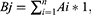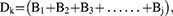Back to Journals » Drug Design, Development and Therapy » Volume 16
Study of the Mechanism of Antiemetic Effect of Lavandula angustifolia Mill. Essential Oil Based on Ca2+/CaMKII/ERK1/2 Pathway
Authors Li J , Wang X, Xun S, Guo Q, Wang Y, Jia Y, Wang W, Wang Y, Li T, Tang T, Zou J, Wang M, Yang M, Wang F, Zhang X, Wang C
Received 4 April 2022
Accepted for publication 14 July 2022
Published 26 July 2022 Volume 2022:16 Pages 2407—2422
DOI https://doi.org/10.2147/DDDT.S366597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Jia Li,1,* Xiao Wang,1,* Shining Xun,2,* Qiuting Guo,3 Yao Wang,1 Yanzuo Jia,1 Wenfei Wang,1 Yujiao Wang,1 Taotao Li,1 Tiantian Tang,1 Junbo Zou,1 Mei Wang,1 Ming Yang,4 Fang Wang,4 Xiaofei Zhang,1,4 Changli Wang1
1Department of Pharmaceutics, The Key Laboratory of Basic and New Drug Research of Traditional Chinese Medicine, Shaanxi University of Chinese Medicine, Xianyang, People’s Republic of China; 2Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, People’s Republic of China; 3Xianyang Vocational Technical College, Xianyang, People’s Republic of China; 4Department of Pharmaceutics, Key Laboratory of Modern Preparation of Traditional Chinese Medicine, Ministry of Education, Jiangxi University of Chinese Medicine, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofei Zhang, Department of Pharmaceutics, College of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang, People’s Republic of China, Tel +86 177 7003 7322, Fax +86 29-38185333, Email [email protected] Changli Wang, Department of Pharmaceutics, College of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang, People’s Republic of China, Tel +86 132 3910 3433, Email [email protected]
Purpose: To investigate the effective components and possible mechanism of action of Lavandula angustifolia Mill. essential oil (LEO) in preventing vomiting through the olfactory pathway.
Materials and Methods: A new network pharmacology–based method was established to analyze main components and pathways of LEO involved in antiemetic effects by introducing component content; biological activities of key proteins of the olfactory pathway and their corresponding compounds were verified by molecular docking technique; and finally pica in a rat model was established to verify the molecular mechanism of antiemetic effects of LEO by enzyme-linked immunosorbent assay (ELISA) to determine the serum 5-HT, substance P, and DA levels in each group and by immunohistochemistry to determine the contents of 5-HT3R, CaMKII and ERK1/2 proteins in the medulla oblongata tissue.
Results: Network pharmacology combined with molecular docking analysis showed that the mechanism of the antiemetic effect of LEO may be related to (2Z)-3,7-dimethyl-2,6-octadienyl acetate, linalyl acetate, butanoic acid, hexyl ester, 4-hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate, .tau.-cadinol and other active ingredients, which regulate the cyclic adenosine monophosphate (cAMP) signaling pathway and the expression of BRAF, PDE and other targets on the pathway. An ELISA revealed that LEO reduced the levels of 5-hydroxytryptamine (5-HT), substance P, and dopamine in serum compared with the model group (P < 0.05). Immunohistochemical analysis showed that LEO decreased the expression of 5-HT3R, CaMKII, and ERK1/2 proteins in the medulla oblongata of rats compared with the model group (P < 0.01).
Conclusion: LEO may achieve the antiemetic effect by reducing the content of 5-HT and inhibiting its related receptors, thereby regulating downstream Ca2+/CaMKII/ERK1/2 pathway of the cAMP signaling pathway.
Keywords: Lavandula angustifolia Mill, vomiting, weight coefficient, molecular docking, mechanism validation
Introduction
According to the Global Cancer Statistics 2020 report, the incidence of cancer is increasing year by year, which seriously affects human health and social development. Specifically, there were 19.3 million new cancer cases and nearly 10 million deaths from cancer in 2020.1,2 Chemotherapy is an important treatment option for cancer patients. However, nausea and vomiting often occur during chemotherapy, which is known as chemotherapy-induced nausea and vomiting (CINV). CINV is one of the most disturbing symptoms of cancer patients after chemotherapy, which reduces their quality of life, and its incidence is as high as 80%–90%.3–5 Severe nausea and vomiting can lead to complications such as water and electrolytic disorders, malnutrition, decreased body immunity, and delayed wound healing, all of which can seriously affect the treatment and prognosis of patients.6 Currently, drugs such as dexamethasone, 5-HT3 and NK-1 receptor antagonists are mostly recommended internationally for the treatment of CINV. However, the efficacy of these drugs is not yet sufficient; if the dose is increased for better efficacy, it may lead to side effects such as constipation, headache, and dizziness, and the high cost of treatment can greatly increase the economic burden of patients.7 Therefore, the search for safe, effective, and inexpensive drugs against CINV has important clinical significance and social benefits.
Plants have long been desired for safer treatment. Aromatherapy, as a standard complementary treatment option for CINV, is effective in reducing the incidence of adverse effects while increasing efficacy. Aromatherapy is a nonpharmacological treatment option based on plant essential oils. It is a feasible, safe, and cost-effective method, and has significant efficacy in alleviating insomnia, anxiety, nausea, and many other conditions.8 Zorba et al demonstrated that aromatherapy by either inhalation or massage reduced nausea and vomiting in chemotherapy patients.9 When essential oils are inhaled, volatile components of the oils stimulate olfactory receptors (OR) and generate chemical signals, which are converted into electrical signals; these signals are then transmitted by olfactory nerve cells to the olfactory center to generate olfactory sensations, thereby eliciting a physical response.10 Compared with traditional clinical drug delivery methods, aromatic active substances transmit nerve signals to the brain through the olfactory pathway. They regulate brain function without liver and kidney metabolism to avoid causing liver and kidney function damage. Therefore, they are simple to use, convenient, safe, and have good patient compliance. Therefore, targeting the olfactory pathway may be a new approach to treat vomiting.
