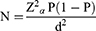Back to Journals » Veterinary Medicine: Research and Reports » Volume 12
Study on Prevalence of Ixodid Ticks of Goats and Acaricide Utilization Practices of Herd Owners in Benatsemay District, South Omo Zone, South-Western Ethiopia
Authors Kifle T, Mathewos M , Fesseha H , Abate A , Wolde A
Received 18 June 2021
Accepted for publication 7 September 2021
Published 16 September 2021 Volume 2021:12 Pages 225—233
DOI https://doi.org/10.2147/VMRR.S324484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Temesgen Kifle,1 Mesfin Mathewos,1 Haben Fesseha,1 Aschenaki Abate,2 Amanuel Wolde3
1School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 2Jinka Agricultural Research Center, Animal Health Research, Jinka, Ethiopia; 3College of Agriculture, Department of Animal Health, Jinka University, Jinka, Ethiopia
Correspondence: Mesfin Mathewos Email [email protected]
Introduction: Ticks are major health problems of goats that contribute to a significant economic loss in Ethiopia.
Methods: A cross-sectional study using a systematic random sampling technique was conducted to determine the prevalence, potential risk factors of hard ticks (Acarina: Ixodidae) of goats, and the acaricide utilization practice of herd owners in the Benatsemay district.
Results: Out of 285 examined goats, 85.26% of goats were found infested by Rhipicephalus and Amblyomma tick genera. The body condition score of goats was the only factor that was significantly (p < 0.05) associated with tick infestations. The frequently identified tick species were Rhipicephalus pulchellus (14.38%), Rh. decoloratus (11.22%), A. cohaerens (5.26%) and A. variegatum (4.21%), and mixed infestation (38.59%). Herd owner’s questionnaire survey revealed that 100% of interviewees responded that tick infestations were frequently encountered throughout the year and prevailed on aged goats (90%) and dry season (60%). Concurrent usage of ethnomedicinal plants and conventional acaricides were dominant practices to control tick infestation as responded by 60% of interviewees. Among the conventional acaricides, Diazinon and Ivermectin were the most practiced acaricides as replied by 90% of the respondents. Moreover, community animal health workers (CAHWs) (43.33%) and owners themselves (33.33%) were primarily responsible for acaricide application to tick-infested animals. About 66.67% of the livestock owner’s responses also disclosed that diazinon was the most effective acaricide followed by ivermectin (16.67%) and amitraz (6.67%). Finally, 56.57% of the respondents replied that acaricidal drugs from private veterinary drug shops were overpriced than the same acaricides from government veterinary clinics.
Conclusion: In this study, there was a high prevalence of hard ticks in goats, and irrational application of acaricides was noted in the Benatsemay district; thus, appropriate tick control measures should be taken to minimize tick burden through using acaricides.
Keywords: acaricide, Benatsemay, goats, Ixodidae ticks
Introduction
In Ethiopia, small ruminants are found in a variety of agro-ecological zones and production systems,1 and they are an important aspect of rural households’ livelihoods and a source of export revenue for the country. Small ruminants play an important role in household income creation and food security, accounting for roughly 23–39% of agricultural cash income and contributing significantly to the growth and development of the national economy.2,3 Small ruminants have a high reproductive rate, making them valuable sources of quick monetary income and meat for domestic consumption.4 However, this massive population’s contribution to food production and export revenue is significantly less than planned.5
Among the numerous causes, one of the associated factors responsible for hampering the expected potential of small ruminant production and productivity is ectoparasite infestation.5,6 When goats are infected with ectoparasites, their ability to deliver the aforementioned products and services is harmed. Goats’ health is also harmed by ectoparasites, which cause them to lose weight, slow their growth, and diminish their output. Arthropod ectoparasites can have several direct and indirect consequences on their hosts as a result of their activity.7
Among ectoparasites, ticks cause a wide range of health problems on small ruminants which results in a direct and indirect loss.7 Tick infestation can result in mechanical tissue damage, irritation, inflammation, hypersensitivity, abscesses, weight loss, lameness, anemia, and, in the worst-case scenario, mortality of infected animals, with socioeconomic consequences.8 They are also to account for significant pre-slaughter and tannery-processed skin flaws, which lead to the downgrading and rejection of small ruminant hides. Furthermore, because of their blood-sucking habit, ticks can transmit a variety of disease infections from animals to animals and from animals to people.9
