Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Study on Biomarkers Related to the Treatment of Post-Stroke Depression and Alternative Medical Treatment Methods
Authors Li M, Ding R, Yang X, Ran D
Received 2 May 2022
Accepted for publication 17 August 2022
Published 26 August 2022 Volume 2022:18 Pages 1861—1873
DOI https://doi.org/10.2147/NDT.S370848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Menghan Li,1,2,* Ran Ding,3,* Xinming Yang,1,2 Dawei Ran1,2
1Acupuncture-Moxibustion Clinical Department, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, 300381, People’s Republic of China; 2Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 3School of Medical Informatics Engineering, Anhui University of Chinese Medicine, Hefei, 230012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Menghan Li, Email [email protected]
Purpose: PSD is a syndrome that occurs after a stroke, which manifests as a series of depressive symptoms and corresponding physiological symptoms. Relevant studies have shown that the drug therapy is often accompanied by drug side effects and patient resistance. Acupuncture has attracted attention as a treatment method without adverse reactions of patients. The purpose of this study was to investigate the possible mechanism of action of acupuncture in PSD.
Patients and Methods: Download depression and stroke datasets from public databases. Bioinformatics methods were used to analyze the key gene targets related to stroke and depression. Functional enrichment analysis assesses important pathways. Further screen PSD-related biological pathways and genes. After the experimental model was established, the expression differences of key genes and related pathways were compared between the model group and the control group through acupuncture treatment and qPCR verification.
Results: Depression and stroke-related genes were obtained by bioinformatics methods, and then important biological processes and biological pathways related to depression and stroke were analyzed by GO and KEGG. And further screen out the disease targets closely related to PSD. In the follow-up animal experiments, we confirmed that acupuncture can intervene on these key pathways and targets, and then play a role in the targeted therapy of diseases.
Conclusion: The results of this study show that five genes (“NRBP1”, “SIRT1”, “BDNF”, “MAPK3”, “CREB1”.) and key biological pathways such as NFkB, PI3K/AKT activation, and MAPK are the keys to the occurrence and development of PSD biomarkers, which can also be therapeutically intervened by acupuncture.
Keywords: PSD, bioinformation, biomarkers, acupuncture
Introduction
PSD is an affective disorder that occurs after acute cerebrovascular injury. It is one of the most common secondary complications of stroke, which has a significant impact on the quality of life of patients and increases the morbidity and mortality of stroke.1 Studies have shown that stroke can increase the risk of depression. Thirty-one percent of stroke patients develop depression within 5 years of stroke. Post-stroke depression has become a serious social public health problem.2 Therefore, exploring the pathogenesis and treatment of PSD is an urgent problem.
Acupuncture, as an inexpensive and safe treatment method, has been widely used for thousands of years to improve certain neurological disorders such as stroke and its sequelae. In addition, multiple clinical studies and systematic reviews suggest that acupuncture,3,4 as a promising intervention, can improve motor and language functions and improve activities of daily living. And there are many studies showing that acupuncture also has the ability to help reorganization,5 which is helpful for patient recovery. With the growing interest in acupuncture therapy, a lot of research has been done on acupuncture therapy after it was introduced into the scientific community. Acupuncture may become an effective adjunctive therapy or an acceptable alternative therapy for the treatment of functional disorders including post-stroke depression.6
Related studies have examined 13 cohort studies involving 3536 participants and found that compared with non-PSD patients, PSD patients had significantly higher C-reactive protein levels on admission, proving that C-reactive protein levels were elevated in the acute phase of stroke. Suggest an increased risk of PSD. The discovery of biomarkers has significant implications for the clinical treatment of PSD.7
The current first-line treatment for PSD is SSRIs, which have some clinical efficacy but cause more cardiovascular and neurological side effects.8 The debate about SSRIs for the treatment of PSD has focused more on safety, especially in stroke patients who often take multiple drugs at the same time, and the harm tends to be greater. Therefore, how to effectively and safely treat PSD is a hot and difficult point in the medical field at home and abroad.
Studies have shown that acupuncture can effectively reduce depression scales such as the HAMD in patients with PSD scores,9 improve depression-related symptoms, improve patients’ quality of life, and regulate patients’ oxidative stress levels and immune environment.10,11 However, its mechanism of action has not been fully elucidated, which affects the application and promotion of acupuncture.
