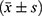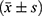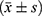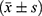Back to Journals » International Journal of General Medicine » Volume 15
Study of Intracranial Hematoma Removal and High Intracranial Pressure Reduction Using a Novel Three-Needle Brain Puncture Technique
Authors Song A, Yang H, Wu G, Ren S, Wang L, Qin G, Mao Y
Received 4 October 2022
Accepted for publication 16 December 2022
Published 30 December 2022 Volume 2022:15 Pages 8797—8805
DOI https://doi.org/10.2147/IJGM.S392149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Anjun Song,1,* Hui Yang,2,* Guofeng Wu,1 Siying Ren,1 Likun Wang,1 Guannan Qin,1 Yuanhong Mao1
1Emergency Department, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 2Guiyang Public Health Treatment Center, Guiyang, Guizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Anjun Song, Email [email protected]
Objective: The present study aimed to evaluate the clinical value of minimally invasive surgery for intracranial hematoma removal and high intracranial pressure (ICP) reduction using a novel three-needle brain puncture technique.
Methods: A total of 202 cases with supratentorial hematoma were analyzed, 54 of whom received three-needle brain puncture (study group), and the remaining cases received single-needle (control groups 1 and 2) and two-needle brain puncture (control group 3). The amount of intracranial hematoma removed, changes in ICP, retention time of puncture needle, volume of residual blood, the National Institute of Health Stroke Scale (NIHSS) score, and postoperative survival rate were used as indexes to evaluate patient outcomes.
Results: We found that three-needle brain puncture (study group) can remove more intracranial hematoma (P < 0.05) and achieve lower ICP (P < 0.05) than single- and two-needle brain puncture (control group). The needle retention time and volume of residual blood significantly decreased in the study group. Additionally, a statistically significant difference was observed in the NIHSS scores and survival rates between the study and control groups (P < 0.05).
Conclusion: These data suggest that three-needle minimally invasive stereotactic puncture can effectively remove hematoma, reduce ICP, decrease the degree of brain damage, and improve prognosis.
Keywords: cerebral hemorrhage, intracranial pressure monitoring, minimally invasive procedures, three-needle puncture, National Institute of Health Stroke Scale
Introduction
Hypertensive intracerebral hemorrhage (HICH) is a devastating event that develops rapidly and is associated with a high mortality rate, primarily due to brain injury caused by increased intracranial pressure (ICP).1 Brain damage caused by medium-to-large volume intracranial hematoma has a high incidence of sequelae and a high mortality rate, posing a huge burden on patients, families, and society.2,3 Currently, the development of effective therapeutic strategies for acute HICH is still challenging. With the constant innovation concept of minimally invasive surgery and the continuous improvement of surgical instruments, minimally invasive surgery has been applied in clinical practice to improve postoperative outcomes. If patients are timely treated with minimally invasive surgery to remove intracranial hematoma and provided general care to reduce ICP, their survival outcomes will be significantly improved. The preoperative period and surgical procedures used for medium-to-large volume ICP are the determinant factors. It is of positive clinical significance to control the preoperative period and improve surgical procedures.4,5 In this paper, clinical data of patients with 30–70 mL of ICH treated with different stereotactic minimally invasive surgeries were collected to determine the optimal treatment for HICH patients. This study describes a novel three-needle brain puncture technique for intracranial hematoma removal and high ICP management. Our findings are expected to provide results in terms of both efficacy and safety of HICH treatment, as well as improve the prognosis of patients.
Materials and Methods
This study of spontaneous ICH treatment (including the use of clinical data and personal patient data) was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All patients or their authorized representatives provided written informed consent to undergo minimally invasive surgery.
Instrument and Reagents
The equipment used for minimally invasive puncture were as follows: Stereotactic device (Xi’an Weisheng Medical Device Co., Ltd., China), disposable intracranial hematoma removal package (Beijing Wantefu Technology Co., Ltd., China), ICP monitoring sensor and ICP monitor (Johnson & Johnson (Shanghai) Medical Device Co., Ltd., USA), miniature skull drill, cerebral drainage bag, silicone syringe, general surgical instruments, disposable applicator, urokinase injection (Guangdong Techpool Bio-Pharma Co., Ltd., China), urapidil injection (Shandong Luoxin Pharmaceutical Group Co., Ltd., China), nitroglycerin injection (Beijing Yimin Pharmaceutical Co., Ltd., China), compound mannitol injection, glycerol, fructose injection, 2% lidocaine hydrochloride injection, and 0.9% sodium chloride injection.
