Back to Journals » OncoTargets and Therapy » Volume 13
Study of the Relationship Between Serum Amino Acid Metabolism and Lymph Node Metastasis in Patients with Colorectal Cancer
Authors Liu J, Wang J, Ma X , Feng Y , Chen Y, Wang Y, Xue D, Qiao S
Received 26 July 2020
Accepted for publication 18 September 2020
Published 13 October 2020 Volume 2020:13 Pages 10287—10296
DOI https://doi.org/10.2147/OTT.S273107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Jinhao Liu,1 Jikun Wang,2 Xueqian Ma,1 Yang Feng,1 Yanlei Chen,1 Yanping Wang,1 Dong Xue,1 Shifeng Qiao1
1The Second Ward of General Surgery, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, People’s Republic of China; 2Oncology Department, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, People’s Republic of China
Correspondence: Shifeng Qiao
The Second Ward of General Surgery, The First Affiliated Hospital of Jinzhou Medical University, No. 2 Section 5, Renmin Street, Guta District, Jinzhou City, Liaoning 121001, People’s Republic of China
Tel +86 15904161717
Email [email protected]
Purpose: Lymph node metastasis is one of the important prognostic factors of colorectal cancer, and an important index of individualized treatment. The purpose of this study is to use metabonomics to identify potential molecular markers of lymph node metastasis in colorectal cancer (CRC).
Patients and Methods: Peripheral blood samples of 223 CRC patients were collected. The metabolic levels of amino acids and carnitine in peripheral blood of CRC patients, with and without lymph node metastasis, were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Results: The results show that there were significant differences in the levels of serum amino acids and carnitine between lymph node metastatic patients and lymph node non-metastatic patients. The diagnostic model that was constructed by 9 types of serum metabolites has a high diagnostic ability.
Conclusion: LC-MS/MS is a detection method that has a broad application in predicting CRC prognosis, individualized treatment, and in studying the mechanism of lymph node metastasis.
Keywords: colorectal cancer, lymph node metastasis, metabonomics, amino acid, liquid chromatography-mass spectrometry
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors, ranking third worldwide. In China, CRC morbidity and mortality are higher than the world average.1 Some studies have shown that in the next few decades, CRC morbidity in China will further increase.2 The main route of cancer metastasis is through the lymph nodes (lymphatic metastasis), as most patients with advanced CRC will have metastatic lymph nodes that are also one of the important factors for CRC prognosis. The five-year survival rate (FYSR) of patients without lymph node metastases or distant metastases is approximatively 90%, but for metastatic CRC patients, this rate is reduced to approximatively 15%.3 Although CRC early screening and treatment technologies have been continuously improving, the mortality rate remains high.4 One of the reasons for the rising mortality rate is metastasis. Unfortunately, the mechanisms of metastasis are still unclear. Therefore, developing a sensitive detection method that can be used to screen lymph node metastasis at an early stage, would be of great significance in setting up individualized treatment plans and in improving patients’ prognosis.
Metabonomics is a new research approach that is used to measure the levels of small molecular substances in biological systems to investigate the effects of cellular metabolisms in the occurrence and development of diseases.5 Amino acids (AA) and carnitine are important metabolites in metabonomics and have recently attracted the attention of an increasing number of medical researchers. Amino acids are one of the important substances in protein synthesis and play important roles in the maintenance of cell morphology and function. Carnitine is a type of amino acid-like substance, that is mainly consumed through diet and that has a variety of biological functions. However, compared with normal people, AA and carnitine metabolisms are dysregulated in cancer.
In this study, the concentrations of amino acids and carnitine in peripheral blood were analyzed using multivariate statistics, with the aim of investigating the metabolic changes of patients with lymph node metastasis and identify these hydrophilic compounds. As far as we know, this is the first metabonomic report that studied CRC lymph node metastasis via investigating the changes of amino acids and carnitine concentrations.