In this study, we eliminated batch effects, combined multiple gene expression profiling dataset, and introduced the concept of weighting. Identification of key gene sets for stroke by weighted gene co-expression network analysis. RRA is an algorithm that integrates sorting to obtain a comprehensively sorted list. Through this algorithm, we obtained the key depression genes in two depression datasets, and combined with the PSD causative genes that have been found in research to obtain five PSD biomarkers and key biological pathways.
Materials and Methods
Data Download and Processing
Stroke and depression gene expression data such as GSE44593, GSE22255, GSE182195, GSE54565, GSE78731, GSE60820, GSE97533 and GSE137595 were downloaded from the GEO database, respectively. Table 1 details the basic information of the selected datasets. Probe expression matrices were extracted and normalized according to the R package affy using RMA. Convert probe expression matrices to gene expression matrices via Platform annotation files. If a gene corresponds to multiple probes, we take the average. Download the latest list of human and mouse homologous genes from NCBI, and use the R package biomaRt to transform the homologous genes. When a gene corresponds to multiple genes, we uniformly select the one with the highest average gene expression. Heterogeneity from different experimental batches and platforms was eliminated with the R package sva. Finally, we transformed the four gene matrices GSE137595, GSE60820, GSE78731, GSE97533 into a normalized gene expression.
 |
Table 1 Summary of the Dataset |
WGCNA Analysis
First, we analyzed the gene expression profiling data after removing batch effects from the normalized gene expression matrix using the limma function package in R. Gene expression data of stroke samples and their corresponding clinical characteristics were used as input data for WGCNA. First, cluster analysis is used to identify and remove outliers. The optimal soft-threshold power value is then determined, which is crucial for guaranteeing a scale-free network. Finally, using the criteria such as minModuleize and FusionCutHeight, potential key gene modules for depression were found. Modules are then selected according to their relationship and clinical characteristics.
DEGs Analysis
The RRA method is an algorithm that integrates the rankings to obtain a comprehensive ranking list, uses the limma package to screen for differentially expressed genes, uses the t-test method to calculate the p-value of the gene, and uses the method of Benjamini and Hochberg to calculate the adjusted p-value. Selection criteria for screening differentially expressed genes were as follows: at least a 1.0-fold change between healthy controls and samples from patients with depression, with an adjusted p-value less than 0.05. According to the difference multiple logFC sorting of the difference analysis, for multiple sorted gene sets, when the intersection is calculated, their sorting situation is also considered. In general, it is to select those genes that show differences in multiple data sets, and those that are ranked high for each difference, their final comprehensive ranking will also be relatively high. In this way, we have identified genes that are key to the development of depression. At the same time, we obtained the overlapping parts of the three key genes of PSD by taking key gene modules of stroke, key DEGs related to depression, and Genecards database, and further refined the key genes of PSD.
Functional Enrichment Analysis
GO terms and KEGG pathway enrichment analysis were performed based on the clusterProfiler package in R language. A significant threshold was set at p < 0.05. FunRich is a stand-alone software tool for functional enrichment and interaction network analysis of genes and proteins. Enrichment analysis is performed through the loaded back-end database.
Predictive Value of Biomarkers
We analyzed human ischemic stroke using the GSE22255 dataset in the GEO database, studied the expression differences of key genes in normal and diseased samples, and determined whether these genes can be used as a basis for determining stroke. AUC value was used to evaluate the predictive validity of the biomarkers, and the ROC curve was derived from the R-package survival ROC. ROC curve analysis was used to assess the ability of key genes to differentiate stroke disease samples.
Single-Sample Gene Set Enrichment Analysis
Bindea et al provided marker genes for many types of immune cells. For each immune-related cell type, enrichment scores were calculated using the ssGSEA technique. Immune cell infiltration levels were calculated using the ssGSEA function of the GSVA package in R. We observed changes in immune cell infiltration between stroke patients and healthy individuals. Pearson correlation was used to analyze the correlation between different infiltration degrees of immune cells and genes.