Lavandula angustifolia Mill. (Labiatae) is a perennial evergreen subshrub with a global distribution, mostly in the Mediterranean region of Europe, and is now widely cultivated in Xinjiang, China.11,12 LEO is an aromatic herbal extract, mainly containing linalool, linalyl acetate, and lavender alcohol and camphor.13,14 It is commonly used in a variety of clinical conditions, such as anxiety, pain, and inflammation.15,16 Several studies have shown that it can block 5-HT and dopamine receptors associated with nausea.17–20 The mechanism of action of current antiemetic drugs also involves inhibition of the binding of transmitters such as acetylcholine, dopamine, histamine, 5-hydroxytryptamine, γ-aminobutyric acid, and substance P to the corresponding receptors. In the first early stage of this study, 2% LEO was applied to Shenque point of pica rats, and the effect of this method on CINV was analyzed. There was no statistical difference between the treatment group and the model group (P> 0.05), and it is speculated that LEO may prevent vomiting via aromatherapy. In vitro experiments in guinea pig ileum and rat uterus by Lis-Balchin and Hart S confirmed that LEO may act as an antispasmodic agent by increasing intracellular levels of cAMP.21 On that basis, it can be inferred that LEO may prevent and treat CINV by increasing the level of intracellular cAMP and eliciting spasmolysis of the gastrointestinal tract. Furthermore, a study evaluating the effect of aromatherapy on nausea and vomiting has shown that symptoms were significantly lower in the Lavandula angustifolia Mill. aromatherapy group than in the placebo group.22 However, no studies have evaluated the mechanism of antiemetic action of LEO through olfactory pathway.
Network pharmacology can reveal the intricate relationship between drugs, targets, and diseases; analyze the molecular basis of diseases at multiple levels; predict the pharmacological mechanism of action of drugs; and compensate for the disadvantages of traditional pharmacological experimental studies that are blind, time-consuming, expensive, and unsystematic.23 However, most of the current studies in network pharmacology focus on the qualitative analysis of the “drug component–target network” while ignoring the effect of the content of each component. This often results in drugs with low component content being considered as active ingredients and drugs with high component content being considered as ineffective ingredients, which makes network pharmacology lose its original meaning. Therefore, there is an urgent need to establish a new network pharmacology method to assess the effects of drugs. In this study, a calculation method was proposed to quantitatively analyze the effects of intervention pathways from the perspective of component weight coefficient and to compare with the signaling pathways of traditional network pharmacology to screen out the key pathways. A rat model of pica was established, and the antiemetic mechanism of LEO delivered through the olfactory pathway was studied. Moreover, the connection between the olfactory pathway and PONV, as well as the possibility to prevent and treat PONV through the olfactory pathway, were explored to provide a theoretical basis for the treatment of vomiting by LEO via the olfactory pathway through aromatherapy. The flowchart of this study is shown in Figure 1.
 |
Figure 1 A detailed flowchart of the study. |
Materials and Methods
Materials and Reagents
LEO was purchased from Xinjiang eprhan Spice Co., Ltd. (number of code 2021-Y-1; Yining, China). All of the other chemicals and solvents were of analytical reagent grade.
LEO was prepared using the following procedure: LEO was extracted by ether, dried, and filtered with anhydrous Na2SO4. Ether was evaporated from the filtrate to obtain colorless volatile oil, which was used as the sample for gas chromatography–mass spectrometry (GC-MS) analysis.24
GC-MS Chromatographic Conditions
LEO was analyzed using GC–MS (model 7890GC/5977MC; Agilent Technologies Co. Ltd., Palo Alto, USA). Chromatographic conditions were as follows: Agilent HP-5 MS capillary column (30 m × 0.25 mm x 0.25 μm), high-purity He carrier gas, injection volume of 1 μL, no splitting, flow rate of 1.2 mL/min. Ramp-up procedure was as follows: initial temperature of 60°C (holding for 5 min), ramp-up to 120°C at 5°C/min (holding for 5 min), and ramp-up to 260°C at 10°C/min (holding for 10 min). The following mass spectrometry conditions were used: ionization mode EI, electron energy of 70 eV, ion source temperature of 230°C, quadrupole temperature of 150°C, scan mass range of 30–400 amu, full scan mode, and solvent delay time of 3.0 min.
Identification of LEO Components
To improve the accuracy of qualitative analysis, the compounds were further identified in combination with retention indices. The retention times (tR) of C8–C20 n-alkane standard solutions were determined according to the analytical conditions under 2.2, and the retention index was calculated as RI=100Z+100×[tR(x)−tR(Z)]/[tR(Z+1)−tR(Z)], where RI is the retention index; x indicates the compound to be analyzed; Z and Z+1 are the number of carbon atoms of n-alkane before and after the peak of the target, respectively; and tR(Z) < tR(x) < tR(Z+1). The retention indices of the compounds to be identified were calculated based on the retention times of the n-alkanes, and the components were selected based on the matches, retention indices, and relevant literature by searching the NIST14 standard mass spectrometry library. The compound with the highest retention index proximity was considered the best identification result.