South Omo is one of Ethiopia’s pastoral zones, with abundant animal resources. In South Omo Zone, small ruminants, especially goats have a major role in supporting the livelihood of pastoral communities. However, the income generated from this small ruminant is marginal due to different constraints. Tick infestation is among the constraint that hampers the productivity of goats in areas.8,10
Chemical treatment is still the most effective tick control approach in Ethiopia. Nonetheless, the uncontrolled and high frequency of commercial acaricide applications may have hastened the emergence of tick resistance to a variety of active chemicals. Following the introduction of acaricide in Africa around 1890, tick treatment based on various application methods became the primary technique of tick control in Africa, resulting in a slew of issues such as pollution, the development of resistant tick strains, and rising expenses11 Similarly, ticks have been mostly controlled in Ethiopia for decades by a range of acaricides, such as organochlorines, organophosphates, macrocyclic lactones, carbamates, amidines, or synthetic pyrethroids.12 Yet, ticks usually develop resistance to acaricides when exposed to favorable factors such as most widespread usage, under concentration, frequent use of organochlorines, and organophosphates compound.11
The inclusion of local indigenous knowledge is ever more becoming a topical subject to enhance livestock veterinary care and helps in decision-making about fundamental aspects in the life of the farmers. Ticks were the major ectoparasites of goats that affect productivity in the KwaZulu-Natal Province of South Africa. The extent of indigenous knowledge utilization is affected by factors such as the type of rangeland, gender, age, residing on a farm, and also having the herbalist in the locality. Thus, this study has revealed that indigenous knowledge should be applied and incorporated in policies including the participation and interaction of indigenous knowledge custodians.13
Moreover, unbalanced demand and supply of acaricide to livestock/goat owners at the veterinary clinic to treat their infected goats; exposing owners to purchase poor quality acaricides from unauthorized sources or vet drug smugglers, which in turn could result in the development of acaricide failure in the study area.10 To conduct out effective tick control and/or tick burden reduction, regular research on the dynamics of tick populations and species composition, as well as the current efficacy status of acaricides against the most prevalent and economically relevant tick species in an area, were required.14 Hence, the goal of this study was to assess the prevalence and potential risk factors of hard ticks in goats, as well as herd owners’ acaricide usage practices in the Benatsemay district.
Materials and Methods
Study Area
Benatsemay is one of the ten districts of the South Omo zone located in Southern Ethiopia. It is located at the latitude ranges between 5º00ʹ31” N - 5º41ʹ47”N and longitudes range between 36º12ʹ13” E - 37º03ʹ50” E. The district is named after Bena and Tsemay people who are living in this district. Benatsemay is bordered on the south by Hamer, on the west by Salamago, on the north by South Ari, on the northeast by the Malle, on the east by Alle districts, and on the southeast by the Oromia Region. The White River separates it from the Alle districts and Oromia Region. The western part of this district is included in the Mago National Park. The administrative center of the Benatsemay district is Keyafer which is located about 702 km southwest of Addis Ababa and 42 km southeast of the zonal town, Jinka. The zone has an area coverage of 24,249 km2 having a total of 1.75 million cattle, 1.55 million sheep, and 2.88 million goats.15
Study Animals
The study animals were indigenous Woito-Guji goat breed that was managed under an agro-pastoral extensive management system. These goat breeds were distinguished by their small body sizes when compared to those found in highland places like Konso and Gamo Gofa.16 In the district, goats were allowed to browse away from the farms throughout the year. All goat populations are watered at the river or community boreholes locally known as “Chirosh” when seasonal rivers dry out. In the district, there is a crash system of housing created from locally available materials that have no roof to protect animals from bad weather but does keep them from walking out at night. The biggest issue faced by goat owners was a lack of quality and quantity feeds as well as water, especially during the dry season. The scoring of the body condition of the goats was determined according to Villaquiran et al,17 with a scale of 1.0 to 5.0. Thus, goats with a score of 1 and 2 were categorized as poor, 3 as a medium, and 4 and 5 as good.