Materials and Methods
Chemicals
Liquid chromatographic (LC) grade acetonitrile, pure water, and methanol were purchased from Thermo Fisher, USA. N-butanol and acetyl chloride were purchased from Sigma-Aldrich, USA. The isotope internal standard 12 amino acids (NSK-A) and the 8 carnitine internal standards (NSK-B) were obtained from Cambridge Isotope laboratories, USA. The Quality Control (QC) standard was purchased from Chromsystems, Germany. The two internal standards were fully mixed with 1mL methanol and diluted 100times to obtain working solutions, that were stored in a refrigerator at 4°C.
Sample Collection
During the 2018–2020 period, the samples from 223 CRC patients were collected. Venous blood was collected in the early morning from each fasting patient and stored in lithium vacuum vessel containing heparin. The vessels were well shaken, and temporarily stored at 4°C, prior sending them to the laboratory within 30 minutes. The study was approved by the Ethics Committee of the first affiliated Hospital of Jinzhou Medical University (Liaoning, China), and all patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki. The study inclusion criteria were: (1) pathological diagnosis of CRC; (2) age 18 to 90 years old; and (3) did not receive other anti-tumor therapies before operation. The study exclusion criteria were: (1) other tumors (primary tumors); (2) patients who received radiotherapy, chemotherapy, or targeted treatments; (3) patients with severe metabolic system diseases; and (4) patients with blood system diseases.
Sample Preparation and LC-MS/MS Analysis
Unless otherwise specified, all operations were carried out at room temperature. Firstly, the blood sample was prepared by dried blood spot (DBS) testing. The blood spot diameter was no less than 8mm to ensure that the paper was fully permeated and pollution-free. All DBS samples were stored at −80°C. Secondly, punch holes in DBS paper were made using a punch, and a 3mm paper tray with a diameter of about 3.2 microliters of human blood was obtained. The paper tray was placed in a 96-well filter plate to prepare amino acids and carnitine. A 100μL of working fluid was added to each hole, followed by shaking at low speed for 20 minutes, then centrifugation at 1500r/min for 2 minutes, and the new filtrate was added into the new 96-well plate. Next, to ensure the accuracy of the experiment, four blank holes were randomly set in each orifice plate, and two high and low concentration QC solutions were added, respectively. The filtrate and QC solution were dried under the condition of pure nitrogen at 50°C. Finally, the dried samples of each orifice plate were used to derive 20 minutes with 60μL acetyl chloride/n-butanol (1:9) mixture at 65°C. After obtention of the derivative solution, this latter was dried under the condition of pure nitrogen at 50°C. A 100μL of 80% acetonitrile aqueous solution was added to each well plate, to fully dissolve and use it for liquid chromatography-tandem mass spectrometry (LC-MS/MS).
The mass spectrometer SCIEXAPI3200MD (ABSCIEX, USA) was used for direct injection mass spectrometry analysis. The instrument was operated under the condition of positive ion electrospray ion source. During each operation, 20μL of the samples were injected and an 80% acetonitrile solution was added for gradient elution. The traffic was initially set to 0.2mL/min, gradually decreased to 0.01mL/min within 0.08min and maintained for 1.5min, and then the traffic increased again to 0.2mL/min and maintained at 0.5min within 0.01min. The ion source spray voltage was set to 4.5kV, the ion source gas pressure was set to 35psi (pounds per square inch), the barrier gas pressure was set to 20psi, and the auxiliary gas temperature was kept at 35°C.
Data Preprocessing and Analysis
The Analystv1.6.0 (ABSCIEX, USA) software was used for system control and data acquisition. The ChmeoView2.0.2 (AB Sciex, USA) preprocessed the data and quantitatively analyzed the metabolites. According to different isotope standards, the corresponding metabolic indexes were obtained.
The data were imported into the SPSS25.0 (IBM) for standardization, and the metabolites were screened by the Mann–Whitney U-test and Stepwise discriminant analysis. The standardized data were imported into the SIMCA-P 14 (Umetrics, Umea, Sweden) software for principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA). An oval region is shown in the model integral diagram, and that defined the 95% confidence interval of the model variables. The model quality is described by R2X or R2Y and Q2. Q2 indicates the model predictability. R2X or R2Y indicates the model fit. To ensure that the data were not over-fitted, a permutation analysis (200 times) was carried out in SIMCA-P to verify the PLS-DA model. The metabolites with variable importance in the projection (VIP) of >1, were screened by SIMCA-P, and the metabolites satisfying a VIP >1 and a Mann–Whitney U-test p <0.05, were regarded as distinguishing metabolites. On this basis, a diagnostic model was constructed using metabolites satisfying stepwise discriminant analysis p <0.05.