Screening of Corresponding Targets for the Active Components of LEO
The two-dimensional structure of the active ingredient was downloaded from PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and the Swiss Target Prediction database was used for target prediction of the ingredients to obtain the corresponding targets of the active ingredient of LEO.25,26
Acquisition of Vomiting Targets and Olfactory Receptor–Related Proteins
Based on GeneCards(https://www.genecards.org/), Comparative Toxicogenomics Database (http://ctdbase.org/), and UniProt(https://www.uniprot.org/) public databases, all vomiting disease targets and OR-related proteins were collected.27,28
Network Construction of Protein–Protein Interaction (PPI)
We used Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) to compare and analyze the potential action targets of LEO with vomiting disease targets and OR-related proteins, and the intersecting targets were screened. The intersecting targets were uploaded to the online STRING (http://string-db.org/) platform with confidence protein parameter score values >0.9 to construct a PPI network.29 The network data were imported into Cytoscape 3.7.2 software for analysis, and the top 10 genes in terms of degree value were screened as key targets using the plugin Cytohubba.30
Active Ingredient–Target Network Construction for LEO
The “active ingredient–antiemetic potential targets” network of LEO was constructed by Cytoscape 3.7.2 software, and the network characteristic parameters were analyzed using the built-in network analyzer tool of the software in order to study the more important ingredients and targets of LEO and the relationship between them.
Establishment of Weighting Coefficients
We calculated the weight coefficient, that is the relative content of the component, for all of the components. Since the drug has the characteristic of multiple components corresponding to multiple targets, the weight coefficients of the targets can be calculated based on the weight coefficients of the components. In addition, the targets act on signaling pathways, so the weight coefficients for each pathway can be calculated and reordered based on the weight coefficients. To better explain the relationship between relative content and active ingredients in terms of pharmacological mechanisms, we correlated the two as follows:
where i,j,k,n=1,2,3, … …, Ai represents the relative content (weighting coefficient) of component i in the total component; 1 represents the uptake rate of the component (we consider that all components are absorbed in full); Bj represents the sum of the weighting coefficients of the active components corresponding to each target, and also represents the weighting coefficient of target j among all targets; and Dk represents the sum of the weighting coefficients of the targets contained in each pathway.
Biological Function and Pathway Analysis
The drug-disease core target genes were converted from Gene Symbol to entrezID by R language, and gene ontology-biological process (GO-BP) analysis and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analyses were performed on the intersecting genes using the clusterProfiler package.31 The enrichment analysis results were obtained at P < 0.05, and were re-ranked according to their weight coefficients (the weight coefficient of each pathway is the sum of the weight coefficients of all targets in the pathway).
Molecular Docking
The three-dimensional crystal structures of key targets (pdb format) were downloaded from the PDB protein database (https://www.rcsb.org/), and the two-dimensional structures of ligands (sdf format) were downloaded from the PubChem database.32 The LibDock tool of Discovery Studio 4.5 software was used to conduct molecular docking of the key target proteins with their corresponding active components and positive drugs.
Experimental Methods
Experimental Animals
Healthy male Wistar rats (180–220 g) without specific pathogens were purchased from Chengdu Dasuo Co., Ltd. (animal qualification certificate No.: SCXK [chuan] 2020–030; Chengdu, China). Standard laboratory temperature and humidity conditions were maintained, and food and water were freely available during the whole study. Animal welfare and experimental process were strictly performed following the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996). The experimental plan was approved by the Experimental Animal Ethics Committee of Shaanxi University of Chinese Medicine (No. SUCMDL20220102001; Xianyang, China).
Animal Grouping and Intervention
After one week of adaptive feeding, the rats were divided into the following four groups (10 rats in each group) based on the random-numbering method: normal control group (CON), model group (MOD), ondansetron group (OND), and LEO treatment group (LEO). After the groups were formed, the rats were kept in single cages; kaolin was administered until the rats no longer gnawed on it; and then the formal experiment was started. The CON and MOD groups were gavaged with distilled water, and the OND group was gavaged with ondansetron at a concentration of 0.08 mg/mL (dose 1.2 mg/kg) once daily for three days. The rats in LEO group were administrated by atomization inhalation. The rats in LEO group were placed in a transparent atomization box (50 cm×36 cm×28 cm), which was directly connected to the ultrasonic atomizer. Then,2% LEO (6 mL/kg diluted with Tween 80) was added into the medicine cup of ultrasonic nebulizer for atomization until the essential oil in the medicine cup was atomized and sniffed once a day for 3 consecutive days.33 After atomization, the animals were kept in the atomization box for 10 min so as to facilitate drug absorption. One hour after the first administration, 6 mg/kg cisplatin (MOD, OND, and LEO groups) or equal volume of normal saline (CON group) was intraperitoneally injected. The daily intake of kaolin, feed, and body weight of rats were recorded at 48 hours and 24 hours before cisplatin was injected, the day of cisplatin was injected, and 24 hours, 48 hours, and 72 hours after cisplatin was injected.
Sample Collection
At 72 hours after modeling, blood was collected from the abdominal aorta of each group of rats after anesthesia with 40 mg/kg sodium pentobarbital i.p. administration. The serum was centrifuged at 4°C 3500 rpm for 20 min. The serum was divided and stored at −80°C cryogenic refrigerator for future use. After blood collection, medulla oblongata tissue was taken immediately, washed with normal saline, and fixed in 4% paraformaldehyde solution for immunohistochemical analysis.
