Back to Journals » International Journal of General Medicine » Volume 14
Study of the Correlation Between the Ratio of Diastolic to Systolic Durations and Echocardiography Measurements and Its Application to the Classification of Heart Failure Phenotypes
Authors Cheng L, Liao K , Wang Y, Lv F, Guo X, Zheng Y , Qin J
Received 14 June 2021
Accepted for publication 19 August 2021
Published 10 September 2021 Volume 2021:14 Pages 5493—5503
DOI https://doi.org/10.2147/IJGM.S324319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Lifang Cheng,1,2,* Kangla Liao,2,* Yingying Wang,2 Fajin Lv,1 Xingming Guo,3 Yineng Zheng,1 Jian Qin2
1Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 2Department of Cardiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 3Key Laboratory of Biorheology Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing, 400044, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yineng Zheng; Jian Qin Tel/Fax +86-23-89012558
Email [email protected]; [email protected]
Background: This study aimed to investigate the correlation between the ratio of diastolic to systolic durations (D/S) and echocardiographic parameters of patients with chronic heart failure (CHF) and evaluate whether the D/S can be used as a supplementary biomarker for the classification of heart failure (HF) phenotypes.
Methods: In total, 122 CHF patients with a left ventricular ejection fraction (LVEF) < 40%, 40%≤LVEF< 50%, or ≥ 50% were categorized as having HF with a reduced ejection fraction (HFrEF) (N=32), HF with a mid-range ejection fraction (HFmrEF) (N=21) or HF with a preserved ejection fraction (HFpEF) (N=69), respectively. All patients underwent echocardiography for assessment of nineteen structural and functional echocardiographic parameters and digital phonocardiography for the measurement of D/S. Spearman correlation was used to analyse the associations between the D/S and echocardiographic parameters. Multivariate logistic regression analysis was performed to examine the associations between the D/S and HF phenotypes, and receiver operating characteristic (ROC) curve analysis was employed to evaluate the predictive value of the D/S in the classification of HF phenotypes.
Results: The D/S values of patients with HFrEF, HFmrEF and HFpEF were 1.32± 0.06, 1.44± 0.11 and 1.54± 0.08, respectively, which were significantly different (All P< 0.05). A close correlation between the D/S and LVEF was found (r=0.777, P< 0.001). The multivariate analysis indicated that the D/S was an independent risk factor for CHF phenotypes (OR=4.927, 95% CI 2.532– 9.587; P< 0.001). The area under the ROC curve for distinguishing between HFmrEF and HFpEF using the D/S was 0.764 (95% CI 0.707– 0.845; P < 0.001) and that for distinguishing between HFmrEF and HFrEF using the D/S was 0.821 (95% CI 0.755– 0.882; P < 0.001).
Conclusion: The D/S was significantly associated with LVEF, and as LVEF decreased, the D/S tended to decrease, which could also serve as a noninvasive supplementary indicator for detecting systolic and diastolic dysfunction.
Keywords: phonocardiogram, heart sounds, ratio of diastolic to systolic durations, D/S, chronic heart failure, CHF
Introduction
Despite the advances in diagnostic technologies and treatments for cardiovascular disease over the past decade, the rates of morbidity, readmission and mortality of chronic heart failure (CHF), which is a complex clinical syndrome characterized by impaired ventricular filling and a reduced ejection fraction resulting from structural and/or functional abnormalities of the heart, remain high worldwide.1,2 Echocardiography is the most common and valuable imaging tool for serial evaluation of heart failure (HF), especially in patients with a reduced ejection fraction (HFrEF).3 However, conventional echocardiographic measurements alone not only have limited capability in the diagnosis of HF with a mid-range ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF)4 but also exhibit varying results that are affected by the skill and judgement of the medical technician performing the examination due to the need for significant professional skill in image acquisition and interpretation. Moreover, echocardiography requires professionally trained technicians because of the complicated procedures involved, which may result in inaccurate diagnoses in primary health-care institutions.5 Therefore, simple indicators that are convenient to obtain and have high repeatability are needed in daily clinical practice.