Study Design and Sampling Techniques
A cross-sectional study design was undertaken from December 2019 to May 2020 to assess the prevalence and potential risk factors of hard ticks of goats and acaricide utilization practice of herd owners in three selected kebeles of Benatsemay district as Diziaman, Luka, and Olkakbo. The research district was purposefully chosen for its accessibility and convenience. Representative kebeles (peasant associations), on the other hand, were chosenrandomly from a list of Kebeles found in the district. By using the Kebeles administration’s lists, representative goat owners were chosen at random from selected Kebeles. To account for these host-related characteristics, goat owners’ herds were stratified based on their sex, age, and body condition score. The research animals were systematically recruited from selected strata (herds) by calculating the interval between the first and second picked animals, and then the nth animal were picked, depending on the size of each herd chosen. Besides, a total of 30 herdsmen were included due to the lack of stable settlement of the pastoralists or the nomadic nature of the herdsmen.
Sample Size Determination
The sample size was calculated using a previously estimated tick prevalence of 85.57% reported by Mebrahtu et al,10 and the desired accuracy level of 5% at the 95% confidence level. As a result, the sample size was calculated using the Thrusfield18 formula.
Where;
N= Total sample size, P= expected prevalence, Zα = Z0.05 = 1.96 (the value of Zα required for confidence=95%), d = desired precision.
Using the formula above, a total of 190 animals would have been the best-predicted sample size for the district’s tick prevalence study. To improve the precision of the study results, the sample size was raised by 50%, resulting in 285 goats being sampled from three study Kebeles. From secondary recorded data, the total goat population was obtained for each study Kebele and the study animal representing the Kebele were taken proportionally based on the goat population of respective Kebele.
Study Methodology
Tick Collection and Laboratory Identification
The goats were visually examined from head to tail by giving attention to the main predilection sites for the presence of ticks. Both engorged and semi-engorged adult or mature ticks were considered during sample collection. Ticks were removed with forceps from various body parts of animals, including the ear, tail, neck, brisket, dewlap, back, hoof, testes, and udder, and treated in 70% ethanol. Animals were properly restrained and all body parts were thoroughly checked before being removed, following the technique used by previous personnel. All ticks were removed from the bodies of cattle using tiny forceps and tick removal safety precautions.19 Tick genus and species were identified using a stereo microscope at the Jinka regional veterinary laboratory.20 All field and laboratory data were recorded on pre-prepared data collection sheet.
Questionnaire Survey
A structured questionnaire was administered to goat owners (pastoralists) to collect data on problems of tick infestation and acaricide utilization practices. Before dissemination of the questionnaire to respective participants, the questionnaire was first translated into the Bena language. Secondary Information from the study district was also recorded and analyzed.
Data Management and Statistical Analysis
All statistical analyses were carried out using STATA version 13 software after the data was loaded into the Microsoft Office Excel 2016 computer program (Stata Corp. College Station, TX). The proportions of variables recorded during the questionnaire survey were analyzed using descriptive statistics (frequencies and percentages). The chi-square test was used to examine the relationship between multiple risk factors (Kebeles, age, sex, BCS, and herd size) and tick infestation. For the presence of a significant connection, a p-value of less than 0.05 was used.
Ethics Approval and Consent to Participate
This research obtained ethical approval from the Wolaita Sodo University, Research Ethics and Review Committee. Before collecting samples, the goat owners’ verbal consent to take samples from their goats was obtained, and ticks were gathered from various body regions while adhering to stringent hygiene measures. The best practice guidelines for veterinary care were followed and the study’s goal was explained to the goat owners, and the Wolaita Sodo University Research Ethics and Review Committee approved the verbal informed consent process in the manuscript.
Results
Questionnaire Survey on Tick Infestation and Acaricide Usage
A total of 30 pastoralists (10 pastoralists from each Kebele) were interviewed about the situations of tick infestation and acaricide utilization in their area. Accordingly, all interviewees (100%) responded that they encounter frequent tick infestations throughout the year which was more prevalent in aged goats (90%) and dry season (60%) but equally infest both sex groups (70%) (Table 1).
 |
Table 1 Interviewee Response on the Frequency of Tick Infestation Among Sex, Age, and Season |
According to the respondents, concurrent usage of traditional (smearing of water solution of different ethnomedicinal plants) and modern acaricides (conventional drugs) (60%) were dominantly applied to control tick infestation. Among the conventional acaricides, interchangeable use (depending on their availability) of Diazinon and Ivermectin was mainly (90%) preferred by the respondents. This study also revealed that governmental veterinary clinic (63.33%) was the dominant source of acaricide in the area followed by authorized private veterinary drug shops (26.66%) (Table 2). Moreover, community animal health workers (CAHWs) (43.33%) and owners themselves (33.33%) were primarily responsible for acaricide application (injection) to infected animals (Table 2).