Enzyme-Linked Immunosorbent Assay
Cisplatin-like cancer chemotherapeutic drugs can induce the release of a variety of neurotransmitters (eg, dopamine, 5-HT, substance P) from the brainstem through a cytoplasmic calcium(Ca2+)-dependent process to cause vomiting.34 Therefore, in this study, the rat 5-HT ELISA kit, rat substance P ELISA kit, and rat dopamine ELISA kit purchased from Jiangsu Enzyme Immunoassay Industrial Co., Ltd. (Yancheng, China) were utilized in accordance with the kit instructions. The serum levels of 5-HT, substance P, and dopamine were determined by double antibody sandwich method, and the absorbance was measured at 450 nm by a microplate reader. The concentrations of 5-HT, substance P, and dopamine in the samples were calculated using the standard curve.
The Contents of 5-HT3R, CaMKII, and ERK1/2 Proteins in Medulla Oblongata Tissue as Detected by Immunohistochemistry
After embedding and sectioning the fixed tissue, the sections were dewaxed to water. After antigen repair, the sections were incubated with 3% hydrogen peroxide at room temperature for 25 min and washed with phosphate buffer saline (PBS) for 5 min (three times). Then, 10% normal goat serum was added and incubated at room temperature for 30 min. After discarding the serum, the sections were incubated with anti-5-HT3R (1:200; Bioss, China), anti-CaMKII (1:200; Bioss, China), and anti-ERK1/2 (1:200; Bioss, China) primary antibodies at 4°C overnight.35 Then, we took the sections from the refrigerator to rewarm them for 30 min, washed them with PBS for 5 min (three times), added a secondary antibody, and incubated it at a room temperature for 50 min. Next, we washed the sections with PBS for 5 min (three times). Diaminobenzidine was used for color rendering, and the color rendering was terminated by washing with pure water. Next steps included hematoxylin counterstaining for 2 min, dehydration, transparency, and sealing.
Statistical Analysis
Data are expressed as mean ± standard error. Statistical differences were determined by one-/two-way ANOVA followed by Tukey’s post hoc test using GraphPad Prism 7.0 statistical software. Data regarding kaolin and food intake and body weight were analyzed by two-way ANOVA, and data regarding ELISA and immunohistochemical assays were analyzed by one-way ANOVA. P < 0.05 was considered statistically significant.
Results
Chemical Composition of LEO
The chemical composition of LEO was determined by GC-MS, as shown in Figure 2A. A total of 21 chemical components of LEO were identified by searching NIST20 standard spectrum library and combining with retention index, as shown in Table 1.
 |
Table 1 Qualitative Results of Lavandula angustifolia Mill. Essential Oil by GC-MS |
Key Target Screening Results
The Swiss Target Prediction database was used to predict each chemical component of LEO, and 293 targets corresponding to the active ingredients of LEO were obtained after de-duplication (See Supplementary Table 1 for components and targets of LEO). A total of 14,199 OR-related targets and 60,492 vomiting disease-related targets were collected from GeneCards, CTD, and UniProt databases, while 12,130 OR-related targets and 29,997 vomiting disease-related targets were obtained after the retrieval results of the three databases were de-duplicated. After mapping by Venny 2.1.0 online website, a total of 134 key targets were obtained (Figure 2B, See Supplementary Table 2 for 134 key targets).
Construction of Key Target PPI Network
The screened key targets were input into String database to obtain protein interaction relationships. There were 134 nodes and 107 edges in the constructed PPI network. The average degree value of the targets in the network was 1.6; the obtained data were imported into Cytoscape software, and the degree value of non-free targets in the network was calculated according to Cytohubha, a plug-in in Cytoscape software (Figure 2C). A higher degree value of the target indicated the higher probability that the active ingredient acts on the target to exert therapeutic effects. Higher Cytohubba-degree scores indicated that these target genes play an important role in the network and can be used as the target genes of the active components. The top 10 key targets included SRC, JUN, ESR1, EGFR, PTK2B, PTPN11, PTK2, CHRM1, JAK1, and GSK3B.
Active Ingredient–Target Network Analysis of LEO
Cytoscape 3.7.2 software was used to construct a network of components and their corresponding targets, as shown in Figure 2D (the orange circles represent the active ingredients contained in the drug, and the purple circles represent the targets of the active ingredients mapped to the vomiting disease and olfactory receptors). There were 154 nodes in the network, including 20 compound nodes, 134 target nodes, and 381 edges. Each edge represented the interrelationship between “LEO components–targets”, and the size of the node was proportional to the degree of the node. In the whole network, the top two targets of degree value were CA2 and CNR2, which can interact with ten and nine compounds respectively.
GO-BP and KEGG Enrichment Analysis
Using R language, the 134 common targets were enriched with GO-BP and KEGG at P < 0.05. A total of 1413 biological processes and 66 KEGG signaling pathways were enriched. The histograms of the top 20 pathways (Figure 3A and B; See Supplementary Tables 3 and 5 for original biological process and signal pathway) were drawn according to the significance of enrichment, including G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger, adenylate cyclase-modulating G protein-coupled receptor signaling pathway, modulation of chemical synaptic transmission, neuroactive ligand–receptor interaction, calcium signaling pathway, and cAMP signaling pathway. Among them, the cAMP signaling pathway is the main signaling pathway for olfactory epidermal perception of odor, and after reordering the enrichment results by weight coefficient, cAMP signaling pathway still ranked in the top (Figure 3C and D; See Supplementary Tables 4 and 6 for biological process and signal pathway after the score is sorted). Therefore, LEO may exert its antiemetic effect by affecting multiple targets such as PDE10A, PDE4B, PDE4D, and BRAF in this pathway.