Quantitative Reverse Transcription Polymerase Chain Reaction
Ten 3-month-old male SD rats were selected for animal modeling. MCAO was used to replicate the ischemic stroke model. The left neck muscle of the rat was bluntly dissected, and the right external carotid artery was ligated. A 5mm gap was cut at the bifurcation of the common carotid artery, a carbon fishing line was inserted, and the fishing line was gently pushed through the internal carotid artery into the skull, resulting in focal cerebral ischemic changes, and the internal carotid artery was ligated. After anesthesia, the right forelimb of the rat was weak and crawled to the right without irritability. After 24 hours, a Longa 5-point neurological evaluation was performed, and rats with a score of 1–3 were selected as stroke model rats.
Modeling of post-stroke depression in rats using the 21-day CUMS. CUMS was performed 2 days after MCAO and consisted of 7 different stimuli in random order: 1) 80dB noise stimulation for 12 hours; 2) in moist litter with cage tilted at about 45 degrees for 24 hours; 3) LED flash stimulation 12 hours; 4) swimming in ice water at 0°C for 5 minutes; 5) fasting and drinking for 12 hours; 6) day-night reversal; 7) tail clipping for 3 minutes. Each of the above stimulation methods was randomly selected daily for 3 consecutive weeks.
After 4 days, the PSD rat model was successfully established, and the intervention was started. After immobilizing the rats, acupuncture treatment was performed. Select points Neiguan (PC6), Shuigou (DU26), Sanyinjiao (SP6), and Baihui (DU20). The location refers to the “Animal Acupuncture Point Map” formulated by the Experimental Acupuncture Branch of the Chinese Acupuncture and Moxibustion Association. Supine fixed on the experimental table, routine disinfection after acupuncture, every morning from 8 to 10 o’clock.
The prefrontal cortex of each rat was collected, 1 mL of TRNzol reagent was added per 100 mg of tissue, and the tissue was quickly and fully homogenized with a high-throughput tissue grinder. After homogenization, no obvious tissue clumps were observed. Reverse transcription was performed using the PrimeScript First Strand cDNA Synthesis Kit (Takara) at 42 and 95 for 60 min and 5 min, respectively. Next, based on the LightCycler 480 II real-time PCR system (Roche), PCR was performed using the SYBR Premix Ex Taq Kit (Takara) at 95°C for 2 min, followed by 40 consecutive cycles. 10 seconds at 95 degrees, 30 seconds at 60 degrees, 15 seconds at 95 degrees, 1 minute at 60 degrees. U6 was used as an internal control. 2. The Ct value of each template was detected by Step One Plus real-time fluorescence quantitative PCR and calculated by the 2-ΔΔCt method. Subtract the Ct value of the target gene from the Ct value of the reference gene to obtain ΔCt. ΔΔCt was obtained by subtracting the mean value of ΔCt from the experimental group. The relative expression changes of target genes in the control and experimental groups were calculated as 2-ΔΔCT.
Results
Data Preprocessing
First, 4 original datasets (GSE137595, GSE60820, GSE78731, GSE97533) including 29 strokes and 21 normal tissues were selected for analysis. The data before and after correction are shown in Figure 1A and B, respectively. The results show that the corrected data can be successfully used for subsequent analysis.
 |
Figure 1 Data principal component analyses (PCA). Before (A) and after (B) batch correction. |
The Identification of Genes Involved in Stroke and Functional Enrichment Analysis
Identification of stroke-related genes using WGCNA analysis using 50 samples from four datasets. The soft threshold is calculated using the scale-free topology criterion. When the soft threshold β = 5, the connectivity between genes in the gene network satisfies a scale-free distribution (scale-free R2 = 0.9) (Figure 2A). Next, hierarchical clustering analysis revealed that modules on the same clade had similar gene expression patterns (Figure 2B). We further assessed the association between different gene modules and different stroke categories and found that the brown module was most closely associated with stroke (Figure 2C). The correlation between the expression of genes in the brown module and stroke is shown in Figure 2D. These findings suggest that genes in the brown module are significantly associated with stroke. In addition, GO and KEGG were performed using these 34 key genes, as shown in Figure 3A–D. Biological processes are mainly enriched in cell chemotaxis, regulation of leukocyte migration, granulocyte migration, and eukaryotic chemotaxis. Chromosomal regions, secretory granules, spindles, collagen-containing extracellular matrix, and molecular functions mainly focus on chemokine activity, cytokine receptor binding, and cardiolipin binding.