Digital phonocardiograms allow graphical visualization and recording of heart sounds derived from the various cardiac structures that pump and move blood.6 Phonocardiography data usually supplement information obtained by cardiac auscultation with a stethoscope and allow quantitative detection of diastole and systole.7 The noticeable advantages of phonocardiography are its low cost, easy operation and time efficiency, which facilitate the possibility of achieving portable, objective and repeatable measurements within a short time. This technique contributes to the assessment of disease severity in hospitalized patients with CHF and to clinical decision-making, given the differences in therapeutic strategies for treating HFpEF and HFrEF, which has led to a progressive increase in the importance of phonocardiography in heart function evaluation in recent years.
The responses of systolic time intervals (STIs) to changes in a variety of haemodynamic states are sensitive and can also noninvasively reflect left ventricular (LV) function. Previous studies have demonstrated the potential of the ratio of diastolic to systolic durations (D/S) in noninvasive evaluation of cardiac reserve.8 The length of diastole determines whether the myocardial perfusion time is sufficient and is associated with cardiac health status and survival status9 because it is related to the level of nutrients and oxygen that will be available during systole and to ventricular filling and cardiac output. When CHF occurs, compromised cardiac filling and function abnormally prolong the systolic duration or shorten the diastolic duration, so that a reduced D/S can be observed in patients with HFrEF.10 However, the D/S has not been studied in relation to HFmrEF and HFpEF. In addition, to further explore the clinical utility of the D/S in the CHF population, an investigation of the correlations between the D/S and echocardiographic parameters is urgently needed to verify our hypothesis that a reduced D/S is associated with an increased risk of occurrence of HFmrEF or HFpEF. Accordingly, the aim of this study was to investigate the relationship between the D/S, as measured by phonocardiography, and the echocardiographic parameters of patients with CHF and evaluate whether the D/S can be used as a supplementary cardiovascular biomarker for the classification of HF phenotypes. Moreover, the agreement between D/S measurements on phonocardiography and echocardiography was also assessed.
Methods
Study Population and Protocol
Our study population consisted of 152 consecutive patients with CHF confirmed by experienced cardiologists according to the established HF diagnostic criteria from the most recent guidelines of the American College of Cardiology/American Heart Association (ACC/AHA)11 between October 23, 2019, and January 16, 2020. HF patients with an LV ejection fraction (LVEF)<40%, 40%≤LVEF<50% and LVEF≥50% were categorized as HFrEF (N=38), HFmrEF (N=31) and HFpEF (N=83), respectively. In addition, 30 patients were excluded because of severe mitral stenosis (N=2), constrictive pericarditis (N=5), atrial fibrillation (N=4), the use of mechanical ventilation, pacemaker implantation (N=7), heart valve replacement (N=2), gravidity (N=1), and a lack of echocardiographic data (N=9). In total, 122 patients with CHF were enrolled. The study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University. Informed consent was obtained from each patient.
Echocardiography
Each patient underwent a standard two-dimensional and Doppler echocardiographic examination (IE33 ultrasound system, Philips Medical Systems, Holland) performed by ultrasound investigators who interpreted the corresponding findings and were blinded to the clinical information and heart sound data. The LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), wall thickness, and LV fractional shortening (FS) were measured by M-mode echocardiography. The LVEF was calculated using the biplane Simpson method. The pulsed-wave Doppler echocardiography sample volume was positioned between the tips of the mitral leaflets to derive the following variables: peak early transmitral filling velocity (E), late transmitral filling velocity (A), and the ratio of E to A (E/A) from the LV filling recordings. Early diastolic mitral annular velocity (e’), late diastolic mitral annular velocity (a’) and the ratio of e’ to a’ (e’/a’) were acquired by the pulsed-wave tissue Doppler method. The ratio of E to e’ (E/e’) was calculated to reflect the LV filling pressure. The pulmonary artery systolic pressure (PASP) was estimated according to the tricuspid valve regurgitation peak velocity and right atrial pressure. For the patients with sinus rhythm, systolic duration was measured by the interval from the end of a’ wave to end of s wave at zero baseline, and diastolic duration was measured by the interval between the end of s wave to the end of a’ wave in the subsequent cardiac cycle. For the patients with atrial fibrillation, systolic duration was measured by the interval from the end of e’ wave to end of s wave at zero baseline, and diastolic duration was measured by the interval between the end of s wave to the end of e’ wave in the subsequent cardiac cycle. Measurements were made online from 5 cardiac cycles, and the results were averaged for analysis in order to reduce the effect of irregular heart rate.
