the young, the post-treatment NIHSS score and the mRS score of patients in the HHCY group were higher than those in the NHCY group. Elevated serum HCY is one of the factors affecting the poor prognoses of rt-PA intravenous thrombolysis in young patients with acute cerebral infarction.17 According to the coronary stent implantation data (cut-off value: 12 µmol/L), HCY was concluded to be an independent risk factor for predicting the recurrence of cardiovascular events in the long-term prognosis after coronary stent implantation as well as being a useful marker for prognostic assessment.12
Tu et al prospectively studied patients with AIS who were admitted within 24 hours after the onset of symptoms. The median serum high sensitivity CRP (Hs-CRP) and HCY levels were significantly higher in patients with AIS compared with normal controls. The levels of Hs-CRP and HCY were independent prognostic markers of functional outcome and death (adjusted for age and the NIHSS) in patients with AIS. In ROC curve analysis, the prognostic accuracy of the combined model (HCY and Hs-CRP) was higher compared with all measured biomarkers individually and the NIHSS score.20,21
Conclusion and Limitations
This study concludes that CRP and HCY can be used as the risk factors for assessing the risk of treating AIS with cerebrovascular stent implantation. They are important factors that affect changes to the degree of neurological deficits after cerebrovascular stent implantation. Furthermore, they can be used as indicators to assess the risk of treating AIS with endovascular stent implantation and as serum markers to predict prognoses. This study provides a single-center retrospective analysis of cases, with neurofunctional changes after stent treatment as the evaluation indicator. The study only assessed short-term prognoses; the research period was for three years, and the observation method was a retrospective analysis of their case history.
The research failed to track long-term prognoses and separately analyze intracranial and extracranial vessels to compare their differences. In the future, data from follow-up visits to patients treated with stents should be further analyzed, the impact of changes in HCY and CRP levels on prognoses and restenosis after stent implantation should be dynamically observed and studied, and whether HCY and CRP can be used as indicators for predicting and observing long-term prognoses and complications of stent treatment should be assessed.
Many studies have confirmed the role of HCY and CRP in the pathogenesis and prognosis of AIS. With the rise of neurointerventional treatment, the prognosis of patients with AIS has been dramatically altered. Intravenous thrombolysis is the first choice for patients with AIS admitted to the hospital within 4.5 hours. In patients with symptomatic non-acute intracranial large artery occlusion admitted beyond the time window of intravenous thrombolysis, endovascular revascularization may be a safe and effective approach in patients with worsening symptoms or recurrent symptoms despite intensive medical therapy and in patients with decompensation on perfusion assessment and collateral circulation assessment. The timing of endovascular revascularization for intracranial large artery occlusion, the opening procedure, and the strict indications for patient enrollment need to be clarified in further large-sample studies.
For patients who exceed the time window at the time of clinical treatment, the Alberta stroke program early CT score determines the option of active angiography to check for revascularization in conjunction with drug therapy and arterial thrombolysis or balloon or stent placement treatment. This treatment decision is based on the ability of the vessel to maintain a state of able blood flow after thrombus extraction or thrombolysis after vascular occlusion. It allows patients to benefit greatly in clinical treatment, but there are no corresponding treatment guidelines to clarify the decision-making process for clinicians. Various solutions are still controversial, and it is the purpose of this study to provide guiding suggestions for clinicians’ treatment decisions. It is hoped that a large-sample, multicenter, long-term follow-up study with multi-point observations of HCY and CRP will follow. It can help clinicians to use HCY and CRP as reference indicators when making treatment decisions to better assess changes in patients’ conditions and bring greater benefits to patients.
Abbreviation
HCY, Homocysteine Amino acid; LDL, Low density lipoprotein; CRP, C-reactive protein; CysC, Cystatin C; Glu, Glucose; NIHSS, National Institutes of Health Stroke Scale; ROC, receiver operating characteristic curve; ICAS, intracranial Atherosclerosis; AIS, acute ischemic stroke.