Molecular Docking Analysis
To further illustrate the binding activity between the target proteins and the corresponding compounds, the key targets (PDE10A, PDE4B, PDE4D, BRAF) and their corresponding compounds in the cAMP signaling pathway were selected for molecular docking using Discovery Studio 4.5 software. The docking results are shown in Table 2, and the interactions are shown in Figure 4. The higher the score of the components, the more important they are for the targets and the greater their regulatory effects on the cAMP signaling pathway. The docking scores of the target proteins and their corresponding small molecule compounds were compared with those of the positive control, and the results showed that PDE10A, PDE4B, PDE4D and BRAF may be the key targets, and linalyl acetate, butanoic acid, hexyl ester, 4-hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate, .tau.-cadinol and (2Z)-3,7-dimethyl-2,6-octadienyl acetate may be the key antiemetic components of LEO.
 |
Table 2 Docking Scores of Target Proteins with Their Corresponding Compounds and Positive Controls |
Effect of LEO on Pica Induced by Cisplatin in Rats
To evaluate the effects of LEO on cisplatin-induced pica behavior in rats, we measured daily kaolin intake, food consumption, and body weight. After three days of adaptation to kaolin, rats rarely ingested kaolin. As shown in Figure 5A, single injection of cisplatin resulted in a significant increase in kaolin intake in rats (P < 0.01), and daily treatment with LEO reduced cisplatin-induced kaolin consumption (P < 0.05). Cisplatin significantly reduced food intake in a time-dependent manner (Figure 5B). Daily treatment with LEO increased food intake after cisplatin modeling (P < 0.05). Cisplatin post-modeling caused a significant decrease in body weight, which was increased by LEO 72 hours after administration (P < 0.05) (Figure 5C).
Effect of LEO on Serum 5-HT, Substance P, and Dopamine Levels in Rats
At 72 hours after intraperitoneal injection of cisplatin, the serum 5-HT, substance P, and dopamine contents in the model group were significantly higher than those in the control group (P < 0.05). Compared with the model group, the serum 5-HT, substance P, and dopamine contents in the LEO group were significantly decreased (P < 0.05). The results showed that LEO was able to decrease the abnormal increase of 5-HT, substance P, and dopamine content in serum, as shown in Figure 6.
Effects on 5-HT3R, CaMKII, and ERK1/2 Proteins in the Medulla Oblongata of Pica Rats
The results of immunohistochemical staining (Figure 7) showed that the expression levels of 5-HT3R, CaMKII, and ERK1/2 proteins were significantly increased in the model group compared with the normal group (P < 0.01); and the expression levels of 5-HT3R, CaMKII, and ERK1/2 proteins were significantly decreased in the LEO group compared with the model group (P < 0.01).
Mechanism Description
The rats were given a single intraperitoneal injection of cisplatin to establish the pica model, and LEO was used for intervention sniffing of the pica model rats. During the experiment, kaolin intake, feed intake, and weight change of the rats were recorded. The contents of 5-HT, substance P, and dopamine in serum of each group were measured by ELISA, and the expression levels of 5-HT3R, CaMKII, and ERK1/2 protein in medulla oblongata tissues were detected by immunohistochemistry. Taking the results of all experiments together, we believe that the mechanism of LEO’s antiemetic action might be through inhibiting 5-HT-related receptors and blocking downstream Ca2+/CaMKII/ERK1/2 pathway of the cAMP signaling pathway (Figure 8).
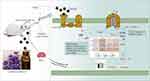 |
Figure 8 Mechanism display of LEO suppressed vomiting via Ca2+/CaMKII/ERK1/2 signaling pathway. *P < 0.05, **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs model group. |
Discussion
In this study, aromatic substances were used to stimulate the release of neurotransmitters by inhaling and sniffing, thus alleviating the pica behavior in rats. Due to the direct anatomical channel between the nasal cavity and the brain tissue, drugs can be absorbed through the nasal mucosa, bypassing the blood-brain barrier and transporting directly into the olfactory bulb or cerebrospinal fluid and then directly into the brain tissue.36 The binding of plant essential oils to olfactory receptors is drug-specific and reaches the brain through specific olfactory transmission pathways, and this binding is faster, safer, easier, and more effective. The cAMP signaling pathway is the main pathway that conducts olfaction in most olfactory neurons in mammals.37,38 The cAMP signaling pathway was ranked in the top both before and after considering the weighting coefficients for the enrichment results, and a review of the literature and analysis of the enrichment results revealed that LEO may act on the cAMP signaling pathway to treat CINV.
The cAMP signaling pathway is the main signaling pathway for the olfactory epithelium to perceive odor. It converts the chemical signal generated by the odor molecule stimulating the olfactory receptor into an electrical signal, which is then transmitted by olfactory nerve cells to the olfactory center to produce olfactory sensation. When an external odor molecule binds to an OR, the odor molecule–OR complex activates the Golf protein.39 Upon activation of the Golf protein, the Gɑ-GTP subunit moves to the adjacent olfactory-specific adenylate cyclase AC3 site, which in turn activates AC3, which catalyzes the removal of a pyrophosphate from ATP to produce large amounts of cAMP.40 cAMP plays a key regulatory role in physiological processes such as metabolism, secretion, calcium homeostasis, and muscle contraction. Cyclic Nucleotide Gated Channel is one of the main targets of direct action of cAMP, mainly regulating calcium ions and activating calmodulin (CaM) and calmodulin-dependent protein kinase (CaMKs), thereby regulating downstream pathways. Extracellular Ca2+ enters the cell and acts as a second messenger to initiate protein phosphorylation and neurotransmitter release.41 Previous studies have shown that Ca2+ mobilization is also an important part of the induction of vomiting.42 An increase in Ca2+ concentration in the free cytoplasm can activate the cytoplasmic Ca2+-sensing protein CaM, a calcium-binding protein present in almost all eukaryotic cells, which is activated by binding Ca2+ under normal conditions. When Ca2+ binds to CaM it generates a Ca2+/CaM complex, which subsequently activates CaMKII and changes its conformation, thereby causing cascade amplification and responding to various extracellular stimuli.43 CaMKII is a downstream kinase that undergoes autophosphorylation in response to elevated intracellular Ca2+, which is essential for the coordination and execution of Ca2+ signaling.44 CaMKII positively regulates the activation of ERK1/2 through binding to Raf-1.45 ERK1/2 is essential for signal transduction from surface receptors to the nucleus, and it plays an important role in cell proliferation, cell differentiation, and apoptosis.46 In addition, Ca2+/CaMKII-dependent ERK1/2 has been reported to be the main intracellular signaling system for 5-HT3R-mediated vomiting.35 Therefore, LEO may achieve an antiemetic effect by inhibiting 5-HT-related receptors and blocking downstream Ca2+/CaMKII/ERK1/2 pathway of the cAMP signaling pathway.