 |
Figure 3 The functional enrichment analysis of brown module genes involved in stroke. (A) biological process. (B) Cell components. (C) Molecular function. (D) KEGG enrichment analysis. |
Afterwards, a total of 184 brown module genes were uploaded using Funrich, of which 177 were selected for further enrichment analysis. As shown in Figure 4, Funrich’s analysis of the enrichment of important gene pathways for stroke development showed that module genes were mainly enriched in the cell cycle, LPS delivered from the LBP carrier to CD14, NFkB and MAP kinase activation mediated by the TLR4 signaling pathway, and DNA unfolding. The specific enrichment fold and the number of enriched genes are shown in Table 2.
 |
Table 2 Brown Module Gene Pathway Enrichment |
 |
Figure 4 Based on the P-value and the percentage of genes, the Funrich software created a bar chart representing 10 biological pathways. |
Identification of DEGs for Depression and Functional Correlation Analysis
DEGs were then identified using two microarray data (GSE44593 and GSE182195). The two datasets were first analyzed independently, and the difference analysis was visualized with volcano plots and plots (Figure 5A–D). GSE44593 identified 1078 differentially expressed genes by differential analysis. There were 259 differentially expressed genes in GSE182195. Subsequently, the difference analysis results of the above two datasets were analyzed using the RRA algorithm, and 83 genes shared by the two datasets were obtained (Figure 5E).
 |
Figure 5 The DEGs identified from GSE182195 (A and B), and GSE44593 (C and D). (E) The upregulated and downregulated DEGs of the three datasets determined by “RRA”. |
Afterwards, a total of 83 brown module genes were uploaded to Funrich, of which 73 were selected for further enrichment analysis. As shown in Figure 6, module genes were mainly enriched in EGFR-dependent Endothelin signaling events, TLR4 signaling-mediated activation of NFkB and MAP kinases, VEGF and VEGFR signaling networks, DNA replication, etc. The specific enrichment folds and the number of enriched genes can be observed in Table 3.
 |
Table 3 Pathway Enrichment of Differentially Expressed Genes in Depression |
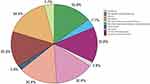 |
Figure 6 Based on the P-value and the percentage of genes, the Funrich software created a pie chart representing 10 biological pathways. |
Screening and Validation of Hub Genes
To further narrow its scope, we analyzed PSD genes, genes in brown modules, and differential genes in depression, and generated intersections between 3 different gene types (Figure 7A), indicating that overlapping genes are related to brain PSD key genes most significantly associated with stroke and depression. The five overlapping genes were “NRBP1”, “SIRT1”, “BDNF”, “MAPK3”, and “CREB1”. and GSE54565 for ROC curve validation of biomarker validity (Figure 7B–F). The area under the curve is close to 1, indicating that the five hub genes have high predictive power. This is a good demonstration of the effectiveness of the model. These results suggest that these five hub genes have the ability to diagnose depression. In addition, using GSE22255 to conduct ROC and gene expression analysis, it was found that these key genes have diagnostic ability for human ischemic stroke disease. and found that their expression levels were different in ischemic stroke (IS) samples and control samples (Figure 8A–E).
 |
Figure 8 (A–E) Diagnostic effectiveness of the biomarkers for stroke: The GSE22255 dataset was used to expression analysis and ROC analysis “NRBP1”,“SIRT1”,“BDNF”,“MAPK3”, and “CREB1”. |
Immune Cell Infiltration Analysis
We used ssGSEA to calculate immune cell infiltration using stroke RNA-sequencing data. Finally, 29 immune cell infiltrating cells were found: aDCs, mast cells, type II IFN response, type I IFN response, DCs, MHC class I, B cells, neutrophils, pDCs, APC costimulators, HLA, T helper cells, iDCs, NK cells, CD8 + T cells, pro-inflammatory, Th1 cells, checkpoints, T cell co-suppression, T cell co-stimulation. We analyzed the infiltration of immune cells in samples from stroke patients (Figure 9A). Immune function was positively correlated with stroke immune function (Figure 9B). The correlation between the Hub gene and each cell was analyzed by Pearson correlation (Figure 9C).