Inclusion and Exclusion Criteria for Patients
The inclusion criteria were as follows: (1) Patients diagnosed with supratentorial cerebral hemorrhage (30–70mL) through cerebral computed tomography (CT) scanning but no obvious subarachnoid hemorrhage; (2) patients with a history of hypertension or hypertension observed upon admission; and (3) patients whose family members provided informed consent for surgery. The preoperative “agreement to puncture” period for the study group was approximately 1 hour (<2 h), while that for the control group was more than 2 hours (≥2 h).
The exclusion criteria were as follows: (1) patients with brain herniation requiring emergency surgery; (2) patients with contraindications or serious complications; (3) patients with cerebral vascular malformations or hemorrhagic neoplasms requiring neurosurgery; (4) patients with hematoma breaking into ventricles with cast formed; (5) patients who died within two weeks; and (6) patients whose family members refused to sign the surgical consent.
Treatment Methods
In accordance with the criteria mentioned above, 202 patients with HICH (volume of intracranial hematoma: 30–70 mL) who received treatment in the emergency department at the affiliated hospital of Guiyang medical college between January 1, 2017 and March 1, 2021 were selected. After admission, the patients were randomized to a study group (54 patients) and a control group (148 patients). Patients in the study group received two-needle minimally invasive stereotactic puncture to evacuate the intracranial hematoma, with an ICP sensor probe with a drainage tube (the third needle) placed into the anterior horn of the lateral ventricle opposite the hematoma to monitor ICP and drain cerebrospinal fluid. The control group was divided into three groups: Control group 1 (39 patients), control group 2 (51 patients), and control group 3 (58 patients). Control group 1 received single-needle minimally invasive stereotactic puncture, and an ICP sensor probe without a drainage tube was placed into the upper lateral edge of the hematoma to monitor ICP. Control group 2 received single-needle minimally invasive stereotactic puncture, and an ICP sensor probe without a drainage tube was placed in the anterior horn of the lateral ventricle opposite the hematoma to monitor ICP. Control group 3 received two-needle minimally invasive stereotactic puncture, and an ICP sensor probe without a drainage tube was placed in the anterior horn of the lateral ventricle opposite the hematoma to monitor ICP (Figure 1). Gender, age, and other general conditions of the patients in the study and control groups had no significant differences (Table 1). All patients received routine treatment such as sedation, hemostasis, blood pressure reduction, anti-infection, dehydration, ICP reduction, and brain cell protection treatment when admitted to the hospital.
 |
Table 1 Comparison of General Clinical Data Between Two Groups |
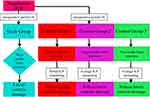 |
Figure 1 The experiment consort diagram. |
Evacuation of Hematoma by a Minimally Invasive Procedure
Before minimally invasive surgery, each patient underwent a repeat CT scan after the location framework was fixed on the patient’s head, and the scan was then printed in actual size. Using the CT scan, the coordinates (X-Y-Z) of the intracranial hematoma and puncture sites were determined without damaging the functional areas. Local anesthesia was then administered at the puncture point before surgery.
The skull was punctured by an electrical drill under the guidance of a stereotactic instrument, and an ICP sensor probe with a drainage tube (the first needle) was inserted slightly into the anterior horn of the lateral ventricle opposite the hematoma (A point in Figure 2) in the study group to monitor ICP and drain cerebrospinal fluid. For the control group, an ICP sensor probe without a drainage tube was placed in the upper lateral edge of the hematoma or in the anterior horn of the lateral ventricle opposite the hematoma to monitor ICP. Next, in the study group, two puncture needles (the second and third needle) were inserted slightly into the upper (B point in Figure 2) and lower (C point in Figure 2) parts of the hematoma (Figure 2), respectively. In the control group, the maximum volume of blood aspirated was approximately 1/3rd of the total amount of intracranial hematoma, and aspiration was stopped if the ICP returned to a normal low limit. Subsequently, the ICP was continuously monitored, and the drainage bags were connected to the needle for intracranial hematoma drainage following the injection of dissolved urokinase into the hematoma area. To dissolve the solid part of the hematoma after surgery, urokinase (50,000 units diluted in 2 mL of 0.9% saline buffer) was injected slowly into the residual hematoma area three times a day. The needle system was closed for 2 h before reopening to allow for spontaneous drainage. Postoperative CT scans were performed on the third, fifth, and seventh day after surgery. The puncture needle system was removed after a brain CT confirmed that the hematoma was either completely or nearly completely removed.