To further identify the key active components in LEO that affect the cAMP signaling pathway, we selected four key targets in this pathway, ie, PDE10A, PDE4B, PDE4D and BRAF, for molecular docking. The interactions between the main active components of LEO and the potential targets were verified by molecular docking. The results indicated that linalyl acetate,4-hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate, .tau.-cadinol, butanoic acid, hexyl ester, and (2Z)-3,7-dimethyl-2,6-octadienyl acetate may be the key antiemetic components of LEO. where BRAF and RAP1 form an activation complex that activates MEK1/2, eventually inducing ETS-domain protein Elk1-mediated gene expression; PDE converts cAMP back to AMP by isolating cAMP activity to terminate cAMP signaling. Furthermore, the putative components (2Z)-3,7-dimethyl-2,6-octadienyl acetate, linalyl acetate, butanoic acid, hexyl ester, 4-hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate, .tau.-cadinol may alleviate vomiting symptoms by targeting BRAF and PDE proteins.The mechanism of CINV and novel antiemetic drugs have mainly been studied in animal models. Rats are the most used laboratory animals, which have no vomiting reflex; however, a large number of studies have shown that after administration of emetic drugs, rats tend to ingest a non-nutritive substance, such as kaolin. Therefore, rats are also often used as experimental animals to study antiemetic drugs, mainly by evaluating the amount of food intake and the amount of heterophilic kaolin.47,48 In this study, a single intraperitoneal injection of cisplatin 6 mg/kg was given to rats to establish a pica model, and LEO was used to intervene in the pica model rats for sniffing. The behavioral results showed that LEO sniffing was able to alleviate the pica behavior of the rats. The ELISA results showed that the serum 5-HT and DA levels of the LEO group were significantly lower than those of the model group, indicating the effectiveness of LEO intervention. The results of our immunohistochemical experiments illustrated that LEO was able to inhibit the expression of 5-HT3R, CaMKII, and ERK1/2 proteins in the medulla oblongata tissue, confirming its direct pharmacological effects through the three core proteins 5-HT3R, CaMKII, and ERK1/2 in the cAMP signaling pathway. This shows that elevated levels of 5-HT and DA and expression of 5-HT3R, CaMKII, and ERK1/2 genes can significantly stimulate afferent nerves to increase nerve impulse production, which in turn increases afferent signals that mediate the central and peripheral nervous system to produce the vomiting reflex and trigger CINV. “Aspiration to the brain” is a nondestructive mode of administration that minimizes toxic side effects, and therefore “aspiration to the brain” through the olfactory route may be an effective route of administration for the control and treatment of vomiting.
Essential oils are refined natural substances, and because they are highly concentrated, they do not perform better the more frequently they are used, even when they are diluted. The most common adverse reactions to the use of essential oils include skin irritation, skin sensitization, mucous membrane irritation. and photosensitivity.49 Empirical evidence suggests that essential oils may have negative effects on children and pregnant and lactating women, as well as on the respiratory, cardiovascular, and central nervous systems.49 Therefore, the use of essential oils must be scientific and prudent. In addition, essential oils must be stored in dark glass bottles that are always well sealed and kept in a cool place.50 The genus Lavandula includes several species; however, the most important are Lavandula angustifolia and Lavandula x intermedia, from which the essential oils are primarily obtained. In this study, essential oil of Lavandula angustifolia flowers from Yili, Xinjiang, was purchased, and the samples of lavender were collected during the peak flowering period in June and extracted by hydrodistillation, with the main component being 1,6-octadien-3-ol, 3,7-dimethyl- and linalyl acetate. The shortcomings of this study are the small sample size and the lack of evaluation of the long-term effects of aromatherapy. Future studies will increase the sample size to evaluate the long-term effects of aromatherapy. The specific mechanism of lavender essential oil inhalation on the improvement of vomiting symptoms still needs to be further investigated, and its efficacy needs to be confirmed by more clinical trials, thus providing a theoretical basis for a new way of olfactory intervention for CINV treatment.
Conclusion
This study confirmed the possibility of olfactory interventions for the treatment of CINV. The results of network pharmacology and mechanism verification based on weight coefficient provided a new insight for comprehensively elucidating the antiemetic effect of LEO. Specifically, LEO reduced the content of 5-HT and inhibited its related receptors. Thus, regulating downstream Ca2+/CaMKII/ERK1/2 pathway of the cAMP signaling pathway may be the potential mechanism of the antiemetic effect of LEO on CINV.