Results
A total of 223 patients were enrolled in this study and their clinical characteristics, according to lymph node metastasis, are shown in Table 1. There were no differences in age (P=0.416, t-test) and gender (P=0.561, χ2 test) between the two groups. According to the TNM staging method that was jointly developed by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), the patients were divided into clinical stages.
 |
Table 1 Clinical Characteristics of the CRC Patients |
Multivariate Statistical Analysis of Metastatic Group and Non-Metastatic Group
The LC-MS/MS-based metabonomics method was used to analyze the peripheral blood metabolic group of CRC patients. First, a principal component analysis was performed on the CRC standardized metabonomic data, with and without lymph node metastasis. Figure 1A (2PCs, R2X= 0.22, Q2=0.0758) shows that most of the metastatic (black dots) and non-metastatic (red dots) groups are scattered in different areas, and that most of the samples are within 95% confidence interval. Therefore, all samples were included in the following analysis to maximize the amount of information. In addition, we also carried out PLS-DA analysis of the data, such as in Figure 1B (2PCs, R2X=0.206 Q2=0.469), and found that there was a significant separation between the metastatic and the non-metastatic groups. After 200-times permutation test (Figure 1C), it was determined that the PLS-DA model is not random or overfitted, indicating that the model is reliable for explaining and predicting variation. To identify the metabolic differences between the metastatic and non-metastatic groups, the OPLS-DA method was used to analyze the two groups’ data (Figure 1D) (2PCs, R2X=0.206 Q2=0.48). It showed that the difference between the metastatic (black dots) and non-metastatic (red dots) groups can be distinguished, which is of certain significance for identifying potential biomarkers and molecular mechanisms of metastatic CRC.
To better understand differences of metabolites between the metastatic and non-metastatic groups, a VIP of >1 was used to screen the metabolites. As shown in Table 2, a total of 25 metabolites were obtained, and there were significant differences between the two groups. Using the Mann–Whitney U-test to analyze the two groups’ data, we found that there was a significant statistical difference in the concentration of most metabolites. Meanwhile, the average concentration of these metabolites in the metastatic group was higher than that in the non-metastatic group. Finally, using stepwise discriminant analysis, 9 metabolites of leucine, free carnitine, methylmalonylcarnitine, isovalerylcarnitine, glutarylcarnitine, tiglylcarnitine, hexanoylcarnitine, dodecanoylcarnitine, and behenic carnitine, were obtained under the conditions of VIP>1 and p < 0.05. To prove the accuracy of the OPLS-DA model, we constructed a diagnostic model using the 9 metabolites and expressed them with receiver operating characteristic (ROC). According to the ROC that was constructed by SPSS, the area under the curve (AUC) was 0.947 (Figure 2), which indicates that the model has a strong ability to distinguish lymph node metastasis.
 |  |  |
Table 2 Metabolites Between Lymph Node Metastasis Group and Lymph Node Non-Metastasis Group |
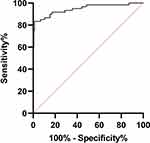 |
Figure 2 ROC analysis between lymph node metastatic and lymph node non-metastatic CRC sample. |
Meanwhile, according to the sample collection method described above, a total of 40 patients’ samples were collected to verify the accuracy of the OPLS-DA model. Table 3 shows the clinical characteristics of the patients in the verification group. There were no differences in age (P=2.252, t-test) and gender (P=0.519, χ2 test) between the two groups. Using SPSS to establish ROC (Figure 3), we determined an AUC of 0.833, which showed that the model ability is good.