 |
Figure 2 The puncture points of three-needle brain puncture are illustrated in the CT scans. |
Criteria to Evaluate Treatment
The following criteria were used to evaluate treatment: (1) The volume of blood evacuated and change in ICP during operation; (2) ICP values at 4, 8, 12, 16, 20, and 24 h on the 1st, 2nd, 3rd, 4th, and 5th day after surgery; (3) the time of needle retention and residual blood volume around the hematoma; (4) the National Institute of Health Stroke Scale (NIHSS) score to evaluate neurological impairment in patients at admission, as well as 1 week and 2 weeks after admission; and (5) survival rates recorded during follow-up visit at 3 weeks and 6 months after surgery.
Statistical Analysis
Statistical analysis was performed using the SPSS 26.0 statistical software. Measurement data conforming to a normal distribution were represented by  . Student’s t-test was used to compare the two groups. Data is represented using %, and the χ2 test was adopted. P < 0.05 was considered statistically significant.
. Student’s t-test was used to compare the two groups. Data is represented using %, and the χ2 test was adopted. P < 0.05 was considered statistically significant.
Ethical Approval and Informed Consent
The study complied with the Declaration of Helsinki and obtained Institutional Review Board (IRB) approval. This study of spontaneous intracerebral hemorrhage treatment (including the use of the clinical data and the personal patient data) was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All the patients’ authorized representatives and the patients who had the ability to communicate with the doctors agreed to undergo minimally invasive surgery. Written informed consent was obtained.
Results
Comparison of Intracranial Hematoma Evacuation and Intraoperative ICP Change
Insertion of two needles into the upper and lower parts of the hematoma achieved better results, especially in the case of blood coagulation. The average volume of intracranial hematoma removed in the study group was 19.80 ± 4.90mL, while the volumes of intracranial hematoma removed in control groups 1–3 were 12.49 ± 4.17 mL, 11.82 ± 3.87 mL, and 19.72 ± 4.40 mL, respectively. The difference between the study group and control groups 1 and 2 was statistically significant (P < 0.05, Table 2). At the end of operation, the average ICP was 8.28 ± 3.39 mmHg (1 mmHg = 0.133 kPa) in the study group, 16.41 ± 4.38 mmHg in control group 1, 15.80 ± 4.54 mmHg in control group 2, and 8.41 ± 3.60 mmHg in control group 3. There was a significant difference in ICP between the study group and control groups 1 and 2 (P < 0.05, Table 2).
 |
Table 2 Comparison of Volume of ICH Clearance and the Change of Intracranial Pressure During Operation Between the Two Groups |
Comparison of Postoperative ICP Change
The ICP was high in all groups on day 1 after surgery, but the study group recorded a lower ICP than the control groups. From day 2 to day 5, the ICP in the study group decreased gradually, indicating that the ICP sensor probe with a drainage tube could better regulate the ICP with small fluctuations. There were statistically significant differences in ICP between the two groups at each time point 5 days after the operation (P < 0.05, Table 3). In the study group, intracranial pressure (mmHg) was effectively reduced on the 5th postoperative day (Figure 3).
 |
Table 3 Comparison of Intracranial Pressure (mmHg) on the First to Fifth Day After Operation Between Two Groups |
 |
Figure 3 Histogram of the mean value of intracranial pressure on the fifth day after operation. |
Comparison of Needle Retention Time and Residual Hematoma
The study group had a significantly shorter needle retention time compared to control groups 1 and 2, but no difference compared to control group 3. On the fifth day post operation, puncture needles were removed from 92.59% (50/54) of patients in the study group, 74.36% (29/39) of patients in control group 1, and 74.51% (38/51) of patients in control group 2 (P < 0.05, Table 4). Meanwhile, the residual hematoma blood volume was lower in the study group (4.25 ± 0.89 mL) than in control groups 1 (6.70 ± 1.55 mL) and 2 (7.12 ± 1.21 mL), and the difference was statistically significant (P < 0.05, Table 4).
 |
Table 4 Comparison of Retention Time of Puncture Needle and Volume of Residual Blood After Operation Between the Two Groups [Case (%)] |
Comparison of NIHSS Score
The NIHSS score was compared among all groups on admission, and there was no significant difference between the study group and control groups 1, 2, and 3 (P > 0.05, Table 5). However, the scores were significantly different at 1 and 2 weeks after admission (P < 0.05, Table 5). In the case of medium- and large-volume HICH patients, a shorter preoperative period (<2 h) is beneficial for removing hematoma, reducing ICP and brain damage, and improving the prognosis of patients by using the two-needle minimally invasive stereotactic brain puncture procedure.