Abbreviations
LEO, Lavandula angustifolia Mill. essential oil; 5-HT, 5-hydroxytryptamine; ELISA, enzyme-linked immunosorbent assay; cAMP, cyclic adenosine monophosphate; CINV, chemotherapy-induced nausea and vomiting; OR, olfactory receptors; GC-MS, gas chromatography–mass spectrometry; PPI, protein–protein interaction; GO-BP, gene ontology-biological process; KEGG, Kyoto encyclopedia of genes and genomes; PBS, phosphate buffer saline; CaM, calmodulin; CaMKs, calmodulin-dependent protein kinase; Ca2+, cytoplasmic calcium.
Data Sharing Statement
The original contributions presented in the study are included in the article and Supplementary Material (See Supplementary Tables 1–6), further inquiries can be directed to the corresponding authors.
Ethics Statement
The human data involved in this study from GeneCards public database and the study meets national and international guidelines for research on humans. Therefore, the ethical approval has been granted an exemption (IEC for Medical of The Second Affiliated Hospital of Shaanxi University of Chinese Medicine).
Funding
This study was supported by the Science and Technology Project of Jiangxi Provincial Department of Education (GJJ150877), the Training plan for young teachers in key disciplines of Jiangxi University of Chinese Medicine (51000112), the 2017 Open Fund of the Key Laboratory of Modern Chinese Medicine Preparation by the Ministry of Education (2017003), the Project of Shaanxi Provincial Department of science and technology (2022JM-555), the Key R & D program of Xianyang City (2021ZDYF-SF-0015), the National Natural Science Foundation of China (Grant no. 82074026), the Education Department of Shaanxi Provincial Government of China (20JK0588), the Shaanxi Provincial Key Laboratory Project of Traditional Chinese Medicine (KF2204), the National Natural Science Foundation of China (81703720), the Scientific Research in the affiliated Hospital of Shaanxi University of Chinese Medicine (2020ZJ005) and the Shaanxi Provincial Key Research and Development Program (2017SF-351).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Liu ZC, Li ZX, Zhang Y, et al. Interpretation on the report of global cancer statistics 2020. J Multidiscip Cancer Manage. 2021;7(02):1–14.
3. Shapiro CL. Highlights of recent findings on quality-of-life management for patients with cancer and their survivors. JAMA oncol. 2016;2(11):1401–1402. doi:10.1001/jamaoncol.2016.3620
4. Zhang QY, Wang FX, Jia KK, Kong LD. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front Pharmacol. 2018;9:1253. doi:10.3389/fphar.2018.01253
5. Wan GS, Sun J. Treatment progress of TCM in chemotherapy-induced nausea and vomiting. J Liaoning Univ Tradit Chin Med. 2009;11(08):76–78. doi:10.13194/j.jlunivtcm.2009.08.78.wangsh.171
6. Molassiotis A, Lee PH, Burke TA, et al. Anticipatory nausea, risk factors, and its impact on chemotherapy-induced nausea and vomiting: results from the Pan European Emesis Registry Study. J Pain Symptom Manage. 2016;51(6):987–993. doi:10.1016/j.jpainsymman.2015.12.317
7. Hsieh RK, Chan A, Kim HK, et al. Baseline patient characteristics, incidence of CINV, and physician perception of CINV incidence following moderately and highly emetogenic chemotherapy in Asia Pacific countries. Support Care Cancer. 2015;23(1):263–272. doi:10.1007/s00520-014-2373-2
8. Fang T, Ma HM, Wang N, Xiao BB, Ruan L, Chen HY. Research progress on the application of aromatherapy. Chin Nurs Res. 2019;33(23):4093–4095.
9. Zorba P, Ozdemir L. The preliminary effects of massage and inhalation aromatherapy on chemotherapy-induced acute nausea and vomiting: a quasi-randomized controlled pilot trial. Cancer Nurs. 2018;41(5):359–366. doi:10.1097/ncc.0000000000000496
10. Huntley A. Aromatherapy science: a guide for healthcare professionals %J focus on alternative and complementary therapies. Perspect Public Health. 2006;11(3):239.
11. Basch E, Foppa I, Liebowitz R, et al. Lavender (Lavandula angustifolia Miller). J Herb Pharmacother. 2004;4(2):63–78.
12. Li H, Bai HT. Romance from the Mediterranean to the foot of Tianshan Mountain-The introduction of lavender resources and breeding of new varieties. Life World. 2019;2019(06):16–21.
13. Tang SY, Shen YP, Liu CB, et al. Determination of volatile chemical components in lavender by supercritical fluid extraction-gas chromatography-mass spectrometry. Acta Agric Jiangxi. 2018;30(03):106–111. doi:10.19386/j.cnki.jxnyxb.2018.03.22
14. Giovannini D, Gismondi A, Basso A, et al. Lavandula angustifolia Mill. essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to Staphylococcus aureus. Immunol Invest. 2016;45(1):11–28. doi:10.3109/08820139.2015.1085392
15. Algieri F, Rodriguez-Nogales A, Vezza T, et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J Ethnopharmacol. 2016;190:142–158. doi:10.1016/j.jep.2016.05.063
16. Karaman T, Karaman S, Dogru S, et al. Evaluating the efficacy of lavender aromatherapy on peripheral venous cannulation pain and anxiety: a prospective, randomized study. Complement Ther Clin Pract. 2016;23:64–68. doi:10.1016/j.ctcp.2016.03.008
17. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23(3):396–404. doi:10.1016/j.ctim.2015.03.009
18. Nasir SS, Schwartzberg LS. Recent advances in preventing chemotherapy-induced nausea and vomiting. Oncology. 2016;30(8):750–762.