 |
Table 2 Interviewee Response on Tick Control Methods, Acaricide Preference, Source of Acaricide, and the Person Responsible for Acaricide Application |
The livestock owner’s response also disclosed that Diazinon (66.67%) was the most effective acaricide followed by Ivermectin (16.67%) and amitraz (6.67%) (Table 3). Deltamethrin is recognized as a costly acaricide in both government veterinary clinics (also rarely available) and private veterinary drug shops. 56.57% of the respondents revealed that acaricidal drugs from private veterinary drug shops are overpriced than the same acaricide from government veterinary clinics (Table 3).
 |
Table 3 Pastoralist’s Response on Efficacy and Cost of Acaricide |
Tick Infestation and Associated Risk Factors
From a total of 285 goats surveyed for tick infestation from three kebeles (peasant association) of the study district, 243 goats were found to be infected with ticks of two genera; Rhipicephalus (Rh.) and Amblyomma (A.) which resulted in an overall prevalence of 85.26%. A high infestation rate was reported in female goats (59.57%) than males (40.43%) and in animals with 1–3-year-old (43.21%) than animals greater than three years old (42.8%). Medium conditioned (51.85%) goats were highly infected than poorly conditioned animals and this was significantly (p< 0.05) associated with the tick infestation. Tick infestations were not significantly associated (p> 0.05) with the sex, age, herd size, and study kebeles (peasant association) (Table 4).
 |
Table 4 Tick Infestation in Each Kebele and Overall Prevalence |
Type of Infestations by Tick Species in the Study Area
Rhipicephalus and Amblyomma were the two tick genera identified in the area during this study. Among the total tick-infected goats, 38.59% were infected with more than one tick genera (mixed infestation), 14.38% were infected with Rh. pulchellus, 11.22% were infected with Rh. decoloratus, 5.26% were infected with A. cohaerens and 4.21% were infected with A. variegatum (Table 5).
 |
Table 5 Type of Infestations by Tick Species in the Study Area |
Correlation Analysis of Tick Prevalence with Associated Risk Factors
The correlation of the tick infestation of goats with factors such as origin, sex, age, and body condition was conducted to investigate the relationship among different variables and summarized in Table 6. Among the associated risk factors, the body condition of the goats has a positive correlation (r= 0.1329) with the prevalence of hard tick infestation. However, the factors such as origin, sex, and age of the animals have an inverse correlation with the hard tick infestation (Table 6).
 |
Table 6 Relationship of Tick Prevalence with Associated Risk Factors |
Discussion
The prevalence of goat tick infestation in the current study was 85.26%. The current prevalence was higher than the findings reported by Fentahun et al,21 20% from Gondar town, Tesfaheywet and Simeon,22 31.3% from Bench Maji Zone, southern Ethiopia, Habtemichael et al23 87.4% from Hargelle District of Afder Zone in Somali Region, Israel et al24 27.5% from Sodo Zuria district, Tefera,25 22.2% from selected districts of Amhara region, Tesfaye et al26 6.3% from Bahir Dar, Zeryehun and Atomsa,27 10.2% from Western Shoa Zone. However, a slightly higher prevalence was reported by Fikre et al28 who reported a prevalence of 90.88% from the pastoral district of Afar. Moreover, the current finding coincides with the report of Mebrahtu et al10 that indicated a prevalence of 85.57% from the selected districts of the South Omo Zone.
The variation in the prevalence of tick infestation in our study as compared to earlier reports could be attributed to differences in control activities among the study areas, differences in agroecology, animal management practices, production systems, and population density. The research area’s increased prevalence of goat tick infestation could be due to goats and other ruminants’ frequent exposure to the same community grazing space, which favored frequent contact with sick animals and questing ticks on the surroundings. In addition, the widespread occurrence of tick infestation in the research area is thought to be related to a poor ectoparasite management system and a lack of awareness among goat owners about the effects of ectoparasites.