Ethics
This study was conducted with approval from the Ethics Committee of Foshan Fosun Chancheng Hospital (CYIRB-LCYJ-2022004). This study was conducted in accordance with the declaration of Helsinki. Verbal informed consent was obtained from all participants. And the verbal informed consent process was approved by the Ethics Committee of Foshan Fosun Chancheng Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42(8):2351–2355. doi:10.1161/STROKEAHA.111.621904
2. Wang Y, Zhao X, Liu L, et al.; CICAS Study Group. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45(3):663–669. doi:10.1161/STROKEAHA.113.003508
3. Procházka V, Jonszta T, Czerny D, et al. Comparison of mechanical thrombectomy with contact aspiration, stent retriever, and combined procedures in patients with large-vessel occlusion in acute ischemic stroke. Med Sci Monit. 2018;22(24):9342–9353. doi:10.12659/MSM.913458
4. Lapergue B, Blanc R, Gory B, et al.; ASTER Trial Investigators. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318(5):443–452. doi:10.1001/jama.2017.9644
5. van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357(6):572–579. doi:10.1056/NEJMcp072057
6. Sacco RL, Adams R, Albers G, et al.; American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113(10):e409–e449.
7. Arikanoglu A, Yucel Y, Acar A, Cevik MU, Akil E, Varol S. The relationship of the mean platelet volume and C-reactive protein levels with mortality in ischemic stroke patients. Eur Rev Med Pharmacol Sci. 2013;17(13):1774–1777.
8. Di Napoli M, Godoy DA, Campi V, et al. C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke. 2011;42(5):1230–1236. doi:10.1161/STROKEAHA.110.604983
9. Wu W, Guan Y, Xu K, et al. Plasma homocysteine levels predict the risk of acute cerebral infarction in patients with carotid artery lesions. Mol Neurobiol. 2016;53(4):2510–2517. doi:10.1007/s12035-015-9226-y
10. Lu SS, Xie J, Su CQ, Ge S, Shi HB, Hong XN. Plasma homocysteine levels and intracranial plaque characteristics: association and clinical relevance in ischemic stroke. BMC Neurol. 2018;18(1):200. doi:10.1186/s12883-018-1203-4
11. Xin XY, Song YY, Ma JF, et al. Gene polymorphisms and risk of adult early-onset ischemic stroke: a meta-analysis. Thromb Res. 2009;124(5):619–624. doi:10.1016/j.thromres.2009.07.007
12. Yeh JK, Chen CC, Hsieh MJ, et al. Impact of homocysteine level on long-term cardiovascular outcomes in patients after coronary artery stenting. J Atheroscler Thromb. 2017;24(7):696–705. doi:10.5551/jat.36434
13. Hassan A, Dohi T, Miyauchi K, et al. Prognostic impact of homocysteine levels and homocysteine thiolactonase activity on long-term clinical outcomes in patients undergoing percutaneous coronary intervention. J Cardiol. 2017;69(6):830–835. doi:10.1016/j.jjcc.2016.08.013
14. Bonaventura A, Liberale L, Vecchié A, et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int J Mol Sci. 2016;17(12):1967. doi:10.3390/ijms17121967
15. Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32(11):2575–2579. doi:10.1161/hs1101.098151
16. Gill D, Sivakumaran P, Wilding P, Love M, Veltkamp R, Kar A. Trends in C-reactive protein levels are associated with neurological change twenty-four hours after thrombolysis for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25(8):1966–1969. doi:10.1016/j.jstrokecerebrovasdis.2016.05.003
17. Yao ES, Tang Y, Xie MJ, Wang MH, Wang H, Luo X. Elevated homocysteine level related to poor outcome after thrombolysis in acute ischemic stroke. Med Sci Monit. 2016;15(22):3268–3273. doi:10.12659/MSM.900010
18. Petras M, Tatarkova Z, Kovalska M, et al. Hyperhomocysteinemia as a risk factor for the neuronal system disorders. J Physiol Pharmacol. 2014;65(1):15–23.
19. Lehotský J, Tothová B, Kovalská M, et al. Role of homocysteine in the ischemic stroke and development of ischemic tolerance. Front Neurosci. 2016;23(10):538.
20. Tu WJ, Zhao SJ, Liu TG, Yang DG, Chen H. Combination of high-sensitivity C-reactive protein and homocysteine predicts the short-term outcomes of Chinese patients with acute ischemic stroke. Neurol Res. 2013;35(9):912–921. doi:10.1179/1743132813Y.0000000228
21. Tu WJ, Chao BH, Ma L, et al. Case-fatality, disability and recurrence rates after first-ever stroke: a study from bigdata observatory platform for stroke of China. Brain Res Bull. 2021;175:130–135. doi:10.1016/j.brainresbull.2021.07.020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.