Verification of Potential Biomarker Expression by qRT-PCR
NF-κB is a key nuclear transcription factor of inflammatory response and one of the important pathways by which microglia induce neuroinflammation. The Sirt1/NF-κB pathway regulates the level of inflammation. Sirt1 can reduce NF-κB binding to nuclear inflammatory genes through deacetylation of a subunit of NF-κB, RelA/P65, thereby reducing the production of inflammatory cytokines such as TNF-α and IL-1β. We can down-regulate the Sirt1/NF-κB pathway by acupuncture to suppress inflammatory responses, exert neuroprotective effects, and improve depression-like behaviors. Recent studies have shown that the Sirt1/NF-κB pathway is involved in the M1/M2 polarization of microglia in ischemic brain tissue. We validated the P65 pathway of key genes of SIRT1 and NF-κB and found that SIRT1 and P65 had high reliability (Figure 10A and B). Then, the selected biomarkers SIRT1 and P65 were validated in TB plasma samples by QRT-PCR analysis. The results were consistent with the predictions. The results showed that the expression levels of plasma SIRT1 (P value ≤0.05) and P65 (P value ≤0.05) after acupuncture treatment were significantly lower than those in the disease control group (Figure 10).
Discussion
Post-stroke depression (PSD) refers to an affective disorder with depression as the main manifestation caused by various factors after a stroke, which must be consistent with the course of the disease for more than 2 weeks.12 The proportion of patients diagnosed with major depression after stroke ranged from 14.3% to 22.9%. Compared with non-depressive stroke patients,8,13 PSD can not only aggravate the cognitive impairment of patients, affect the recovery effect of patients, prolong the average length of hospital stay, but also increase the 10-year mortality rate by 3 to 4 times, accelerate hypertension, diarrhea, development and other diseases.14,15 Due to the high incidence of PSD, it is easy to cause serious impact on all aspects of the body,16 and there is a lack of clinical treatment methods without side effects.17 Its prevention and effective treatment have received more and more attention. The key targets of PSD, related pathways and the development of new alternative treatments are urgently needed to be found.
Several relevant studies have shown that disease-targeted therapy can be carried out through some biomarkers, such as elevated serum MDA levels are positively associated with an increased risk of depression after acute stroke, affecting the most important marker of PSD at 3 months.18 Some compounds such as fibrinogen in men, free T3, magnesium in women are closely related to BDNF in PSD, inflammation balance, oxidative stress, glutamate neurotransmission, neurotrophic factor production.19 Research in the field of post-stroke biomarkers has the potential to provide personalized treatment for stroke patients, as well as help diagnose and understand the pathophysiology of this common neuropsychiatric complication. However, related research mainly focuses on intervening diseases through drugs, which will inevitably lead to clinical side effects of pharmaceutical drugs and drug resistance.
Therefore, in order to improve the therapeutic selectivity of PSD, there is a need to investigate new,20 effective biomarkers that can help to develop new treatments with no or few side effects.21 In this study, we screened out five biomarkers and biological pathways that were significantly expressed in stroke and depression samples, and these five biomarkers could also be used to predict patient outcomes. Our study may provide some guidance for patient prognosis and personalized treatment.
Studies have shown that inflammation caused by disturbance of M1/M2 polarization of microglia is an important factor in PSD symptoms.22 Sirt1/NF-κB pathway is associated with M1/M2 polarization of microglia in ischemic brain tissue. Among them, high expression of Sirt1 can reduce the degree of inflammatory response, reduce M1 polarization of microglia, and promote M2 polarization.23 At the same time, M2 microglia also release brain-derived neurotrophic factor (BDNF) to promote synapse formation.23 Therefore, we hypothesized that acupuncture might ameliorate the PSD inflammatory environment, modulate the M1/M2 polarization direction of microglia, upregulate the NRBP1-CREB-BDNF pathway in microglia, and promote synaptic motility by producing more BDNF, thereby improving PSD symptoms,24 which has also been verified experimentally and subsequently clinically. Combined with relevant literature, the polarization direction of microglia M1/M2 is closely related to the treatment of ischemic PSD,22,25 Sirt1 can inhibit the expression of IL-1, IL-6, TNF-α pro-inflammatory factors, and Sirt1/NF- The κB pathway also plays an important role in regulating microglial M1/M2 polarization.26–28
In conclusion, this study analyzed depression and stroke separately through bioinformatics analysis, combined with existing post-stroke depression studies, and identified 5 disease-related genes (“NRBP1”, “SIRT1”, “BDNF” ‘, “MAPK3”, “CREB1”.) and PSD-related pathways, the selected biomarkers SIRT1 and P65 were validated by QRT-PCR in plasma samples. We found that these biomarkers can be used as therapeutic targets for post-stroke depression, and acupuncture therapy can effectively intervene in post-stroke depression and serve as a side-effect-free alternative to clinical drugs.