The prevalence of tick infestation was not significantly (p> 0.05) associated with putative risk factors such as Kebeles, sex, age, BCS, and herd size. This might be associated with the study district (Benatsemay) is categorized under a pastoral production system with lowland altitude coupled with unrestricted animal movements. Therefore, the dynamics and frequent mobility of flocks involving different animal species of all ages, sex, and BCS groups together and frequent exposure to the same open rangeland in pastoral areas increase the chance of direct contact between animals. Therefore, this favored the transmission of tick parasites from animal to animal regardless of Kebele, sex, age, BCS, and herd size.29,30
In this study, seven species of ticks belonging to two genera (Rhipicephalus and Amblyomma) were identified in the area. Infestation with similar compositions of tick genera was reported by Fikre et al28 from Afar region, Fentahun et al21 from Gondar town, Tesfaheywet and Simeon,22 from Bench Maji Zone, southern Ethiopia, Habtemichael et al23 from Hargelle District of Afder Zone in Somali Region, Israel et al24 27.5% from Sodo Zuria district, Tefera,25 22.2% from selected districts of Amhara region, Tesfaye et al26 from Bahir Dar, Zeryehun and Atomsa,27 from Western Shoa Zone on small ruminants from different parts of Ethiopia. Among the species identified, Rhipicephalus pulchellus was the most dominant tick species followed by Rh. decoloratus, Rh. evertsi evertsi and A. cohaerens.
This species composition was in line with previous studies conducted in selected districts of the South Omo Zone.10 Also, previous works of31 and32 reported Rhipicephalus pulchellus was the dominant tick species, research was conducted in other parts of the country. Regarding specific tick species prevalence, higher prevalence of different Rhipicephalus species; Rhipicephalus pulchellus (47.7%), Rh. decoloratus (29.3%), Rh. evertsi evertsi (23.3%) and Rh. pravus (22.8%) were reported previously in studies in selected districts of the South Omo Zone.10 However, specific Amblyomma species prevalence of current study finding was slightly higher than previously reported study result on the above-mentioned area by the same authors as 3.9%, 1.4%, and 0.99%, respectively, for A. variegatum, A. cohaerens, and A. gemma.
The correlation of the tick infestation of goats with factors such as origin, sex, age, and body condition showed that the body condition of the goats has a positive correlation (r= 0.1329) with the prevalence of hard infestation whereas origin, sex, and age of the animals has an inverse correlation with the hard tick infestation. This might be due to tick attributed to the loss of body weight as they suck blood, and induction of nuisances as tick feed on the different body surface of the animal and this, in turn, lead the goats spent a long period on rubbing against inanimate objects.
The questionnaire survey of herd owners on acaricide efficacy revealed that diazinon (66.7%) was the most practiced acaricide as compared with other acaricide followed by ivermectin (6.7%) against Amblyomma and Rhipicephalus. This finding was inconsistent with the previous reports of Sajid et al;33 Turkson and Botchey,34 who reported a field strain of A. variegatum were resistant to Diazinon in In vivo application as compared with ivermectin which showed greater efficacy against A. variegatum at the recommended dose after 7 days post-exposure. Similarly, Tessema and Gashaw35 and Habtemichael et al23 reported that ticks have developed resistance against organophosphate acaricides. The disparities in the efficacy of these acaricides were most likely due to their widespread use, irregular application, insufficient spraying, and incorrect mixing, as well as the usage of acaricides that had been held for a long period after dilution.
Conclusion
This study revealed a high prevalence of tick infestation on the goats of the study district and identified two major tick genera of Rhipicephalus and Amblyomma. Among identified tick species, Rh. pulchellus, Rh. decoloratus, A. cohaerens, and A. variegatum were the most prevalent tick species. Mixed infestation of different tick species was dominantly detected on infected goats. Among conventional acaricides, Diazinon and Ivermectin were preferred and frequently practiced over deltamethrin by the goat owners due to their easy availability in the pharmaceutical market and affordable price. Although goat owners use conventional acaricides as a control option, they also use ethnobotanicals to control ticks and other ectoparasites. Furthermore, community animal health workers (CAHWs) and owners were responsible for the injection or application of acaricides to tick-infected animals. Thus, awareness creation should be created to herd owners on rational usage of acaricides to hold out resistance development, and also on ethnobotanical plants that were traditionally used by pastoralists for further determination of the drug efficacy and toxicity. The indigenous knowledge of the pastoral peoples should be included in government policies and implemented to promote its utilization and enhance livestock veterinary care.