Phonocardiography and D/S Measurement
Patients underwent a simultaneous phonocardiogram/electrocardiogram recording within 5 hours of undergoing an initial echocardiographic examination. The digital phonocardiogram was obtained by a multi-channel physiological measurement system (RM-6240BD, Chengdu Instrument Factory, China) on a 120-second strip with a simultaneous electrocardiogram (ECG), which was recorded from the V3/V4 standard precordial position. The automatic measurement of the D/S over an average of 30 cardiac cycles was performed by a computerized algorithm (patent number: ZL201710698340.X).
Statistical Analysis
Normally distributed continuous variables are presented as the mean ± standard deviation (SD), whereas variables with skewed distributions are presented as the median with the interquartile range [M (Q1, Q3)]. Categorical variables are expressed as frequencies (proportions), and the chi-square test was used for data comparisons between groups. Comparisons of the D/S and echocardiography measurements among the different CHF subtype groups were performed using the Kruskal–Wallis test or one-way analysis of variance (ANOVA), where appropriate. Within-group post hoc comparisons were performed with the Bonferroni-corrected Mann–Whitney U-test or Student’s t-test when significant differences were observed among the three-group comparisons. The intraclass correlation coefficient was used to assess the agreement of each indicator. Correlations between the D/S and echocardiography parameters were analysed using the Spearman rank correlation. Receiver operating characteristic (ROC) curve analysis was used to calculate the area under the curve (AUC) of the D/S to aid in the classification of CHF phenotypes and was also used to identify the cut-off values of the D/S that best predicted CHF phenotypes. Specificity and sensitivity were also calculated. The optimal cut-off value was defined as the highest Youden index [(specificity + sensitivity) – 1]. Univariate logistic regression was used to evaluate whether a variable was independently associated with CHF phenotypes, and statistically significant risk factors were further included and examined in a multivariable logistic regression model to establish a predictive model. The Bland-Altman plot was used to assess the agreement of D/S measurements obtained by phonocardiography and echocardiography. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed with R software (version 3.4.2).
Results
Demographic and Baseline Clinical Characteristics of the Study Population
The demographics and baseline clinical parameters of the patients in the HFpEF, HFmrEF and HFrEF subgroups are summarized in Table 1. Of the patients included in this analysis, 68 (55.74%) patients had coronary artery disease (CAD), 73 (59.8%) patients had hypertension, 52 (42.6%) patients had type 2 diabetes mellitus (T2DM), 16 (13.1%) patients had dilated cardiomyopathy (DCM), and 28 (22.9%) patients had pulmonary arterial hypertension (PAH). There were no statistically significant differences in age, heart rate, New York Heart Association (NYHA) classification, CAD, hypertension and T2DM, among the groups.
 |
Table 1 Baseline Characteristics |
D/S Among Different CHF Phenotypes and Its Associations with Echocardiographic Measurements
The D/S and echocardiographic measurements for different CHF phenotypes are presented in Table 2. The illustrations of LVEF measurement using the Simpson method and the systolic and duration measurement are shown in Figures 1 and 2, respectively. Compared with patients in the HFmrEF and HFpEF groups, those with HFrEF had the lowest D/S values (1.32±0.06; P < 0.001). As the LVEF decreased, the D/S tended to decrease. The results of the correlation analysis between the D/S and echocardiographic measurements are shown in Figure 3. There was a strong positive correlation between the D/S and LVEF (r=0.777, P < 0.001) and a negative correlation between the D/S and LVESD (r=−0.702, P < 0.001). In addition, the D/S was significantly correlated with some echocardiographic indexes of the quantitative assessment of systolic function, such as LV posterior wall motion amplitude (LVPWMA) (r=0.690, P < 0.001), FS (r=0.680, P < 0.001), and IVSE (r=0.677, P < 0.001), and inversely correlated with those of diastolic function, such as LVEDD (r=−0.598, P < 0.001), LAAD (r=−0.359, P < 0.001) and E/e’ (r=−0.267, P=0.036). There was no significant correlation (P=0.084) between the D/S and heart rate in the patients with CHF.