 |
Table 3 Clinical Characteristics of the CRC Patients in Verification Group |
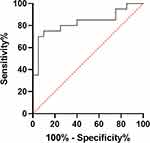 |
Figure 3 ROC analysis between lymph node metastatic CRC and lymph node non-metastatic CRC sample in verification group. |
Discussion
Colorectal cancer incidence and mortality remain high worldwide.1 Although enteroscopy and tumor markers can help clinicians diagnose colorectal cancer, the early detection of lymph node metastasis is difficult. The emergence of metabonomics can effectively solve this problem. Cancer, as a metabolic disease,6 is often caused by genetic mutations that can cause metabolic changes in small molecules, leading to metabolic disorders. Cancer development can be effectively evaluated by the concentration of free amino acids in peripheral blood. Studies have shown that metabonomics can be used for early diagnoses of breast, colorectal, and liver cancers, but also for other related diseases.7–10 Moreover, metabonomics have also a strong ability to recognize cancer metastases.11–13
In this study, we used LC-MS/MS to analyze the metabolic levels of amino acid and carnitine in peripheral blood of CRC patients, with and without lymph node metastasis. In this study, a total of 50 metabolites were analyzed, and there were 25 significant metabolic differences between the two groups. Finally, the diagnostic model that was constructed by 9 selected metabolites, had an excellent diagnostic ability. It is well known that lymph node metastasis is related to CRC prognosis, and therefore, this study focused on analyzing the metabolites’ differences between the lymph node metastatic group and the lymph node non-metastatic group, to find potential molecular markers that can be used in the future studies on the mechanism of CRC metastasis.
Methionine, as an essential amino acid in the human body, can only be supplied by food. After methionine enters the human body, S-Adenosyl Methionine (SAMe) is formed under the action of methionine adenosine transferase (MAT). SAMe can provide active methyl groups for the methylation of histone, phosphate, DNA, RNA, and other proteins, forming a variety of active substances and participating in human metabolism. SAMe has a strong hepatoprotective effect, as it increases glutathione content, and reduces inflammatory responses and liver injury.14 DNA methylation is closely related to tumor development and its status requires active methyl that are provided by SAMe. Some studies have found that there are widespread defects in the DNA methylation of malignant tumors, and that SAMe participates in DNA methylation through the catalytic activity of DNA methyltransferase. Moreover, abnormal DNA methylation has been shown to be one of the markers of lung cancer.15 It has also been found that SAMe can inhibit the proliferation of CRC,16,17 breast,18 gastric,19 and liver cancer cells,20 and promote the apoptosis of cancer cells. Meanwhile, Nakamura et al found that CRC aberrant methylation was closely related to lymph node metastasis.21
We found that the methionine concentration in the metastatic group was significantly higher than that in the non-metastatic group. According to the methionine metabolic pathway in the human body, choline can be oxidized into betaine, that provides methyl for homocysteine, and form methionine and N, N-Dimethylglycine Ethyl Ester (DMG). Due to the limitation of the experimental methods, we could not directly observe the concentration of choline in peripheral blood, but we can speculate that the concentration of choline will also increase significantly. Choline is not only a necessary nutrient for the cell membrane phospholipid metabolism but is also an important methyl donor. Studies had shown that choline plays a key role in tissue malignant transformation22 and in the production of methylamine. This later is often considered non-toxic for the human body, however, some studies had found that the accumulation of methylamine in rats can induce the occurrence of liver cancer.23 The liver is the main organ of CRC metastasis. The liver is the main organ of CRC metastasis and when methylamine accumulates in the human body, it indicates that the liver’s metabolic level has been dysregulated during CRC development. Although methionine is not the amino acid that is used as a diagnostic model, there is also a significant difference in its metabolic level between the two groups. Therefore, methionine can be used as a potential molecular marker for CRC lymph node metastasis, but its mechanism needs to be further explored.