Abbreviations
BCS, Body Condition Score; BHC, Benzene-hexachloride; CAHWs, Community Animal Health Workers; CSA, Central Statistical Agency of Ethiopia; DDT, Dichlorodiphenyltrichloroethane; SNNPR, South Nations Nationalities and Peoples Region; SOZLFD, South Omo Zone Livestock and Fishery Department.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This research obtained ethical approval from the Wolaita Sodo University, Research Ethics and Review Committee. Before collecting samples, the goat owners’ verbal consent to take samples from their goats was obtained, and ticks were gathered from various body regions while adhering to stringent hygiene measures. The best practice guidelines for veterinary care were followed and the study’s goal was explained to the goat owners, and the Wolaita Sodo University Research Ethics and Review Committee approved the verbal informed consent process in the manuscript.
Acknowledgments
The authors would like to acknowledge the regional veterinary laboratory of Sodo.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was not supported by any funding source or institution.
Disclosure
All authors declared no competing conflicts of interest for this work.
References
1. Mengesha M, Tsega W. Indigenous sheep production in Ethiopia: a review. Iran J Appl Anim Sci. 2012;2(4):311–318.
2. Asresie A, Zemedu L, Adigrat E. The contribution of livestock sector in Ethiopian economy. Adv Life Sci Technol. 2015;29:79–90.
3. Galiè A, Mulema A, Benard MAM, Onzere SN, Colverson KE. Exploring gender perceptions of resource ownership and their implications for food security among rural livestock owners in Tanzania, Ethiopia, and Nicaragua. Agric Food Secur. 2015;4(1):1–14. doi:10.1186/s40066-015-0021-9
4. Tolera A, Abebe A. Livestock production in pastoral and agro-pastoral production systems of southern Ethiopia. Livest Res Rural Dev. 2007;19(12):4–7.
5. Debele G, Guru M, Hundessa F, Duguma M. Assessment of farmers’ management practices and factors affecting goats’ production system in Adami Tulu Jido Kombolcha district of EastShawa zone, Ethiopia. Agric Biol JN Am. 2013;4(5):520–526.
6. Legesse G, Siegmund-Schultze M, Abebe G, Zárate AV. Economic performance of small ruminants in mixed-farming systems of Southern Ethiopia. Trop Anim Health Prod. 2010;42(7):1531–1539. doi:10.1007/s11250-010-9603-5
7. Tolossa YH. Ectoparasitism: threat to Ethiopian small ruminant population and tanning industry. J Vet Med Anim Health. 2014;6(1):25–33. doi:10.5897/JVMAH2013.0253
8. Getaneh D, Tesfaye T, Alemayehu Y. Characterization of farming and agricultural production systems, production constraints and need identification In Debub Ari and Benatsemay Districts of Southern Ethiopia. Int Res J Sci Technol. 2021;2(2):359–373.
9. Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012;28(10):437–446. doi:10.1016/j.pt.2012.07.003
10. Mebrahtu K, Tesfaye T, Getachew S, Alaro T, Dachaw B. Prevalence of small ruminant ectoparasites in selected Districts of South Omo Zone, Southern Ethiopia. Adv Biol Res. 2018;12(2):91–96.
11. Admasu P, Wakayo BU, Megersa M, Feyera T. In vitro and in vivo acaricidal efficacy study of amitraz and diazinon against some tick species infesting Camelus dromedarius around Jigjiga, Eastern Ethiopia. Afr J Pharm Pharmacol. 2015;9(34):850–855. doi:10.5897/AJPP2015.4425
12. Etana A, Tadesse E, Mengistu A, Hassen A. Advanced evaluation of cowpea (Vigna unguiculata) accessions for fodder production in the central rift valley of Ethiopia. J Agric Ext Rural Dev. 2013;5(3):55–61.
13. Mkwanazi M, Ndlela S, Chimonyo M. Utilisation of indigenous knowledge to control ticks in goats: a case of KwaZulu-Natal Province, South Africa. Trop Anim Health Prod. 2020;52(3):1375–1383. doi:10.1007/s11250-019-02145-0
14. Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(S1):S3–S14. doi:10.1017/S0031182004005967
15. Central Statistical Agency. Agricultural sample survey. Report on livestock and livestock characteristics. 2017:1–194.