 |
Comparison of Survival Rates
The survival rates of patients in the two groups were compared within the third week and at 6 months after surgery. The survival rates of patients within the third week after surgery was not significantly different between the study group and the control group 1, 2 and 3 (P > 0.05, Table 6); meanwhile, there was no significant difference between the study group and the control group 3 (91.38%, 53/58) (P > 0.05, Table 6); however, the difference between the study group (92.59%, 50/54) and the control group 1 (76.92%, 30/39) and 2 (78.43%, 40/51) was statistically significant (P < 0.05, Table 6) at 6 months after surgery.
 |
Table 6 Comparison of Survival Rate Between the Two Groups [Case (%)] |
Discussion
Primary and secondary brain damage may be consequences of intracranial hematoma. Primary brain damage, such as intracranial hypertension or cerebral herniation, can be caused by mechanical compression of hematoma, whereas secondary brain damage can be caused by toxic substances, inflammatory mediators, and free radicals released by hematoma.6,7 In recent decades, minimally invasive procedures have been used to treat patients with medium- and large-volume intracranial hematoma.5 Brain stereotactic minimally invasive surgery is one of the popular surgical options for minimally invasive intracerebral hematoma removal.8,9 However, it is still necessary to constantly improve the treatment strategy because timely removal of intracranial hematoma and reduction of ICP can reduce the risks for primary and secondary brain damages mentioned above.
In this study, the patients with supratentorial HICH of 30–70 mL, the preoperative “agreement to puncture” period is controlled in approximately 1 hour in the study group, and intracranial hematoma was removed using the two-needle minimally invasive stereotactic brain puncture procedure, an ICP sensor with a drainage tube (the third needle) was used to timely regulate the changes in ICP. This new three-needle minimally invasive technique is advanced and precise. The ICP will not be too high or too low due to irregular cerebral drainage volume. Compared to the control groups 1 and 2, removal of hematoma in the study group by selecting puncture sites from the upper and lower parts of the hematoma could help aspirating the liquid part of the hematoma and reduce ICP. The ICP in the study group was lower than that in the control groups 1 and 2, and it decreased gradually (P<0.05) from day 2 to day 5 after surgery. Meanwhile, the NIHSS scores in the study group improved remarkably at 1 and 2 weeks after admission, indicating that the study group had a better prognosis. The comparison of needle retention time, residual hematoma blood, and survival rate indicated that the three-needle minimally invasive stereotactic brain puncture procedure can achieve better results.
In this study, cerebral drainage in the study group could be controlled through the open paracele which makes the intracranial pressure decreased smoothly and improve the patients’ prognosis by reducing brain damage compared to the control group 3. The intracranial hematoma can be effectively removed using the two-needle minimally invasive stereotactic brain puncture procedure in the control group 3. Though the survival rate of the patients is high in this group, the neurological deficits are severe for improperly drain cerebrospinal fluid without a fluid drainage tube. This finding fully demonstrates the importance of the three-needle minimally invasive stereotactic brain puncture procedure.
In patients with medium- and large-volume intracranial hematoma, the fatality and disability rates caused by brain damage are very high. The therapeutic time window for HICH is approximately 6–8 hours; early surgeries performed to remove most of the hematoma and reduce the high ICP caused by compression of hematoma are believed to be optimal in terms of minimizing the damage to brain tissue.10–13 Therefore, physician-patient communication should be effective and timely, and the preoperative period should be controlled within 1 hour for patients with large volumes of intracranial hematoma (>50 mL). The use of two-needle brain puncture to clear intracranial hematoma and an ICP sensor probe with a drainage tube (the third needle) to drain cerebrospinal fluid and regulate high cranial pressure can reduce brain injury after intracranial hematoma. The results of this study are consistent with previously reported relevant literature.14–17 This provides an effective reference for the treatment of medium- and large-volume intracranial hematoma.
Conclusions
In conclusion, for patients with supratentorial HICH of 30–70 mL, an effective preoperative procedure followed by three-needle minimally invasive stereotactic brain puncture could effectively remove hematoma, reduce ICP, decrease the degree of brain damage, thereby improving the prognosis of patients, and has a very wide popularization and application prospects.