Cancer is a metabolic disease and the local metabolism of cancer patients is dysregulated compared to that of healthy people. The proliferation of cancer cells requires large amounts of glucose and protein and the PI3K/Akt/mTOR signaling pathway plays an important in these processes. Its activation can promote protein production, cancer cell proliferation, and inhibit cancer cell apoptosis, which consequently promotes cancer occurrence and development.24 Extracellular signals can induce the phosphatidylinositol 3-kinase (PI3K) through tyrosine kinase receptor phosphorylation and Akt activation, that promote the activity of mammalian target of rapamycin (mTOR) to regulate protein synthesis and cell proliferation.25 Branched chain amino acids can be used as cellular signal molecules to activate the mTOR pathway.26,27 Among the 3 branched chain amino acids, leucine has the strongest ability to activate the mTOR pathway and promote protein synthesis in various tissues.28,29 Leucine can also promote insulin production by islet B cells, which promotes the activation of the mTOR pathway. Xiao et al30 observed a significant decrease in the proliferation ability of breast cancer cells in the absence of leucine, in vivo, and in vitro. Liu et al,31 also observed similar results in a mouse model of pancreatic cancer. Therefore, the elevated level of leucine plays an important role in the development of CRC. In this study, we found that the concentration of leucine in the metastatic group was higher than that in the non-metastatic group, confirming the conclusions of Xiao et al and Liu et al who demonstrated that leucine can promote the proliferation and metastasis of tumor cells. However, other scholars found the opposite result. Viana et al32 and others showed that the tumor weight of mice in the tumor group that was fed with a leucine-rich diet, was significantly lower than that in the blank group. Vaughan et al33 reached a similar conclusion. The difference in the experimental results may be due to different types of tumors and research methods.
Carnitine is a type of amino acid that is mostly found in human muscles. Mitochondrial fatty acid β-oxidation is the main energy supply pathway of the human body. However, fatty acids cannot freely pass through the mitochondrial membrane, and requires carnitine to enter the mitochondria and participates in oxidation and energy supply. In this process, carnitine is dehydrated and esterified, with corresponding fatty acids, by the acyltransferase to form acyl carnitine. Carnitine and acyl carnitine play important roles in the cellular distribution of energy and in ensuring the transport of fatty acids from the mitochondrial membrane to the metabolic oxidation site. In recent years, researchers are paying increasing attention to the clinical value of carnitine.
In addition, there are significant changes in carnitine and acylcarnitine levels in the peripheral blood of many cancer patients.34–36 In esophageal squamous cell carcinoma, a study found that the level of plasma L-carnitine in cancer patients was significantly increased compared with the healthy group, while the levels of acylcarnitine, such as C8 and undecanoylcarnitine, significantly decreased.36 Carnitine and acylcarnitine are essential for the cytoplasmic entry of long-chain fatty acids into the mitochondria. Since acylcarnitine is produced by carnitine through acetyl-CoA, acylcarnitine low levels may indicate a decrease in acetyl-CoA activity and β-oxidation in patients with esophageal squamous cell carcinoma. Malaguarnera et al34 found that carnitine level was significantly decreased in peripheral blood of patients with gastrointestinal malignant tumors and cachexia. The authors compared the total carnitine, free carnitine, long-chain acetyl carnitine, and short-chain acetyl carnitine, in peripheral blood of healthy subjects and patients with malignant digestive tract tumors, malignant non-digestive tract tumors, and non-malignant tumors. Finally, it was found that the level of all carnitine was significantly decreased in malignant digestive tract tumors. This may be due to the decrease of the eating ability and the disorder of carnitine metabolism in patients with cachexia. Furthermore, it was also observed that the levels of palmitic acid, myristic acid, and carnitine in peripheral blood of patients with CRC, were lower than those of healthy subjects.35
In the tumor microenvironment of cancer patients, even if there is plenty of oxygen, cancer cells still provide energy for themselves in the form of glycolysis, an event that is called the Warburg effect, and that can be regarded as the main marker of cancer.37,38 In this study, the levels of free carnitine, methylmalonylcarnitine and other carnitines, in the lymph node metastatic group, were significantly higher than those in the non-metastatic group. Among them, succinyl carnitine comes from succinyl coenzyme A in the TCA cycle, and which indicates that, in the non-tumor part of the body, the sugar aerobic oxidation pathway is activated to meet its own energy needs. Therefore, the increase in the level of carnitine indicates that other routes of energy supply are more active. It is well known that most patients enter a state of cachexia in the late stage of cancer, in which the metabolic pathway is dysregulated. At this time, a variety of active ways of energy supply will consume a large amount of patients’ energy supply substances, which may be a potential sign of cachexia. However, this inference needs further research to be proved.