At present, according to our data analysis and experimental verification, these genes are key target genes of post-stroke depression and their expression can be affected by acupuncture treatment. The expression of “SIRT1”, “BDNF”, “MAPK3”, and “CREB1” can play a role in the treatment of PSD, which has important guiding significance for the future clinical treatment of PSD. However, we need to collect more datasets for further analysis to validate our findings, and to further study the biological functions of these key genes. In the future, more functional studies are needed to better characterize the roles of key genes in PSD. Second, we did not collect more clinical follow-up data related to post-stroke depression. These factors can help us to more comprehensively characterize the role of these key genes. We strongly recommend further research on this topic to progressively improve the scholarly impact of these genetic biology evidence.
Conclusion
The results of this study show that five genes (“NRBP1”, “SIRT1”, “BDNF”, “MAPK3”, “CREB1”) and activation of key biological pathways, NFkB, PI3K/AKT, and MAPK are PSD biomarkers. The key to the occurrence and development of the disease provides a basis for elucidating the pathogenesis of the disease and establishing therapeutic targets from a new perspective, and they can also be therapeutically intervened by acupuncture. This study further revealed the role of key pathways and targets in PSD and comprehensively demonstrated that acupuncture can be used as a new treatment method for PSD.
Abbreviations
PSD, Post-stroke depression; DEGS, differential expression analysis; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; SSRIs, Selective serotonin reuptake inhibitors; HAMD, Hamilton Depression Scale; RMA, Robust Multiple Array Average; AUC, The area under the ROC curve; ROC, Receiver operating characteristic; ssGSEA, Single-Sample Gene Set Enrichment Analysis; qRT-PCR, Quantitative reverse transcription polymerase chain reaction; MCAO, Middle cerebral artery occlusion; CUMS, Chronic Mild Stress Method.
Ethics Approval and Informed Consent
The animal study was reviewed and approved by Laboratory Animal Ethics Committee of Institute of Radiation Medicine, Chinese Academy of Medical Sciences and the First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine. The procedures involving animals were in compliance with the Consensus Author Guidelines on Animal Ethics and Welfare.
Acknowledgments
The authors thank all the participants for their cooperation.
Funding
The study was financially supported by Tianjin Municipal Education Commission Scientific Research Project (Natural Science) (Grant Nos. 2021KJ150).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Robert G, Robinson MD, Ricardo E, et al. Post-stroke depression: a review. Am J Psychiatry. 2016;173(3):221–231. doi:10.1176/appi.ajp.2015.15030363
2. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102–109. doi:10.1016/j.arr.2019.01.013
3. Cai W, Shen WD. Anti-apoptotic mechanisms of acupuncture in neurological diseases: a review. Am J Chin Med. 2018;46(03):515–535. doi:10.1142/S0192415X1850026X
4. Liu L, Tian T, Li X, et al. Revealing the neural mechanism underlying the effects of acupuncture on migraine: a systematic review. Front Neurosci. 2021;15:674852. doi:10.3389/fnins.2021.674852
5. Zhang J, Lu C, Wu X, et al. Neuroplasticity of acupuncture for stroke: an evidence-based review of MRI. Neural Plast. 2021;2021:1–14. doi:10.1155/2021/2662585
6. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374–383. doi:10.7326/0003-4819-136-5-200203050-00010
7. Yang Y, Zhu L, Zhang B, et al. Higher levels of C-reactive protein in the acute phase of stroke indicate an increased risk for post-stroke depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;134:104309.