 |
Table 2 Heart Sound Feature and Echocardiographic Indexes |
 |
Figure 2 The measurement of systolic and diastolic durations from pulsed tissue Doppler for (A) patients with sinus rhythm or (B) atrial fibrillation. |
Determination of the Predictive Value of D/S for CHF Phenotypes
ROC curve analysis was performed to identify the optimal value of the D/S for the classification of CHF phenotypes in hospitalized patients. As shown in Figure 4, the AUC for the distinction between HFmrEF and HFpEF using the D/S was 0.764 (95% CI 0.707–0.845; P < 0.001) and that for the distinction between HFmrEF and HFrEF using the D/S was 0.821 (95% CI 0.755–0.882; P < 0.001). Table 3 shows the optimal cut-off values, accuracy, sensitivity and specificity for distinguishing HFmrEF from HFpEF and HFmrEF from HFrEF using the D/S measured by phonocardiography.
 |
Table 3 The Result of ROC Curve Analysis |
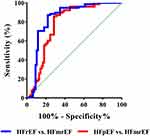 |
Figure 4 Receiver operating characteristic (ROC) curve for predicting CHF phenotypes. |
D/S is an Independent Risk Factor for CHF Phenotypes
We next performed univariate and multivariate logistic regression analyses to identify whether the D/S is an independent predictive factor for CHF phenotypes. Clinical indicators, such as sex, DCM and PAH, were analysed for statistically significant differences using univariate logistic regression, and the results are shown in Table 4. Variables with significant differences among groups revealed in the univariate analysis were included in the multivariable logistic regression analysis for further exploration. The D/S was transformed into an ordered hierarchical variable according to the two optimal cut-off values. Multivariate logistic regression analysis demonstrated that the D/S (HFrEF vs HFpEF: OR 4.927, 95% CI 2.532–9.587, P < 0.001; HFmrEF vs HFpEF: OR 1.832, 95% CI 0.847–4.779, P=0.008) was an independent risk predictor of the CHF phenotypes. Compared with the HFmrEF and HFpEF groups, patients with HFrEF were more likely to have the depressed D/S.
 |
Table 4 Univariable and Multivariable Predictors of HFrEF, HFmEF and HFpEF |
Agreement Evaluation
To assess the reliability of the D/S measurement, we compared the D/S measured by phonocardiography and that measured by echocardiography in the same patients. The plot depicted in Figure 5A directly compare D/S values measured by phonocardiography (x-axis) versus those measured by echocardiography (y-axis). The Bland-Altman analysis (Figure 5B) showed that the average discrepancy between the two technologies was small, at −0.08, with 95% limits of agreement ranging between −0.13 and 0.13. All plots showed good agreement between the measurements.
Discussion
In this study, the correlations between the D/S and echocardiographic indexes were investigated, and we demonstrated that the D/S was significantly different among HFrEF, HFmrEF and HFpEF patients and that it is an independent risk factor for CHF phenotypes. Furthermore, the D/S can be used as a supplementary cardiovascular biomarker for the classification of CHF phenotypes.
The 2016 European Cardiology Association HF guidelines assigned new cut-off values for the classification of CHF and highlighted the need to accurately distinguish CHF phenotypes because patients with different CHF phenotypes seem to benefit from different treatment strategies.4,12 Although LVEF is a commonly used clinical indicator for the differential diagnosis of CHF, echocardiographic assessment of LVEF requires professionally skilled physicians. Therefore, rapid, convenient, effective and reliable cardiovascular indicators are needed for preliminary screening and diagnosis in primary health-care institutions in remote areas.
A previous study showed that the D/S is a noninvasive indicator for evaluating cardiac reserve. Application of the D/S to cardiac function assessment in various populations, such as preterm infants,13 neonates,14 healthy young people15 and pregnant women,16 has been studied. Nevertheless, measurement of the D/S among different CHF phenotypes has not been investigated, and its associations with echocardiography measurements has received little attention and has not previously been revealed. Our study demonstrated that the D/S was not only significantly different among HFrEF, HFmrEF and HFpEF patients but also strongly correlated with LVEF. As LVEF decreases, the D/S tends to decrease.