As far as we know, this is the first study that analyzed the metabolic differences between lymph node and non-lymph node metastatic CRCs, through investigating the peripheral blood changes in amino acids and carnitine. However, additional clinical data are needed to analyze and prove the medical value of the identified metabolites in CRC development and metastasis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Zhang L, Cao F, Zhang G, et al. Trends in and predictions of colorectal cancer incidence and mortality in China from 1990 to 2025. Front Oncol. 2019;9:98. doi:10.3389/fonc.2019.00098
3. Jones P, Cade JE, Evans CEL, Hancock N, Greenwood DC. Does adherence to the World Cancer Research Fund/American Institute of Cancer Research cancer prevention guidelines reduce risk of colorectal cancer in the UK Women’s Cohort Study? Br J Nutr. 2018;119(3):340–348. doi:10.1017/S0007114517003622
4. van der Werf A, Arthey K, Hiesmayr M, et al. The determinants of reduced dietary intake in hospitalised colorectal cancer patients. Support Care Cancer. 2018;26(6):2039–2047. doi:10.1007/s00520-018-4044-1
5. Van QN, Veenstra TD. How close is the bench to the bedside? Metabolic profiling in cancer research. Genome Med. 2009;1(1):5. doi:10.1186/gm5
6. Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–527. doi:10.1093/carcin/bgt480
7. Cubiella J, Clos-Garcia M, Alonso C, et al. Targeted UPLC-MS metabolic analysis of human faeces reveals novel low-invasive candidate markers for colorectal cancer. Cancers. 2018;10(9):300. doi:10.3390/cancers10090300
8. Miyagi Y, Higashiyama M, Gochi A, et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. 2011;6(9):e24143. doi:10.1371/journal.pone.0024143
9. Davis VW, Schiller DE, Eurich D, Sawyer MB. Urinary metabolomic signature of esophageal cancer and Barrett’s esophagus. World J Surg Oncol. 2012;10:271. doi:10.1186/1477-7819-10-271
10. Liu XN, Cui DN, Li YF, Liu YH, Liu G, Liu L. Multiple “Omics” data-based biomarker screening for hepatocellular carcinoma diagnosis. World J Gastroenterol. 2019;25(30):4199–4212. doi:10.3748/wjg.v25.i30.4199
11. Goto R, Nakamura Y, Takami T, Sanke T, Tozuka Z, Coleman WB. Quantitative LC-MS/MS analysis of proteins involved in metastasis of breast cancer. PLoS One. 2015;10(7):e0130760. doi:10.1371/journal.pone.0130760
12. Engin A, Gonul II, Engin AB, Karamercan A, Sepici Dincel A, Dursun A. Relationship between indoleamine 2,3-dioxygenase activity and lymphatic invasion propensity of colorectal carcinoma. World J Gastroenterol. 2016;22(13):3592–3601. doi:10.3748/wjg.v22.i13.3592
13. Chen YL, Wu WL, Jang CW, et al. Interferon-stimulated gene 15 modulates cell migration by interacting with Rac1 and contributes to lymph node metastasis of oral squamous cell carcinoma cells. Oncogene. 2019;38(23):4480–4495. doi:10.1038/s41388-019-0731-8
14. Martínez-Uña M, Varela-Rey M, Mestre D, et al. S-Adenosylmethionine increases circulating very-low density lipoprotein clearance in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):673–681. doi:10.1016/j.jhep.2014.10.019
15. Greenberg AK, Rimal B, Felner K, et al. S-adenosylmethionine as a biomarker for the early detection of lung cancer. Chest. 2007;132(4):1247–1252. doi:10.1378/chest.07-0622
16. Lin PC, Lin JK, Lin CH, et al. Clinical relevance of plasma DNA methylation in colorectal cancer patients identified by using a genome-wide high-resolution array. Ann Surg Oncol. 2015;22(Suppl S3):S1419–1427. doi:10.1245/s10434-014-4277-2
17. Michailidi C, Theocharis S, Tsourouflis G, et al. Expression and promoter methylation status of hMLH1, MGMT, APC, and CDH1 genes in patients with colon adenocarcinoma. Exp Biol Med (Maywood). 2015;240(12):1599–1605. doi:10.1177/1535370215583800