Ethical Approval and Informed Consent
This study of spontaneous intracerebral hemorrhage treatment (including the use of the clinical data and the personal patient data) was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All the patients’ authorized representatives and the patients who had the ability to communicate with the doctors agreed to undergo minimally invasive surgery. Written informed consent was obtained.
Funding
Guiyang science and technology plan projects ([2017] 30-11, GY17014).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. 2019;19(7):679–694. doi:10.1080/14737175.2019.1623671
2. Donkor ES. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165. doi:10.1155/2018/3238165
3. Burns JD, Fisher JL, Cervantes-Arslanian AM. Recent advances in the acute management of intracerebral hemorrhage. Neurosurg Clin N Am. 2018;29(2):263–272. doi:10.1016/j.nec.2017.11.005
4. Scaggiante J, Zhang X, Mocco J, Kellner CP. Minimally invasive surgery for intracerebral hemorrhage. Stroke. 2018;49(11):2612–2620. doi:10.1161/STROKEAHA.118.020688
5. Wang W, Zhou N, Wang C. Minimally invasive surgery for patients with hypertensive intracerebral hemorrhage with large hematoma volume: a retrospective study. World Neurosurg. 2017;105:348–358. doi:10.1016/j.wneu.2017.05.158
6. Song A, Wu G, Hang H, Wang L. Rosiglitazone pretreatment influences thrombin-induced anti-oxidative action via activating NQO1and gamma-GCS in rat microglial cells. Neurol Res. 2018;40(2):139–145. doi:10.1080/01616412.2017.1417686
7. Zhang Y, Chen Y, Wu J, et al. Activation of dopamine D2 receptor suppresses neuroinflammation through alphaB-crystalline by inhibition of NF-kappaB nuclear translocation in experimental ICH mice model. Stroke. 2015;46(9):2637–2646. doi:10.1161/STROKEAHA.115.009792
8. Yuan J, Lv Y, Zhang S, Li Y, Jiao X. A comparative study on the clinical efficacy of stereotaxic catheter drainage and conservative treatment for small and medium amount intracerebral hemorrhage in the Basal Ganglia. Evid Based Complement Alternat Med. 2022;2022:7393061. doi:10.1155/2022/7393061
9. Zhang J, Lu S, Wang S, Zhou N, Li G. Comparison and analysis of the efficacy and safety of minimally invasive surgery and craniotomy in the treatment of hypertensive intracerebral hemorrhage. Pak J Med Sci. 2018;34(3):578–582. doi:10.12669/pjms.343.14625
10. Menacho ST, Grandhi R, Delic A, et al. Impact of intracranial pressure monitor-guided therapy on neurologic outcome after spontaneous nontraumatic intracranial hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(3):105540. doi:10.1016/j.jstrokecerebrovasdis.2020.105540
11. Wu G, Wang L, Wang F, Feng A, Sheng F. Minimally invasive procedures for intracerebral hematoma evacuation in early stages decrease perihematomal glutamate level and improve neurological function in a rabbit model of ICH. Brain Res. 2013;1492:140–147. doi:10.1016/j.brainres.2012.11.023
12. Wang L, Luo S, Ren S, et al. Irregular-shaped hematoma predicts postoperative rehemorrhage after stereotactic minimally invasive surgery for intracerebral hemorrhage. Front Neurol. 2022;13:727702. doi:10.3389/fneur.2022.727702
13. Yu SX, Zhang QS, Yin Y, Liu Z, Wu JM, Yang MX. Continuous monitoring of intracranial pressure for prediction of postoperative complications of hypertensive intracerebral hemorrhage. Eur Rev Med Pharmacol Sci. 2016;20(22):4750–4755.
14. Al-Shahi Salman R, Klijn CJM, Selim M. Minimally invasive surgery plus alteplase for intracerebral haemorrhage. Lancet. 2019;393(10175):965–967. doi:10.1016/S0140-6736(19)30309-5
15. Shi J, Zou X, Jiang K, et al. Intracerebral hemorrhage with tentorial herniation: conventional open surgery or emergency stereotactic craniopuncture aspiration surgery. Transl Neurosci. 2021;12(1):198–209. doi:10.1515/tnsci-2020-0173
16. Kellner CP, Song R, Pan J, et al. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. 2020;12(5):489–494. doi:10.1136/neurintsurg-2019-015528
17. Al-Kawaz MN, Li Y, Thompson RE, et al. Intracranial pressure and cerebral perfusion pressure in large spontaneous intracranial hemorrhage and impact of minimally invasive surgery. Front Neurol. 2021;12:729–831. doi:10.3389/fneur.2021.729831
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.