For patients with CHF, cardiac dysfunction is a consequence of progressively impaired cardiac contractility17 coupled with abnormal haemodynamic conditions. Since the monitoring of systolic and diastolic durations can reflect cardiac haemodynamics,18 there is also accumulating evidence that systole and diastole are fundamental to the coronary flow reserve because the functional systolic and diastolic reserves are decreased, even in the early subclinical stages of cardiac dysfunction.19,20 Nevertheless, the durations of systole and diastole are not routinely evaluated for different CHF subtypes. In this study, our results revealed that the D/S was different among HFpEF, HFmrEF and HFrEF and confirmed that a reduced D/S was associated with a worse LVEF and worse systolic function. Our findings are consistent with previous studies and offer a plausible explanation for the physiological mechanism that causes a prolonged systolic duration and shortened diastolic duration due to the degree of impaired ventricular function and decreased cardiac output.20–22 The D/S could be a particularly effective indicator because it may monitor not only prolongation of isovolumic contraction and relaxation periods but also shortening of the diastole filling period. Although durations of systole and especially diastole are strongly affected by heart rate, the correlation between D/S and heart rate in the patients with CHF, and the result shows no significant correlation. This may be because D/S is a relative value that may equalize the influence of differing heart rates on systolic/diastolic durations.
Since 2006, researchers have focused on studies involving the systolic-to-diastolic duration ratio (S/D),20,23–27 which was measured by echocardiography. The S/D was observed to be abnormally increased in children with dilated and restrictive cardiomyopathy.23,24 Friedberg et al26 investigated S/D values in children with hypoplastic left-heart syndrome and suggested that the S/D can be used as a promising novel indicator of global right ventricular (RV) function. In addition, the S/D was used to evaluate PAH severity.20,27 Alkon et al20 found that an increased S/D > 1.4 inversely correlated with survival in children with PAH and suggested that the S/D was beneficial for more detailed assessments of ventricular performance. Ghio et al28 identified that the correlates of RV function impairment in HFrEF were different from those in HFpEF and HFmrEF. The S/D can be used to reflect global RV performance in PAH.19 Our study indicates that the D/S is an independent risk factor for CHF phenotypes and can also be used to aid in the classification of HF phenotypes. This observation is in agreement with previous studies19,20,26,27 indicating that the S/D can reflect global RV dysfunction, of which the correlates in HFrEF were different from those in HFpEF and HFmrEF. A study conducted by Robaeys et al29 demonstrated that RV dysfunction was frequently present in HF, irrespective of LVEF. Additionally, the S/D is a sensitive indicator for measuring global RV dysfunction,19,20,26,27 and this may explain the phenomenon in which some patients have a normal LVEF but reduced D/S.
The essence of the two indicators, D/S and S/D, is the same since they are mutually reciprocal. Our study also demonstrated good agreement between D/S measurements obtained by phonocardiography and echocardiography for the first time. This indicates that the results of the D/S and S/D measured by these two technologies can be easily shared and are beneficial for facilitating the potential of the D/S or S/D in clinical applications. Phonocardiography, as a visualization of digital cardiac auscultation, also has potential in serving as one of the first examinations performed for primary screening of various cardiovascular diseases. Therefore, the D/S obtained from digital phonocardiograms has the advantages of speed, convenience, simple operation and repeatable measurement and can be used widely in primary medical institutions. The D/S will facilitate a timely differential diagnosis, classification and response evaluation to significantly reduce the risk of morbidity and mortality, especially when echocardiography is not immediately available.
Several limitations should be mentioned for the present study. First, the study population was enrolled from a single hospital institution and the number of patients included in our study was relatively small. Next, external validation data from multiple institutions should be used to verify the effectiveness of indicator. Second, due to the retrospective nature of the study, there were some unidentified confounding factors that could not be controlled. Although the CHF cohort of this study was selected from clinically stable inpatients, time-dependent variables such as medications that can affect heart sound variables may have impacted our results, and the results are not applicable to all patients with CHF. Third, some laboratory data such as brain natriuretic peptide (BNP), high-sensitivity troponin T (hs-TNT), and secreted frizzled-related protein 5 (SFRP5) were not evaluated. For some readmitted patients with a past medical history of CHF, BNP and hs-TNT testing was not performed routinely, and SFPR5 was not detected in most subjects because of the retrospective study. The further prospective study should involve laboratory biomarkers and perform subgroup analysis to establish their associations with D/S and explore the corresponding physiological mechanism. In future work, the results derived from this study should be further validated externally using multi-centre and large-sample randomized control trials. Then, an in-depth study including outcome assessment of treatment through follow-up visits is needed to further establish whether the D/S or another heart sound feature alert may improve patient prognosis when correlated with appropriate intervention tactics.