18. Pakneshan P, Szyf M, Farias-Eisner R, Rabbani SA. Reversal of the hypomethylation status of urokinase (uPA) promoter blocks breast cancer growth and metastasis. J Biol Chem. 2004;279(30):31735–31744. doi:10.1074/jbc.M401669200
19. Luo J, Li YN, Wang F, Zhang WM, Geng X. S-adenosylmethionine inhibits the growth of cancer cells by reversing the hypomethylation status of c-myc and H-ras in human gastric cancer and colon cancer. Int J Biol Sci. 2010;6(7):784–795. doi:10.7150/ijbs.6.784
20. Pascale RM, Simile MM, De Miglio MR, Feo F. Chemoprevention of hepatocarcinogenesis: S-adenosyl-L-methionine. Alcohol (Fayetteville, NY). 2002;27(3):193–198. doi:10.1016/S0741-8329(02)00227-6
21. Nakamura K, Yamashita K, Sawaki H, et al. Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 2015;35(3):1411–1421.
22. Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11(12):835–848. doi:10.1038/nrc3162
23. Lin JK, Ho YS. Hepatotoxicity and hepatocarcinogenicity in rats fed squid with or without exogenous nitrite. Food Chem Toxicol. 1992;30(8):695–702. doi:10.1016/0278-6915(92)90165-H
24. Hao J, Yang Z, Wang L, et al. Downregulation of BRD4 inhibits gallbladder cancer proliferation and metastasis and induces apoptosis via PI3K/AKT pathway. Int J Oncol. 2017;51(3):823–831. doi:10.3892/ijo.2017.4081
25. Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021–1031. doi:10.1158/1535-7163.MCT-13-0639
26. Bajotto G, Sato Y, Kitaura Y, Shimomura Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur J Appl Physiol. 2011;111(8):1815–1828. doi:10.1007/s00421-010-1825-8
27. Blomstrand E, Eliasson J, Karlsson HK, Köhnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136(1):269s–273s. doi:10.1093/jn/136.1.269S
28. Sans MD, Tashiro M, Vogel NL, Kimball SR, D’Alecy LG, Williams JA. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr. 2006;136(7):1792–1799. doi:10.1093/jn/136.7.1792
29. Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004;313(2):429–436. doi:10.1016/j.bbrc.2003.07.015
30. Xiao F, Wang C, Yin H, et al. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget. 2016;7(39):63679–63689. doi:10.18632/oncotarget.11626
31. Liu KA, Lashinger LM, Rasmussen AJ, Hursting SD. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metabol. 2014;2(1):6. doi:10.1186/2049-3002-2-6
32. Viana LR, Tobar N, Busanello ENB, et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci Rep. 2019;9(1):15529. doi:10.1038/s41598-019-52112-w
33. Vaughan RA, Garcia-Smith R, Gannon NP, Bisoffi M, Trujillo KA, Conn CA. Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids. 2013;45(4):901–911. doi:10.1007/s00726-013-1538-5
34. Malaguarnera M, Risino C, Gargante MP, et al. Decrease of serum carnitine levels in patients with or without gastrointestinal cancer cachexia. World J Gastroenterol. 2006;12(28):4541–4545. doi:10.3748/wjg.v12.i28.4541
35. Qiu Y, Cai G, Su M, et al. Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS. J Proteome Res. 2009;8(10):4844–4850. doi:10.1021/pr9004162
36. Xu J, Chen Y, Zhang R, et al. Global and targeted metabolomics of esophageal squamous cell carcinoma discovers potential diagnostic and therapeutic biomarkers. Mol Cell Proteomics. 2013;12(5):1306–1318. doi:10.1074/mcp.M112.022830
37. Zheng J. Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (Review). Oncol Lett. 2012;4(6):1151–1157. doi:10.3892/ol.2012.928
38. Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13(1):89. doi:10.1186/1475-2867-13-89
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