16. Molla B. The health performance of imported Boer goat (Capra hircus) and their crosses with Woito-guji goat breeds in South Omo Zone, South-Western Ethiopia. Trop Anim Health Prod. 2016;48(4):855–861. doi:10.1007/s11250-016-1018-5
17. Villaquiran M, Gipson TA, Merkel RC, Goetsch AL, Sahlu T. Body condition scores in goats. Langston University: American Institute for Goat Research; 2004.
18. Thrusfield M. Veterinary Epidemiology. John Wiley & Sons; 2018.
19. Soulsby E, Lloyd S. Passive immunization in cystercosis: characterization of antibodies concerned. 1982.
20. Walker AR. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Edinburgh: Bioscience Reports; 2003.
21. Fentahun T, Woldemariam F, Chanie M, Berhan M. Prevalence of ectoparasites on small ruminants in and around Gondar Town. Am Eurasian J Sci Res. 2012;7(3):106–111.
22. Tesfaheywet Z, Simeon H. Major ectoparasites of small ruminants in Bench Maji Zone, southern Ethiopia. Livest Res Rural Dev. 2016;28(4):63.
23. Habtemichael Y, Alemu A, Adem A, Felek B. Epidemiological and therapeutics studies on tick species of small ruminants in Hargelle District, Afder Zone, Somali Region, Ethiopia. EntomolOrnithol Herpetol. 2020;9(234):2161–0983.
24. Israel Y, Abera T, Wakayo BU. Epidemiological study on ectoparasite infestation of small ruminants in Sodo Zuria District, Southern Ethiopia. J Vet Med Anim Health. 2015;7:140–144. doi:10.5897/JVMAH2014.0358
25. Tefera S. Investigation on Ectoparasites of Small Ruminants in Selected Sites of Amhara Regional State and Their Impact on the Tanning Industry. Bishoftu, Ethiopia: Faculty of Veterinary Medicine, Addis Ababa University; 2004.
26. Tesfaye D, Assefa M, Demissie T, Taye M. Ectoparasites of small ruminants presented at Bahir Dar veterinary clinic, Northwest Ethiopia. Afr J Agric Res. 2012;7(33):4669–4674. doi:10.5897/AJAR12.599
27. Zeryehun T, Atomsa M. Ectoparasite infestations of sheep and goats. Eurasian J Vet Sci. 2012;28(4):185–189.
28. Fikre Z, Hailegebrael B, Mu’uz G, Ahmed S, Ashenafi G. Epidemiology of major small ruminant ectoparasites and effectiveness of the control approaches employed in selected pastoral districts of Afar, Northeastern Ethiopia. J Biol Agric Healthc. 2015;5(14):63–72.
29. Hussen AH, Agonafir A. A study on ticks affecting camels (Camelus dromedarius) in Jigjiga district of Somali region, Eastern Ethiopia. Int J Adv Res Biol Sci. 2018;5(9):121–130.
30. Akande F, Adebowale A, Idowu O, Sofela O. Prevalence of ticks on indigenous breed of hunting dogs in Ogun State, Nigeria. Sokoto J Vet Sci. 2018;16(3):66–71. doi:10.4314/sokjvs.v16i3.10
31. Ahmed J, Wendemagegn D, Tsehay A, Silesh S, Abebe H. Prevalence of tick infestation on small ruminants in and around Dire Dawa, Eastern Ethiopia. Int J Res Granthaalayah. 2017;5(5):326–336. doi:10.29121/granthaalayah.v5.i5.2017.1864
32. Eyob E, Matios L. Preliminary survey on the distribution of ixodid ticks in small ruminants of Dhas District of Borena pastoral area, Southern Rangelands of Ethiopia. Adv Biores. 2014;5(1):87–91.
33. Sajid MS, Iqbal Z, Khan MN, Muhammad G. In vitro and in vivo efficacies of ivermectin and cypermethrin against the cattle tick Hyalomma anatolicum anatolicum (Acari: Ixodidae). Parasitol Res. 2009;105(4):1133–1138. doi:10.1007/s00436-009-1538-2
34. Turkson P, Botchey M. Acaricide resistance in the cattle tick, Amblyomma variegatum, in the coastal savanna zone of Ghana. Ghana J Agric Sci. 1999;32(2):199–204. doi:10.4314/gjas.v32i2.1902
35. Tessema T, Gashaw A. Prevalence of ticks on local and crossbred cattle in and around Asella town, southeast Ethiopia. Ethiop Vet J. 2010;14(2):79–89.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.