19. Drozdoff L, Klein E, Kiechle M, Paepke D. Use of biologically-based complementary medicine in breast and gynecological cancer patients during systemic therapy. BMC Complement Altern Med. 2018;18(1):259. doi:10.1186/s12906-018-2325-3
20. Chumpitazi BP, Kearns GL, Shulman RJ. Review article: the physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment Pharmacol Ther. 2018;47(6):738–752. doi:10.1111/apt.14519
21. Lis-Balchin M, Hart S. Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller). Phytother Res. 1999;13(6):540–542. doi:10.1002/(sici)1099-1573(199909)13:6<540::aid-ptr523>3.0.co;2-i
22. Karaman S, Karaman T, Tapar H, Dogru S, Suren M. A randomized placebo-controlled study of aromatherapy for the treatment of postoperative nausea and vomiting. Complement Ther Med. 2019;42:417–421. doi:10.1016/j.ctim.2018.12.019
23. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi:10.1038/nchembio.118
24. Huang KJ. A GC-MS analysis of the volatile components of curcuma Zedoaria. J Shaanxi Univ Chin Med. 2020;43(04):68–73. doi:10.13424/j.cnki.jsctcm.2020.04.015
25. Kim S, Chen J, Cheng T, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49(D1):D1388–D1395. doi:10.1093/nar/gkaa971
26. Gfeller D, Michielin O, Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29(23):3073–3079. doi:10.1093/bioinformatics/btt540
27. Stelzer G, Dalah I, Stein TI, et al. In-silico human genomics with GeneCards. Hum Genomics. 2011;5(6):709–717. doi:10.1186/1479-7364-5-6-709
28. Bateman A, Martin M-J, Orchard S. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49(D1):D480–D489. doi:10.1093/nar/gkaa1100
29. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
30. Kohl M, Wiese S, Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi:10.1007/978-1-60761-987-1_18
31. Yu G, Wang LG, Han Y, He QY. clusterprofiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
32. Burley SK, Bhikadiya C, Bi C, et al. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021;49(D1):D437–D451. doi:10.1093/nar/gkaa1038
33. Kim JT, Wajda M, Cuff G, et al. Evaluation of aromatherapy in treating postoperative pain: pilot study. Pain Pract. 2006;6(4):273–277. doi:10.1111/j.1533-2500.2006.00095.x
34. Darmani NA, Zhong W, Chebolu S, Vaezi M, Alkam T. Broad-spectrum antiemetic potential of the L-type calcium channel antagonist nifedipine and evidence for its additive antiemetic interaction with the 5-HT(3) receptor antagonist palonosetron in the least shrew (Cryptotis parva). Eur J Pharmacol. 2014;722:2–12. doi:10.1016/j.ejphar.2013.08.052
35. Zhong W, Hutchinson TE, Chebolu S, Darmani NA, Guerrero-Hernandez A. Serotonin 5-HT3 receptor-mediated vomiting occurs via the activation of Ca2+/CaMKII-dependent ERK1/2 signaling in the least shrew (Cryptotis parva). PLoS One. 2014;9(8):e104718. doi:10.1371/journal.pone.0104718
36. Xiang Y, Long Y, Feng LL, Li N. Research progress on antifatigue mechanism and volatile oil of aromatherapy l. J Chin Med Mater. 2018;41(12):2953–2957. doi:10.13863/j.issn1001-4454.2018.12.047
37. Wong ST, Trinh K, Hacker B, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27(3):487–497. doi:10.1016/s0896-6273(00)00060-x
38. Munger SD, Lane AP, Zhong H, et al. Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation. Science. 2001;294(5549):2172–2175. doi:10.1126/science.1063224
39. Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244(4906):790–795. doi:10.1126/science.2499043
40. Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347(6289):184–187. doi:10.1038/347184a0
41. Peng W, Chen Y, Tumilty S, et al. Paeoniflorin is a promising natural monomer for neurodegenerative diseases via modulation of Ca(2+) and ROS homeostasis. Curr Opin Pharmacol. 2022;62:97–102. doi:10.1016/j.coph.2021.11.009
42. Zhong W, Chebolu S, Darmani NA. Thapsigargin-induced activation of Ca(2+)-CaMKII-ERK in brainstem contributes to substance P release and induction of emesis in the least shrew. Neuropharmacology. 2016;103:195–210. doi:10.1016/j.neuropharm.2015.11.023
43. Lu XZ, Bi XY, Yu XJ, Zang WJ. Research progress of calcium/calmodulin-dependent protein kinase II and cardiovascular diseases. Prog Physiol Sci. 2014;45(01):32–36.
44. Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol Motil. 2011;23(7):e282–e293. doi:10.1111/j.1365-2982.2011.01673.x
45. Illario M, Cavallo AL, Bayer KU, et al. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J Biol Chem. 2003;278(46):45101–45108. doi:10.1074/jbc.M305355200
46. Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21–44. doi:10.1080/02699050500284218
47. Sanger GJ, Holbrook JD, Andrews PL. The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci. 2011;32(7):402–409. doi:10.1016/j.tips.2011.03.009
48. Batra VR, Schrott LM. Acute oxycodone induces the pro-emetic pica response in rats. J Pharmacol Exp Ther. 2011;339(3):738–745. doi:10.1124/jpet.111.183343
49. Buckle J. Aromatherapy for health professionals. Beginnings. 2003;23(1):6–7.
50. Efe Ertürk N, Taşcı S. The effects of peppermint oil on nausea, vomiting and retching in cancer patients undergoing chemotherapy: an Open Label Quasi-Randomized Controlled Pilot Study. Complement Ther Med. 2021;56:102587. doi:10.1016/j.ctim.2020.102587
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.