8. Medeiros GC, Roy D, Kontos N, et al. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. 2020;66:70–80. doi:10.1016/j.genhosppsych.2020.06.011
9. Man SC, Hung BHB, Ng RMK, et al. A pilot controlled trial of a combination of dense cranial electroacupuncture stimulation and body acupuncture for post-stroke depression. BMC Complement Altern Med. 2014;14(1):1–8. doi:10.1186/1472-6882-14-255
10. Pavão TS, Vianna P, Pillat MM, et al. Acupuncture is effective to attenuate stress and stimulate lymphocyte proliferation in the elderly. Neurosci Lett. 2010;484(1):47–50. doi:10.1016/j.neulet.2010.08.016
11. Kwon S, Lee B, Yeom M, et al. Modulatory effects of acupuncture on murine depression-like behavior following chronic systemic inflammation. Brain Res. 2012;1472:149–160. doi:10.1016/j.brainres.2012.07.009
12. Das J, Rajanikant GK. Post stroke depression: the sequelae of cerebral stroke[J]. Neurosci Biobehav Rev. 2018;90:104–114. doi:10.1016/j.neubiorev.2018.04.005
13. Shao A, Lin D, Wang L, et al. Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 2020;11(6):1537. doi:10.14336/AD.2020.0225
14. Zhu Z, Guo D, Shi M, et al. Effect of immediate blood pressure reduction on post-stroke depression in ischemic stroke patients: a substudy of CATIS trial. J Affect Disord. 2022;300:195–202. doi:10.1016/j.jad.2021.12.120
15. Xu M, Wu G. The clinical significance of serum IL-33 and sST2 alterations in the post-stroke depression. J Multidiscip Healthc. 2021;14:2009. doi:10.2147/JMDH.S310524
16. Mortensen JK, Andersen G. Pharmacological management of post-stroke depression: an update of the evidence and clinical guidance. Expert Opin Pharmacother. 2021;22(9):1157–1166. doi:10.1080/14656566.2021.1880566
17. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–144. doi:10.1016/j.pharmthera.2017.11.005
18. Liu Z, Zhu Z, Zhao J, et al. Malondialdehyde: a novel predictive biomarker for post-stroke depression. J Affect Disord. 2017;220:95–101. doi:10.1016/j.jad.2017.05.023
19. Qiu X, Wang H, Lan Y, et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry. 2022;22(1):1–10. doi:10.1186/s12888-022-03805-6
20. Zhao H, Mo M, Miao C, et al. Association of serum biomarker neurofilament light concentration with post-stroke depression: a preliminary study. Gen Hosp Psychiatry. 2020;64:17–25. doi:10.1016/j.genhosppsych.2020.01.006
21. Liang H, He J, Tu X, et al. MicroRNA-140-5p: a novel circulating biomarker for early warning of late-onset post-stroke depression. J Psychiatr Res. 2019;115:129–141. doi:10.1016/j.jpsychires.2019.05.018
22. Nagy EE, Frigy A, Szasz JA, et al. Neuroinflammation and microglia/macrophage phenotype modulate the molecular background of post-stroke depression: a literature review. Exp Ther Med. 2020;20(3):2510–2523. doi:10.3892/etm.2020.8933
23. Fu CY, Zhong CR, Yang YT, et al. Sirt1 activator SRT2104 protects against oxygen-glucose deprivation/reoxygenation-induced injury via regulating microglia polarization by modulating Sirt1/NF-κB pathway. Brain Res. 2021;1753:147236. doi:10.1016/j.brainres.2020.147236
24. Cuadros MA, Sepulveda MR, Martin-Oliva D, et al. Microglia and microglia-like cells: similar but different. Front Cell Neurosci. 2022;16. doi:10.3389/fncel.2022.816439
25. Chen Y, Huang W, Li Z, et al. The effect of acupuncture on the expression of inflammatory factors TNF-α, IL-6, IL-1 and CRP in cerebral infarction: a protocol of systematic review and meta-analysis. Medicine. 2019;98:24.
26. Cosma NC, Üsekes B, Otto LR, et al. M1/M2 polarization in major depressive disorder: disentangling state from trait effects in an individualized cell-culture-based approach. Brain Behav Immun. 2021;94:185–195. doi:10.1016/j.bbi.2021.02.009
27. Tao Y, Gao K, Shen B, et al. MicroRNA-135b-5p downregulation causes antidepressant effects by regulating SIRT1 expression. Biochem Genet. 2021;59(6):1582–1598. doi:10.1007/s10528-021-10076-5
28. Song Y, Wu Z, Zhao P. The protective effects of activating Sirt1/NF-κB pathway for neurological disorders. Rev Neurosci. 2021;33:427–438. doi:10.1515/revneuro-2021-0118
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