Conclusion
In summary, we demonstrated that the D/S was significantly different among HFrEF, HFmrEF and HFpEF patients and was highly correlated with LVEF. We also identified that the D/S was an independent risk factor for CHF phenotypes and can be used as a supplementary cardiovascular biomarker for the classification of CHF phenotypes. This suggests that the D/S could serve as a noninvasive indicator for systolic and diastolic dysfunction detection and may be useful for providing initial guidance for cardiovascular disease management in CHF patients.
Abbreviation
CHF, Chronic heart failure; ESC, European Society of Cardiology; HFrEF, Heart failure with a reduced ejection fraction; HFmrEF, Heart failure with a mid-range ejection fraction; HFpEF, Heart failure with a preserved ejection fraction; HS, Heart sound; S1, The first heart sound; S2, The second heart sound; S3, The third heart sound; LV, Left ventricular; STIs, Systolic time intervals; SDI, Systolic dysfunction index; EMAT, Electromechanical activation time; LVEF, Left ventricular ejection fraction; D/S, Ratio of diastolic to systolic durations; E/A, Ratio of the peak early diastolic velocity (E) to the peak atrial filling velocity (A); E/e’, Ratio of E to the early diastolic mitral annular velocity (e’); ARD, Aortic root diameter; RATD, Right atrium transverse diameter; LAAD, Left atrium anteroposterior diameter; RVAD, Right ventricular anteroposterior diameter; LVEDD, Left ventricular end-diastolic diameter; LVESD, Left ventricular end-systolic diameter; IVSEDT, Interventricular septum end-diastolic thickness; LVPWMA, Left ventricular posterior wall motion amplitude; IVSE, Interventricular septum excursion; LVPWEDT, Left ventricular posterior wall end-diastolic thickness; PASP, Pulmonary arterial systolic pressure; FS, Fractional shortening; e’/a’, Ratio of e’ to the late mitral annular velocity (a’); E/e’, Ratio of E to e’; TAPSE, Tricuspid annular plane systolic excursion; TAPSE-s’, Tricuspid lateral annular systolic velocity; CAD, Coronary artery disease; AF, Atrial fibrillation; DCM, Dilated cardiomyopathy; PAH, Pulmonary arterial hypertension; NYHA, New York Heart Association; SFRP5, Secreted frizzled-related protein 5; HS-TNT, High-sensitivity troponin T; OR, Odds ratio; CI, Confidence interval.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31800823 and No. 31870980) and the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0395).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378. doi:10.1038/nrcardio.2016.25
2. Dharmarajan K, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin. 2017;13(3):417–426. doi:10.1016/j.hfc.2017.02.001
3. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7(9):782–794.
4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200.
5. Robertson C, Rose S, Kesselheim AS. Effect of financial relationships on the behaviors of health care professionals: a review of the evidence. J Law Med Ethics. 2012;40(3):452–466. doi:10.1111/j.1748-720X.2012.00678.x
6. Yamashita K. New non‐invasive approach to detect cardiac contractility using the first sound of phonocardiogram. Acute Medicine Surgery. 2020;7(1):e483. doi:10.1002/ams2.483
7. Shuvo SB, Ali SN, Swapnil SI, Al-Rakhami MS, Gumaei A. CardioXNet: a novel lightweight deep learning framework for cardiovascular disease classification using heart sound recordings. IEEE Access. 2021;9:36955–36967. doi:10.1109/ACCESS.2021.3063129
8. Xiao S, Guo X, Sun X, Xiao Z. A relative value method for measuring and evaluating cardiac reserve. Biomed Eng Online. 2002;1(1):1–5. doi:10.1186/1475-925X-1-6
9. Abe M, Tomiyama H, Yoshida H, Doba N. Diastolic fractional flow reserve to assess the functional severity of moderate coronary artery stenoses: comparison with fractional flow reserve and coronary flow velocity reserve. Circulation. 2000;102(19):2365–2370. doi:10.1161/01.CIR.102.19.2365
10. Zheng Y, Guo X, Qin J, Xiao S. Computer-assisted diagnosis for chronic heart failure by the analysis of their cardiac reserve and heart sound characteristics. Comput Methods Programs Biomed. 2015;122(3):372–383. doi:10.1016/j.cmpb.2015.09.001
11. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803.
12. Lyu S, Yu L, Tan H, et al. Clinical characteristics and prognosis of heart failure with mid-range ejection fraction: insights from a multi-centre registry study in China. BMC Cardiovasc Disord. 2019;19(1):1–12. doi:10.1186/s12872-019-1177-1
13. Yang X, Zeng W. Determination of cardiac reserve in preterm infants. Turkish J Pediatr. 2011;53(3):308–313.
14. Yang X, Zeng W. A relative value method for measuring and evaluating neonatal cardiac reserve. Indian J Pediatrics. 2010;77(6):661–664. doi:10.1007/s12098-010-0058-5
15. Xie M, Xiao S, Liu T, et al. Multi-center, multi-topic heart sound databases and their applications. J Med Syst. 2012;36(1):33–40. doi:10.1007/s10916-010-9443-x
16. Shao Y, Zhang Y, Liu OM. Using phonocardiography to investigate maternal cardiac reserve function in gestational hypertension and pre‐eclampsia. J Obstetrics Gynaecol Res. 2013;39(1):53–60. doi:10.1111/j.1447-0756.2012.01897.x
17. Norman HS, Oujiri J, Larue SJ, Chapman CB, Margulies KB, Sweitzer NK. Decreased cardiac functional reserve in heart failure with preserved systolic function. J Card Fail. 2011;17(4):301–308. doi:10.1016/j.cardfail.2010.11.004
18. Urbaszek A, Kirchner J, van Ooyen A, Skerl O. Hemodynamic Monitoring with an Implantable Pressure Monitor is Improved by Additional Detection of Heart Sounds. Biomed Eng-Biomed Te. 2012;57:740–742.
19. McCabe C, Vranesic II, Verdes MC, et al. Right ventricular systolic to diastolic duration ratio: a novel predictor of outcome in adult idiopathic pulmonary arterial hypertension. Int J Cardiol. 2019;293:218–222. doi:10.1016/j.ijcard.2019.05.019
20. Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am J Cardiol. 2010;106(3):430–436. doi:10.1016/j.amjcard.2010.03.048
21. Mondal T, Slorach C, Manlhiot C, et al. Prognostic implications of the systolic to diastolic duration ratio in children with idiopathic or familial dilated cardiomyopathy. Circ Cardiovasc Imaging. 2014;7(5):773–780.
22. Xu B, Kawata T, Daimon M, et al. Prognostic value of a simple echocardiographic parameter, the right ventricular systolic to diastolic duration ratio, in patients with advanced heart failure with non-ischemic dilated cardiomyopathy. Int Heart J. 2018;59(5):968–975. doi:10.1536/ihj.17-475
23. Friedberg MK, Silverman NH. Cardiac ventricular diastolic and systolic duration in children with heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97(1):101–105. doi:10.1016/j.amjcard.2005.07.127
24. Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with heart failure secondary to restrictive cardiomyopathy. J Am Soc Echocardiography. 2006;19(11):1326–1331. doi:10.1016/j.echo.2006.05.024
25. Sarnari R, Kamal RY, Friedberg MK, Silverman NH. Doppler assessment of the ratio of the systolic to diastolic duration in normal children: relation to heart rate, age and body surface area. J Am Soc Echocardiography. 2009;22(8):928–932. doi:10.1016/j.echo.2009.05.004
26. Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with hypoplastic left heart syndrome: a novel Doppler index of right ventricular function. J Am Soc Echocardiography. 2007;20(6):749–755. doi:10.1016/j.echo.2006.11.014
27. Sehgal A, Athikarisamy SE, Adamopoulos M. Global myocardial function is compromised in infants with pulmonary hypertension. Acta Paediatrica. 2012;101(4):410–413. doi:10.1111/j.1651-2227.2011.02572.x
28. Ghio S, Guazzi M, Scardovi AB, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19(7):873–879. doi:10.1002/ejhf.664
29. Robaeys W, Bektas S, Boyne J, et al. Pulmonary and right ventricular dysfunction are frequently present in heart failure irrespective of left ventricular ejection fraction. Heart Asia. 2017;9(2):548. doi:10.1136/heartasia-2017-010914
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.